Overview
The future of clinical research in Latin America is poised for significant growth, driven by increased investments, regulatory improvements, and advancements in digital health technologies. The article highlights key trends such as the emergence of Colombia as a research hub, the streamlined regulatory processes introduced by Brazil’s ANVISA, and the vital role of Contract Research Organizations (CROs) in enhancing efficiency and patient recruitment, all of which collectively position the region favorably for innovative healthcare solutions.
Introduction
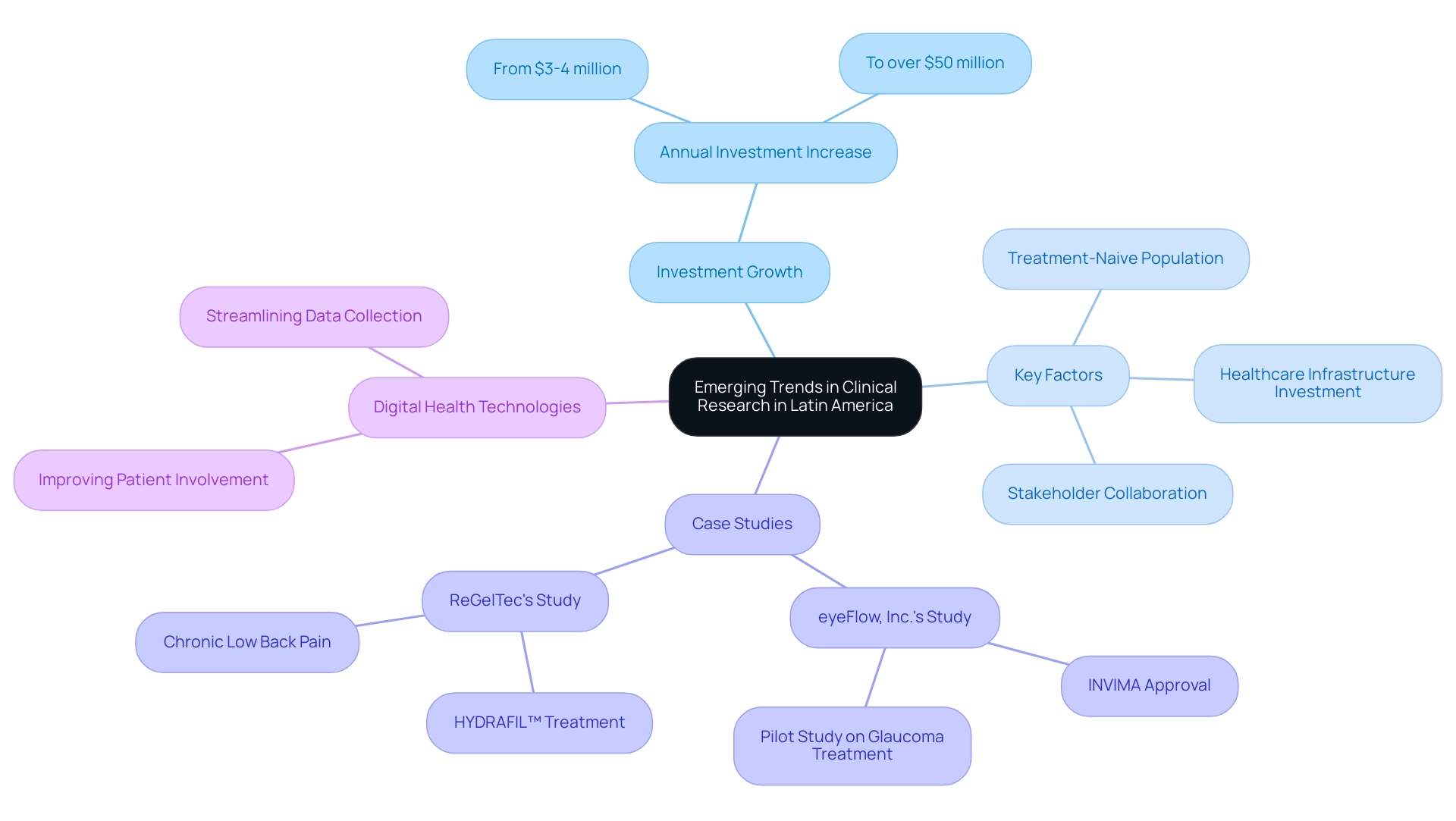
Latin America is rapidly emerging as a key player in the global clinical research landscape, driven by significant investments and a commitment to innovation. With annual investments in the sector skyrocketing from a mere $3-4 million to over $50 million, particularly in the Andean Region, the region is witnessing a surge in clinical trials and research activities.
