Overview
Exploring medtech collaboration between the US and Latin America is essential for fostering innovation and enhancing healthcare outcomes by leveraging advanced technologies and diverse patient populations. The article illustrates this through examples of successful partnerships, such as Avantec Vascular's trials and the regulatory harmonization efforts, which together streamline processes and accelerate the development of new medical technologies, ultimately benefiting both regions.
Introduction
As the MedTech sector continues to evolve, the collaboration between the United States and Latin America emerges as a critical driver of innovation and improved healthcare outcomes. This partnership leverages the advanced technological capabilities of the US alongside the diverse patient populations of Latin America, creating a fertile ground for groundbreaking medical advancements.
With notable projects such as Avantec Vascular's pioneering clinical studies and PAVmed's successful implantations, the potential for impactful collaborations is becoming increasingly evident.
However, challenges such as regulatory disparities and cultural differences must be navigated to fully realize the benefits of these partnerships. By fostering open communication and mutual understanding, stakeholders can enhance their efforts to address healthcare needs and drive economic growth across the Americas, ultimately leading to a more equitable and efficient healthcare landscape.
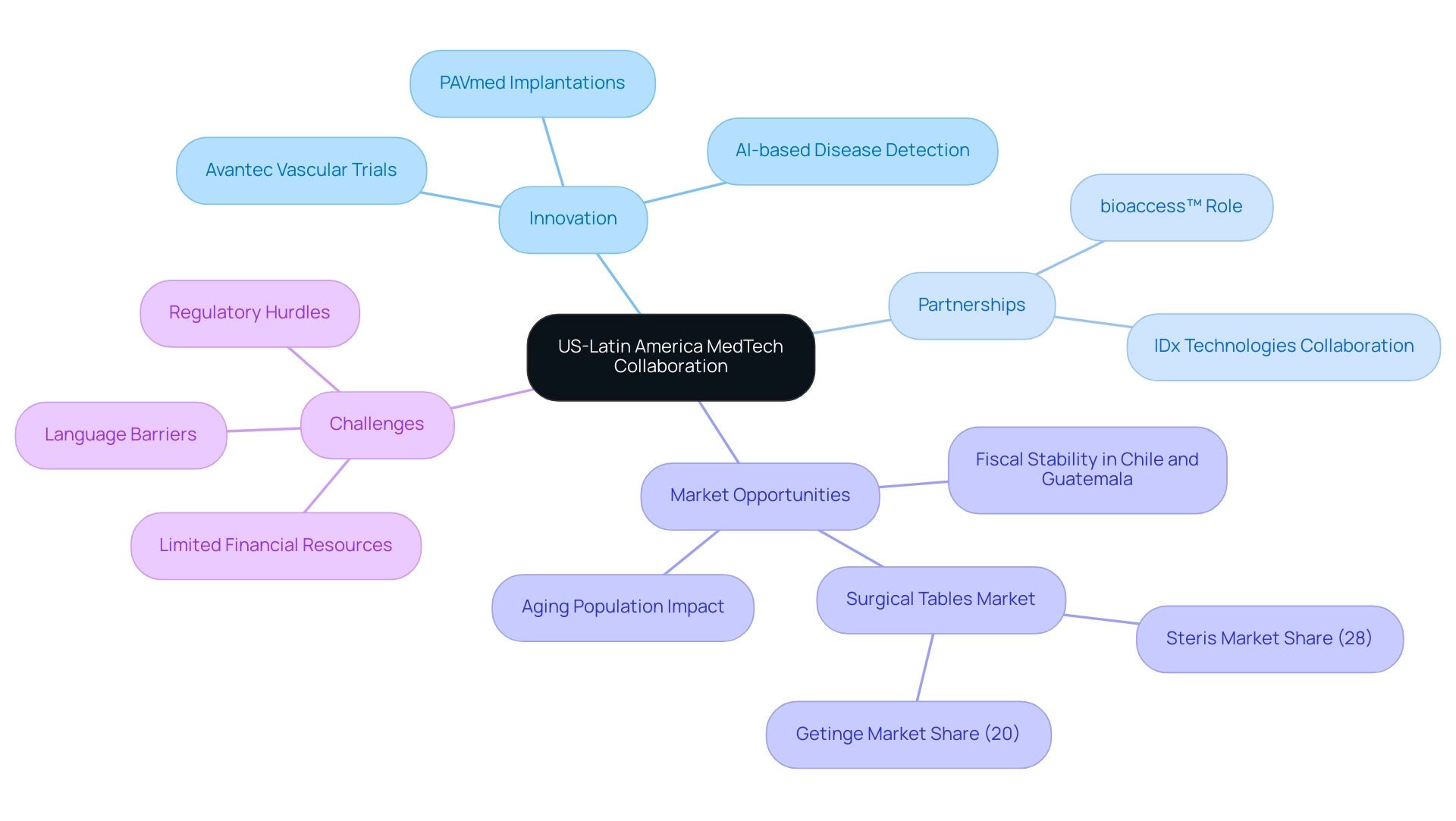
The Importance of US-Latin America MedTech Collaboration for Innovation
The essential role of exploring medtech collaboration between us and Latin America lies in fostering innovation and enhancing healthcare outcomes. The US is distinguished by its advanced technological capabilities and substantial investments in medical research, while Latin America presents a burgeoning market characterized by diverse patient populations. Significantly, Avantec Vascular is carrying out its first-in-human trial of an innovative vascular device in the region with the assistance of bioaccess™, which plays a vital role in choosing principal investigators and managing regulatory approvals.