This growth is underpinned by:
- Enhanced healthcare infrastructure
- A treatment-naive patient population
- Collaborative efforts between local and international stakeholders
As countries like Colombia, especially Barranquilla, become prominent hubs for clinical trials, the landscape is evolving with:
- New regulatory frameworks
- The pivotal role of Contract Research Organizations (CROs)
- Strategic patient recruitment initiatives
This article delves into the multifaceted trends shaping clinical research in Latin America, exploring the challenges and opportunities that lie ahead while highlighting the critical role of ethical considerations and technological advancements in driving successful outcomes.
Emerging Trends in Clinical Research Across Latin America
In recent years, the future of clinical research in Latin America has shown significant growth in research activities, particularly in the Andean Region, where annual investment in the sector soared from $3-4 million to over $50 million. This expansion is attributed to several factors, including:
- Increased investment in healthcare infrastructure
- A growing population of treatment-naive patients
- Strengthened collaboration between [local and international stakeholders
Significantly](https://blog.bioaccessla.com/understanding-research-trends-in-latin-america-an-in-depth-tutorial), Colombia—particularly Barranquilla—has emerged as a key center for research, emphasized by eyeFlow, Inc.'s recent INVIMA approval for an 18-month pilot study on a groundbreaking glaucoma treatment, aiming to recruit 60 participants.
The importance of INVIMA's regulatory endorsement highlights the dedication to safety and efficacy in research studies, which is essential for fostering trust among stakeholders. This, along with ReGelTec's initial feasibility study where eleven individuals were successfully treated with HYDRAFIL™ for chronic low back pain, which reported significant pain reduction in these individuals, highlights the potential success stories in the region. The strategic geographic position, varied demographic profiles, and adherence to regulatory compliance further enhance Barranquilla's reputation as a premier destination for medical studies.
Furthermore, the incorporation of digital health technologies plays a vital role in improving patient involvement and streamlining data collection processes, thus speeding up the progress of research across the region. Subscribing to resources like the Horizon Databook provides stakeholders with access to comprehensive market intelligence, enabling informed decision-making and a strategic advantage in anticipating industry shifts. Furthermore, advancements in the pharmaceutical industries of Mexico and Brazil enhance the overall expansion and appeal of Latin America for research studies, which is vital for the future of clinical research in Latin America as it creates opportunities for innovative healthcare solutions and significant returns for stakeholders.
Navigating the Regulatory Landscape for Clinical Trials in Latin America
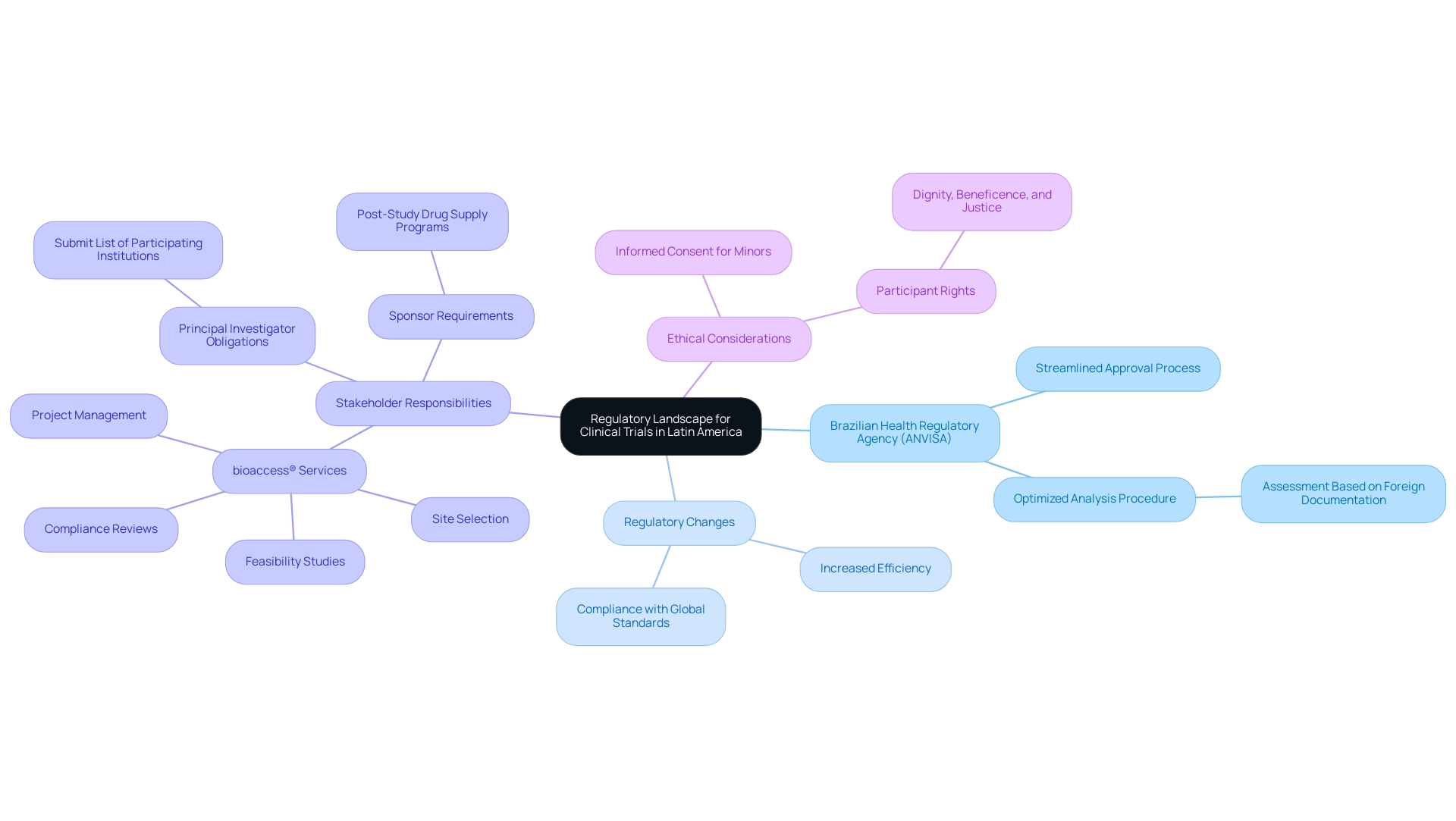
The regulatory environment is undergoing notable changes, which will significantly impact the future of clinical research in Latin America, especially in Brazil. The Brazilian Health Regulatory Agency (ANVISA) has enacted new laws aimed at streamlining the approval process for medical studies, enhancing transparency and efficiency—critical factors in attracting international sponsors to the region. In 2023, ANVISA introduced an optimized analysis procedure that enables the assessment of research applications based on documentation from comparable foreign regulatory authorities.
This initiative has markedly increased the efficiency of regulatory processes while ensuring that health products comply with established safety and efficacy standards. As Brazil modifies its regulations, nearby nations such as Argentina and Mexico are also updating their frameworks to comply with global standards, which is essential for the future of clinical research in Latin America. Within this context, organizations like bioaccess® are positioned to offer extensive research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Their leadership in medtech research, combined with Katherine Ruiz's expertise in regulatory affairs for medical devices and in vitro diagnostics in Colombia, highlights their commitment to the future of clinical research in Latin America and regulatory excellence in the region. Significantly, the Principal Investigator (PI) is now obligated to submit a list of participating institutions and related protocols for multicenter research studies, ensuring comprehensive oversight and adherence. Furthermore, sponsors must guarantee access to post-study drug supply programs for participants enrolled in research studies, which is crucial for participant welfare.
Researchers must also be aware that if a child reaches the legal age of consent during a study, their informed consent must be obtained for further participation, emphasizing the ethical responsibilities in medical studies. As President Luiz Inacio Lula da Silva aptly stated, 'a fixed deadline would violate participant rights and ethical standards of dignity, beneficence, and justice', underscoring the importance of ethical considerations in the context of regulatory changes. These components are essential for guaranteeing that studies are carried out with the highest regard for participant rights and well-being.