This underscores the potential for impactful partnerships. Likewise, PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia showcase successful medical outcomes that benefit both regions. This dynamic combination provides invaluable insights into medical needs, making it imperative for these regions to collaborate.
By leveraging each other's strengths and exploring medtech collaboration between us and Latin America, they can accelerate the development of new medical technologies, streamline regulatory processes, and enhance the efficiency of clinical trials. Partnerships like those between IDx Technologies and bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection are crucial for exploring medtech collaboration between us and Latin America. This partnership not only promotes innovation but also addresses critical health disparities, ensuring that advancements are accessible to a broader audience.
Moreover, as nations such as Chile and Guatemala attained fiscal deficit stability and reverted to pre-pandemic economic conditions, the medical technology sector is positioned for expansion, offering distinctive opportunities for US investment in innovative health solutions. The recent pension reforms advocated by President Lacalle Pou in Uruguay, which raised the retirement age to 65 and permitted retirees to keep working, illustrate the continuous changes required to assist an aging population—a key element in influencing the future of medical services in Latin America. Additionally, the surgical tables market, with Steris leading at 28% and Getinge at 20%, illustrates the competitive landscape within the medical technology sector, underscoring the significance of collaboration and innovation in addressing healthcare challenges.
However, healthcare technology startups in Latin America continue to face challenges such as regulatory hurdles, limited financial resources, and language barriers, which necessitate a solution-driven approach to bridge the gaps in research and innovation.