The Role of CROs in Shaping the Future of Clinical Research
Contract Research Organizations (CROs) play a crucial role in shaping the future of clinical research in Latin America, especially in Colombia, which provides considerable competitive benefits for first-in-human (FIH) studies. These advantages include:
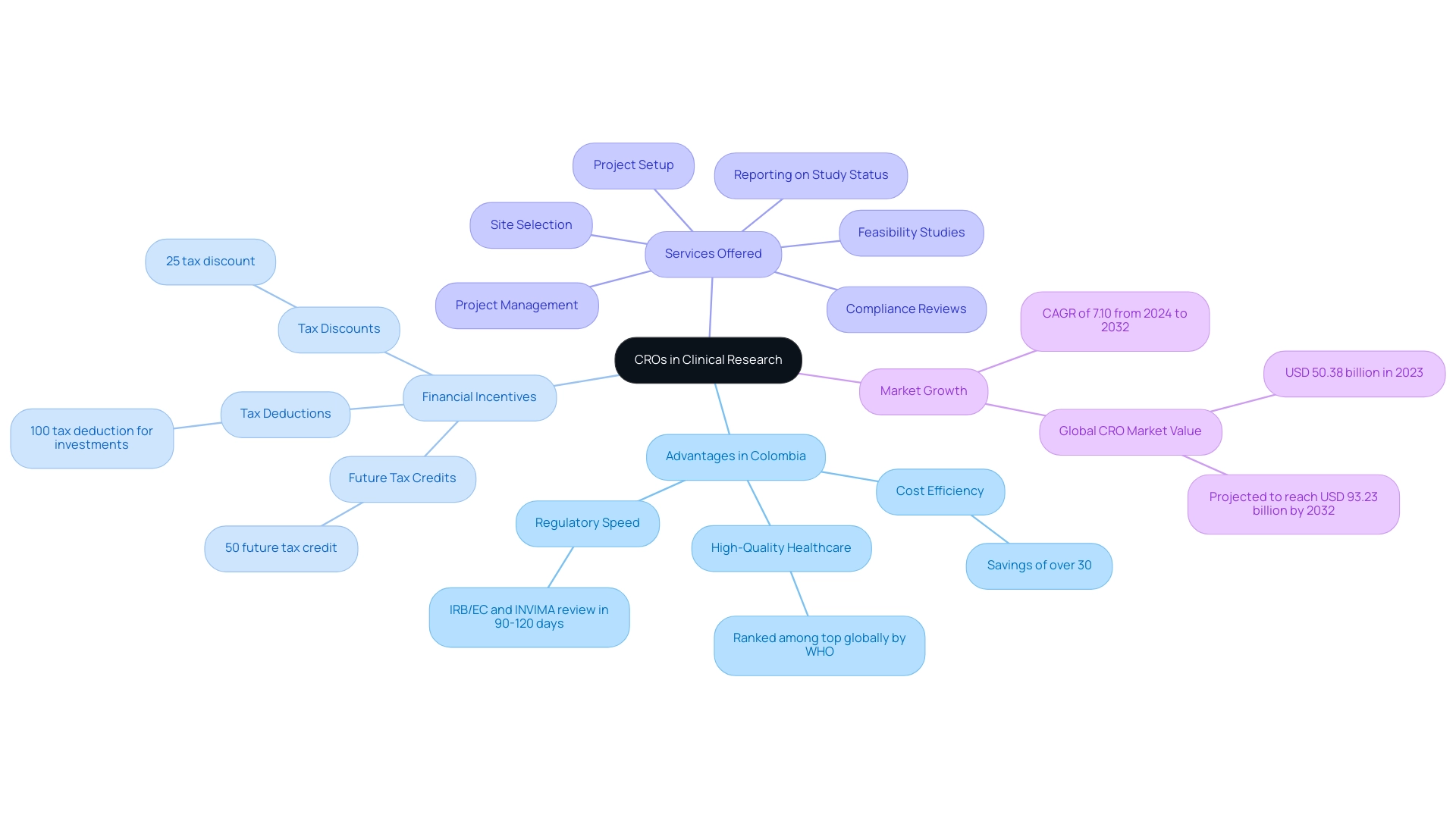
- Cost efficiency, with savings of over 30% compared to studies in North America or Western Europe.