Unlocking Opportunities: Benefits of MedTech Partnerships in the Americas
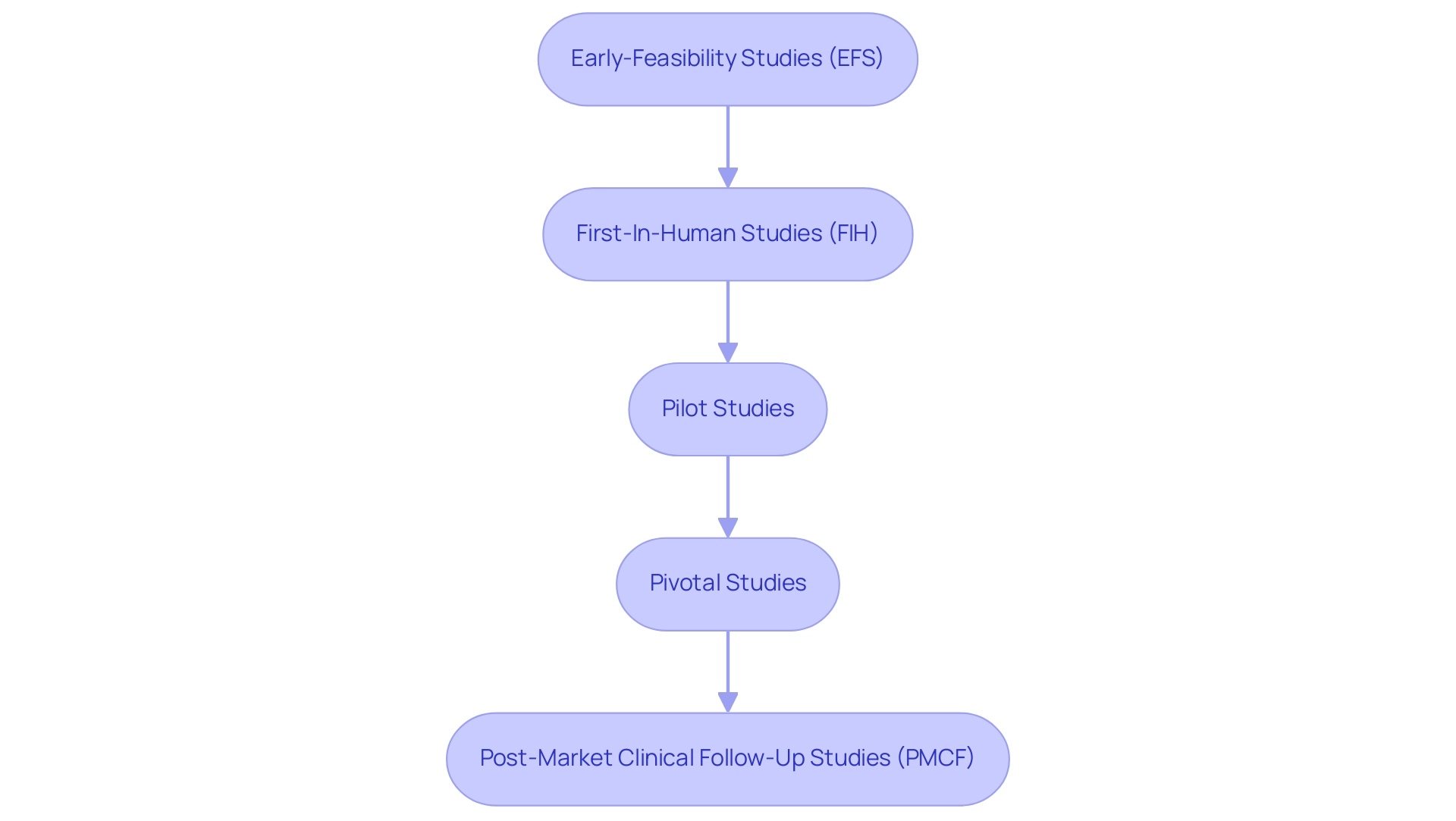
Exploring medtech collaboration between us and Latin America is emerging as a pivotal source of innovation and growth in the sector. These partnerships facilitate shared resources and knowledge exchange, providing access to diverse patient populations that are essential for the development and testing of new technologies. Notably, joint research initiatives, such as those managed by bioaccess®, which include:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
can significantly enhance clinical trial efficiency, leading to faster outcomes and reduced costs.
Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, emphasizes this impact, stating, "Our partnership with bioaccess® during the first human trial in Colombia was instrumental in navigating the complexities of the regulatory landscape and achieving timely results." Moreover, the shift towards direct-to-consumer markets, exemplified by the increasing demand for continuous glucose monitors (CGMs), demonstrates how MedTech companies are utilizing these partnerships to engage hyper-conscious health care consumers, generating new revenue streams. This trend is particularly relevant as industry experts like Conor Stewart note that while the US and Latin America are making strides, Asia, especially China, is poised to play a more prominent role in the future.
Such partnerships not only enhance the global competitiveness of participating companies but also drive economic growth and improvement in local communities. According to a statistic from December 2021, a significant share of clinicians believes that in ten years' time, the majority of medical services will be provided in patients' homes rather than conventional settings, underscoring the urgency and importance of these partnerships. Ultimately, exploring medtech collaboration between us and Latin America through the synergy fostered by medical technology partnerships not only enhances the capabilities of the companies involved but also leads to improved healthcare solutions and patient outcomes across the Americas.
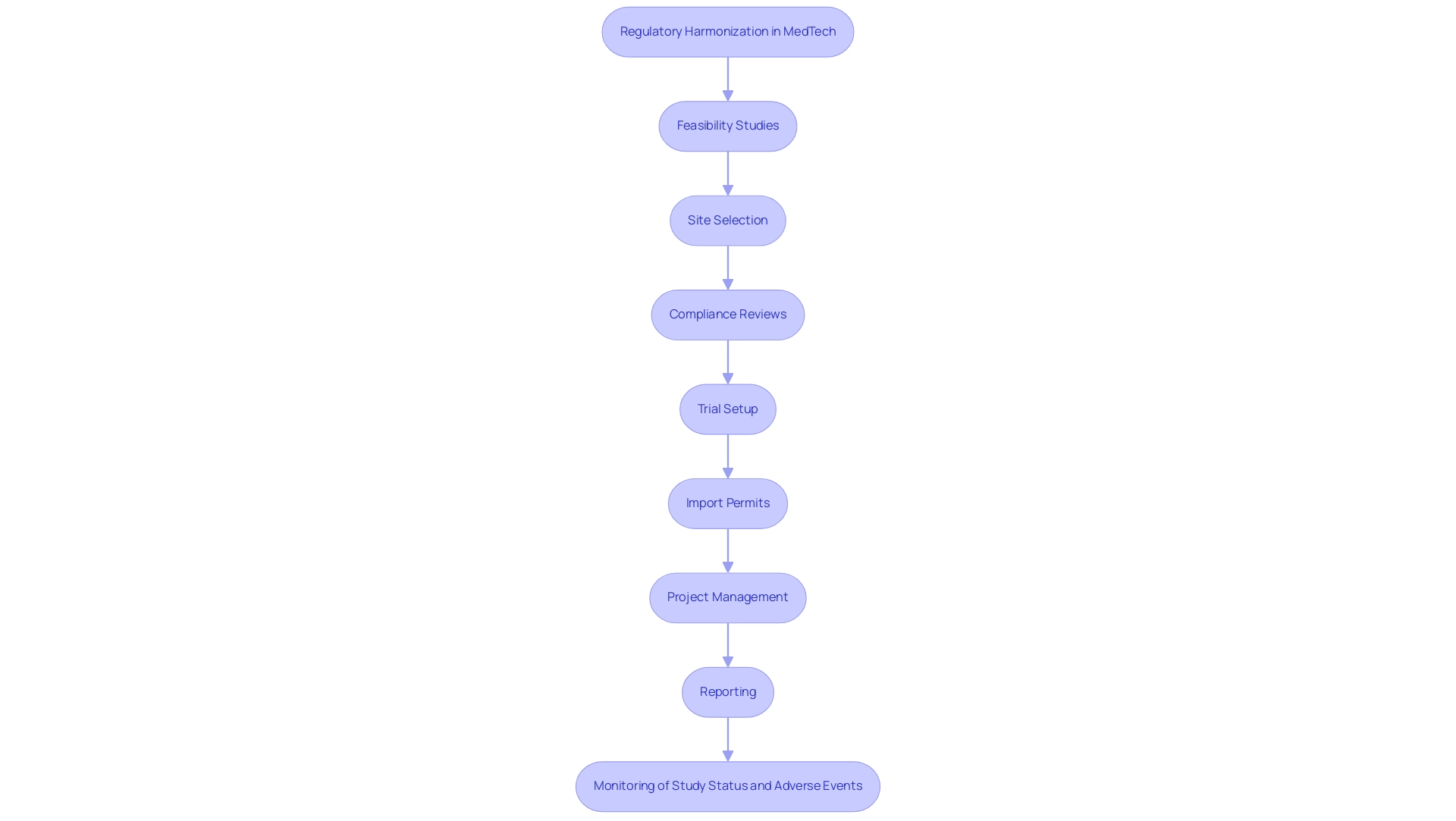
Regulatory Harmonization: A Pathway to Successful Collaboration
A main hurdle to exploring medtech collaboration between us and Latin America in the medical technology sector is the disparities present within their regulatory frameworks. Regulatory harmonization is vital for streamlining processes, allowing for smoother cross-border product movement. Our comprehensive trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
- Monitoring of study status and adverse events
All designed to facilitate this alignment.