- Regulatory speed, as the total IRB/EC and INVIMA review typically takes only 90-120 days.
- High-quality healthcare, with Colombia's healthcare system ranked among the top globally by the World Health Organization.
Furthermore, Colombia's universal healthcare coverage aids in patient recruitment, which is essential for the future of clinical research in Latin America, while strong R&D tax incentives improve the financial feasibility of conducting studies.
Investments in science, technology, and innovation projects receive:
- A 100% tax deduction
- A 25% tax discount
- A 50% future tax credit
This makes Colombia an appealing location for the future of clinical research in Latin America. The worldwide Healthcare Contract Research Organization market is valued at USD 50.38 billion in 2023 and is expected to increase to USD 93.23 billion by 2032, indicating a rising demand for clinical studies. According to Akash Anand, Head of Business Development & Strategy, this growth underscores the increasing dependency on CROs to assist both local and international sponsors, which is pivotal for the future of clinical research in Latin America by leveraging their in-depth understanding of regional regulations and patient demographics.
CROs offer extensive services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Project setup
- Project management
- Reporting on study status and adverse events
This expertise not only streamlines execution but also enhances data quality, crucial for successful outcomes. The complexities inherent in Phase III studies, which dominate the research landscape, further drive the necessity for specialized CROs skilled at managing logistics, participant recruitment, and regulatory compliance.
Leading players such as ICON plc, IQVIA, and Thermo Fisher Scientific Inc. are at the forefront of this evolving field. In tackling ongoing challenges, CROs are increasingly embracing technological innovations to enhance processes and facilitate communication, which is vital for the future of clinical research in Latin America across the region.
Strategies for Effective Patient Recruitment and Retention in Clinical Trials
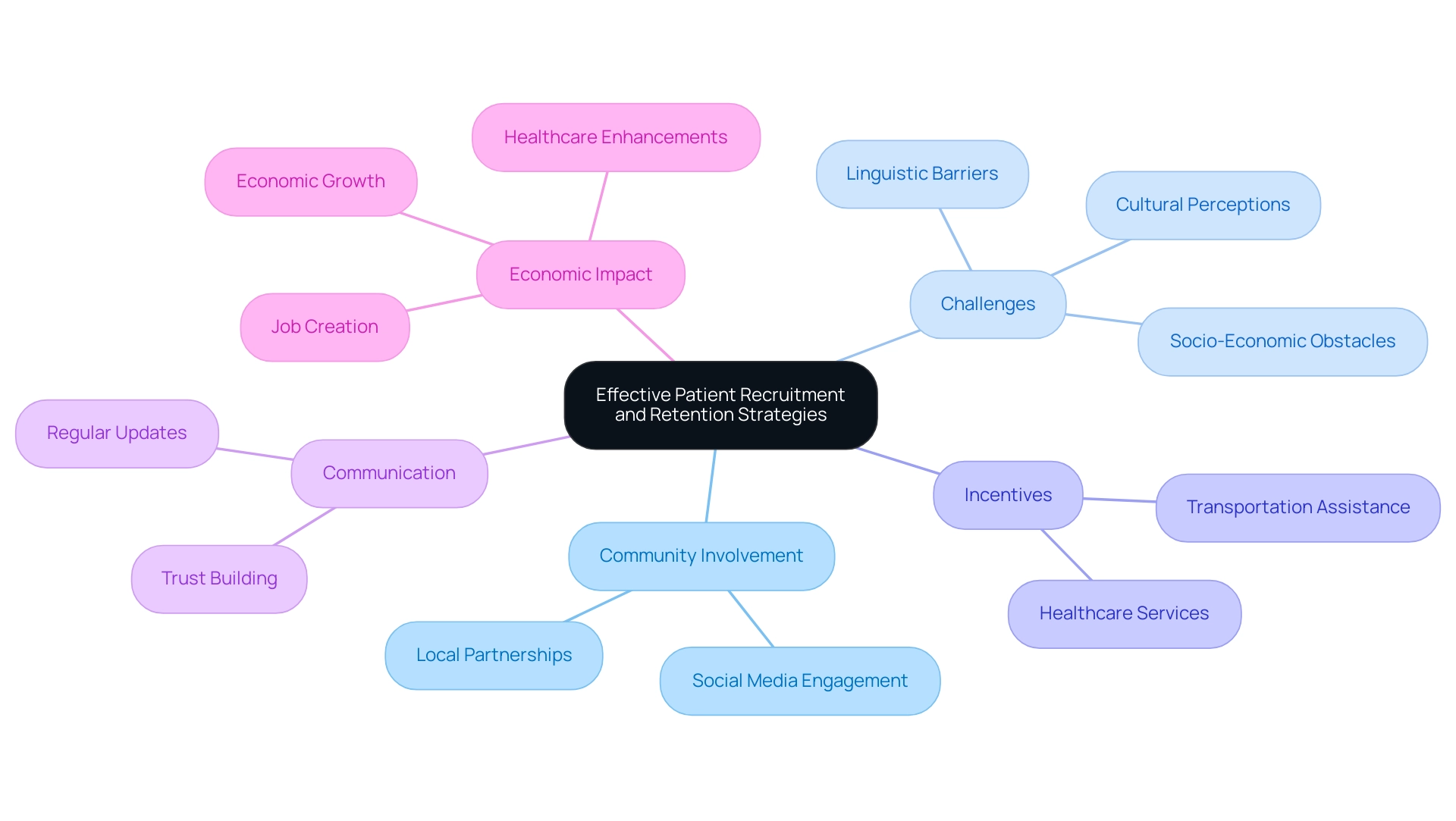
The execution of efficient volunteer recruitment and retention approaches is essential for the success of medical studies, which ultimately impacts the future of clinical research in Latin America. As emphasized by Clinical Leader, which has reported extensively on the future of clinical research in Latin America, there is a growing acknowledgment of the significance of customized participant engagement initiatives. With the research study participant recruitment services sector expected to attain USD 2.28 billion by 2032, a growing focus is placed on community involvement strategies that incorporate partnerships with local health organizations and the utilization of social media platforms.
These approaches not only improve understanding of research studies and their advantages but also assist in overcoming the linguistic, cultural, and socio-economic obstacles that can hinder informed consent, especially as studies grow in nations such as Peru, Colombia, and Chile. Challenges such as language differences, cultural perceptions, and varying economic conditions must be navigated to ensure that participants are fully informed and comfortable with their involvement.
Incentives such as transportation assistance and healthcare services have proven to enhance participant retention rates significantly. Moreover, maintaining regular communication with participants is crucial; it fosters trust and encourages ongoing involvement throughout the study.
A recent case study emphasized that the future of clinical research in Latin America requires successful patient recruitment by addressing minority underrepresentation and integrating studies with medical practice. By adopting comprehensive strategies that promote inclusivity and cultural sensitivity, researchers can advance medical science and enhance healthcare results while ensuring that studies are representative and effective. This approach not only fosters trust among participants but also corresponds with the insights from Clinical Leader concerning the economic impact of Medtech research studies on local economies, which include job creation, economic growth, and healthcare enhancements.
Furthermore, comprehensive trial management services, including feasibility studies, site selection, compliance reviews, and project management, are essential in navigating these challenges and maximizing the benefits of trials for local communities.
Challenges and Opportunities in Latin American Clinical Research
Despite the significant potential for the future of clinical research in Latin America, numerous challenges continue to impede progress. Infrastructure limitations, particularly in laboratory facilities and access to advanced technology, pose substantial barriers to conducting high-quality research. Socio-economic factors, such as disparities in healthcare access and varying levels of education, complicate efforts in recruitment and retention of individuals.
Notably, GlobalCare Clinical Trials, in partnership with bioaccess™, has achieved a remarkable reduction in clinical trial subject recruitment time by over 50% and a subject retention rate exceeding 95% in Colombia, exemplifying effective strategies in overcoming these hurdles. Furthermore, a collaborative approach is seen with IDx Technologies, which is actively seeking partnerships with Latin American ophthalmology centers to advance AI-based disease detection. According to a recent survey targeting physicians involved in screening, diagnosis, treatment, or follow-up of individuals with cancer, these disparities highlight the urgent need for innovative solutions.