According to recent statistics, aligning regulations could reduce the time to market by up to 30% and lower costs by approximately 25%, underscoring the potential benefits of regulatory alignment. Initiatives focused on exploring medtech collaboration between us and Latin America not only facilitate collaboration but also foster greater trust among stakeholders involved in the MedTech ecosystem. For instance, the recent case study on the EPA's final rule regarding ethylene oxide emissions illustrates the real-world implications of regulatory changes, as manufacturers must adapt to new standards that can impact product availability.
This cooperative method, supported by specialists such as Katherine Ruiz in Regulatory Affairs for medical devices in Colombia, plays a crucial role in exploring medtech collaboration between us and Latin America, leading to more effective trials and accelerated product approvals, ultimately improving patient access to innovative medical solutions. Moreover, the successful execution of these clinical trials contributes to local economies by creating jobs, boosting economic growth, and improving healthcare outcomes. As Jim Welch, EY Global Healthcare Leader, emphasizes, 'Understanding the state of the industry and its regulatory challenges is essential for leveraging opportunities in this evolving landscape.'
This insight emphasizes the critical need for stakeholders to navigate the complexities of regulatory frameworks to promote successful partnership between the regions.
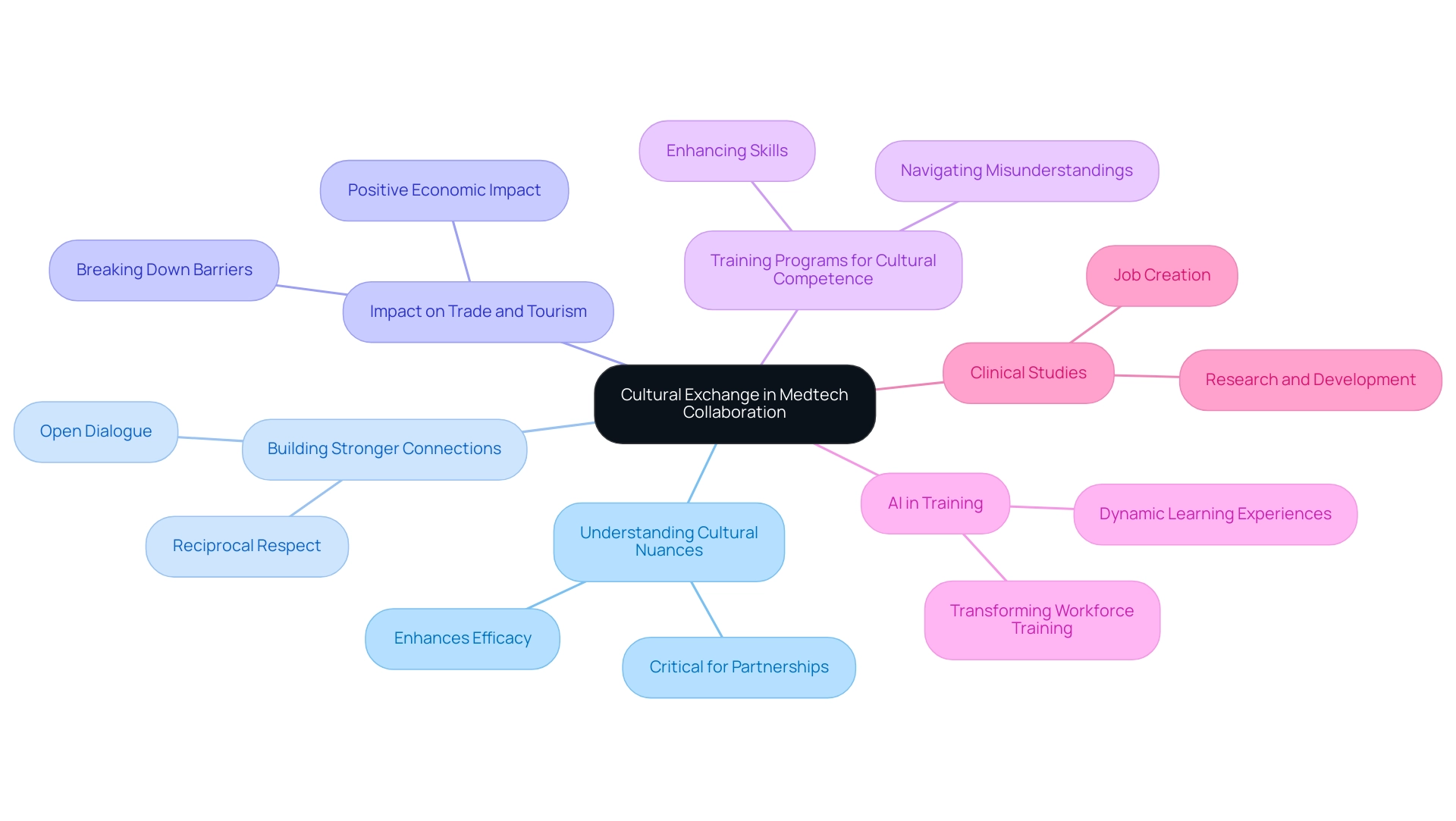
Cultural Exchange: Enhancing Understanding and Collaboration