Nevertheless, these challenges also open avenues for innovation. Strategic alliances between academic institutions and private organizations can significantly improve investigative capabilities, as shown by WellSpan Health's collaboration with the JHCRN, which has successfully enrolled over 100 individuals in multiple studies, contributing to the network's investigative initiatives. Moreover, the adoption of digital health technologies offers a transformative chance to bridge geographical barriers and enhance patient involvement in trials.
By harnessing these innovative solutions, stakeholders can not only address existing challenges but also contribute to the future of clinical research in Latin America, positioning the region as a leader in global clinical research initiatives.
Conclusion
The dynamic landscape of clinical research in Latin America is marked by significant advancements and opportunities driven by strategic investments and collaborative efforts. The surge in funding, particularly in the Andean Region, has positioned countries like Colombia as emerging hubs for clinical trials, with innovative studies demonstrating promising results in patient care. The evolution of regulatory frameworks, particularly through ANVISA's reforms in Brazil, has further streamlined the approval processes, enhancing transparency and fostering a conducive environment for international sponsors.
Contract Research Organizations (CROs) are integral to this growth, providing essential expertise that facilitates efficient trial execution while ensuring compliance with evolving regulations. Their role in patient recruitment and retention is paramount, as tailored engagement strategies not only address cultural and socio-economic barriers but also enhance the inclusivity and effectiveness of clinical trials. Successful case studies from the region exemplify how innovative approaches can drive participant engagement and improve healthcare outcomes.
However, challenges remain, including infrastructure limitations and socio-economic disparities that can hinder progress. The path forward lies in fostering strategic partnerships and leveraging digital health technologies to bridge these gaps. By embracing innovation and collaboration, stakeholders in Latin America can overcome existing obstacles and solidify the region's position as a key player in the global clinical research arena. The commitment to ethical considerations and participant welfare will be crucial in ensuring that this growth translates into meaningful advancements in healthcare for diverse populations.
Frequently Asked Questions
What recent trends have been observed in clinical research in Latin America, particularly in the Andean Region?
Clinical research in Latin America has experienced significant growth, with annual investment in the Andean Region rising from $3-4 million to over $50 million, driven by increased healthcare infrastructure investment, a growing population of treatment-naive patients, and strengthened collaboration between local and international stakeholders.
Why has Colombia, specifically Barranquilla, become a key center for clinical research?
Barranquilla has emerged as a key center for research due to its strategic geographic position, varied demographic profiles, and adherence to regulatory compliance. The recent INVIMA approval for an 18-month pilot study on a glaucoma treatment by eyeFlow, Inc. emphasizes its growing reputation in the field.
What role does INVIMA play in clinical research in Colombia?
INVIMA's regulatory endorsement is crucial for ensuring safety and efficacy in research studies, which fosters trust among stakeholders and supports the integrity of clinical research.
Can you provide examples of successful clinical studies in the region?
One example is ReGelTec's initial feasibility study where eleven individuals were treated with HYDRAFIL™ for chronic low back pain, reporting significant pain reduction. This highlights the potential for successful clinical outcomes in the region.
How are digital health technologies influencing clinical research in Latin America?
Digital health technologies improve patient involvement and streamline data collection processes, thereby accelerating the progress of research across the region.
What recent regulatory changes are impacting clinical research in Brazil?
The Brazilian Health Regulatory Agency (ANVISA) has enacted new laws to streamline the approval process for medical studies, enhancing transparency and efficiency, which is critical for attracting international sponsors.
What specific initiatives has ANVISA introduced to improve regulatory processes?
In 2023, ANVISA introduced an optimized analysis procedure that allows for the assessment of research applications based on documentation from comparable foreign regulatory authorities, increasing the efficiency of regulatory processes.
How are neighboring countries responding to Brazil's regulatory changes?
Countries like Argentina and Mexico are updating their regulatory frameworks to comply with global standards, which is essential for the future of clinical research in the region.
What services does bioaccess® offer to support clinical research?
Bioaccess® provides extensive research management services including feasibility studies, site selection, compliance reviews, project management, and reporting.
What ethical considerations are emphasized in the context of clinical research?
Researchers must ensure informed consent is obtained if a child reaches the legal age of consent during a study. Ethical responsibilities are highlighted by the requirement for comprehensive oversight and participant welfare assurances, such as access to post-study drug supply programs.