Cultural exchange is essential for exploring medtech collaboration between us and Latin America. As highlighted by Patrick Pui Kin Kor, understanding cultural nuances is critical; it can significantly enhance the efficacy of partnerships. By encouraging open dialogue and reciprocal respect, stakeholders can build stronger connections that enable effective teamwork, ultimately fostering economic growth and enhancing health results.
Clinical studies conducted in these regions not only create jobs but also promote research and development, leading to increased international recognition for local economies. The insights from Haase et al. regarding digital diagnostic devices during the pandemic further demonstrate the necessity for cultural competence in adapting to diverse medical needs.
Moreover, statistics indicate that cultural exchange programs can positively impact trade and tourism by breaking down barriers, reinforcing their importance in medical technology collaborations. In 2024, the emphasis on cultural exchange is expected to grow, as training programs designed to enhance cultural competence will equip teams to navigate potential misunderstandings and foster inclusivity. This cultural synergy not only strengthens partnerships but also catalyzes innovative solutions that resonate with varied populations, ultimately leading to improved patient care.
Additionally, the scoping exercise conducted by Pagliari et al. highlights the varying definitions of e-health, which underscores the complexities of cultural exchange in medical services. The case study titled 'AI Revolutionizing Training' illustrates this evolution, as AI is set to transform workforce training, moving from passive data analysis to dynamic, interactive learning experiences, enhancing learning outcomes and changing how industries approach training and knowledge retention.
Through these collaborative efforts, we are exploring medtech collaboration between us and Latin America, positioning the medical technology sector to drive global health improvement and innovation, particularly with bioaccess® leading the charge in clinical research focused on regulatory excellence.
Future Trends: The Evolving Landscape of MedTech Collaboration
The transformation of the MedTech collaboration environment is focused on exploring medtech collaboration between us and Latin America, driven by rapid technological advancements and changing medical needs. A notable trend is the increasing investment in telemedicine and digital wellness solutions, fueled by a rising demand for remote medical services. The recent partnership between GE Healthcare and Radnet exemplifies this shift, as they aim to integrate AI capabilities into mammography imaging, demonstrating the potential of data analytics and artificial intelligence in enhancing clinical research efficiency and product development.
Furthermore, the growth of at-home diagnostics, which emerged as a significant trend during the pandemic, has highlighted the potential for self-testing devices beyond COVID-related applications. Despite a slowdown post-pandemic, this market is expected to expand, improving healthcare access and opening consumer markets for medical device sales. However, it's essential to consider the regulatory context; the FDA has yet to authorize any tools that continuously adapt or utilize generative AI, which may shape future innovation pathways and pose challenges for stakeholders.
As highlighted by Ginger Pigott, product liability concerns are vital in this changing environment, stressing the necessity for strong legal structures in medical technology partnerships. Furthermore, INVIMA plays a crucial part in supervising medical device regulations in Colombia, categorized as a Level 4 health authority by PAHO/WHO, ensuring compliance and safety in trials. The economic influence of MedTech clinical studies is also substantial, aiding in job creation, economic growth, and healthcare enhancement in local economies, promoting international partnerships.
As the sector evolves, stakeholders must adopt a flexible and proactive mindset, leveraging new technologies and methodologies to remain competitive. This collaboration holds vast promise for innovation and improved patient outcomes, particularly in exploring medtech collaboration between us and Latin America, with M&A transactions likely to deliver immediate returns to investors through mechanisms such as premium valuations and stock swaps, as highlighted by David J. Dykeman.

Conclusion
The collaboration between the United States and Latin America in the MedTech sector represents a transformative opportunity for innovation and enhanced healthcare outcomes. By leveraging the advanced technological capabilities of the US alongside the diverse patient populations in Latin America, significant strides can be made in medical advancements. Successful projects, such as those undertaken by Avantec Vascular and PAVmed, illustrate the promising potential of these partnerships, demonstrating that effective collaboration can lead to groundbreaking clinical studies and improved patient care.
However, the path to realizing these benefits is not without challenges. Regulatory disparities and cultural differences must be addressed to facilitate smoother collaborations. The importance of regulatory harmonization cannot be overstated, as it is essential for accelerating the time to market and reducing costs for new medical technologies. Additionally, fostering cultural exchange is crucial for building trust and ensuring that partnerships are productive and inclusive, ultimately leading to a more equitable healthcare landscape.
Looking ahead, the evolving landscape of MedTech collaboration is poised for growth, driven by emerging trends such as telemedicine and digital health solutions. As stakeholders adapt to these changes, the potential for innovation and improved healthcare delivery across the Americas will continue to expand. By embracing a collaborative spirit and focusing on shared goals, the US and Latin America can work together to overcome obstacles, enhance healthcare outcomes, and drive economic growth, paving the way for a healthier future for all.
Frequently Asked Questions
What is the significance of medtech collaboration between the US and Latin America?
Medtech collaboration between the US and Latin America is essential for fostering innovation and enhancing healthcare outcomes, leveraging the US's advanced technology and investment in research alongside Latin America's diverse patient populations.
What role does bioaccess™ play in medtech collaborations?
Bioaccess™ plays a crucial role in selecting principal investigators and managing regulatory approvals for clinical trials, facilitating partnerships that enhance research and innovation in medical technology.
Can you provide examples of successful medtech collaborations in Latin America?
Notable examples include Avantec Vascular's first-in-human trial of a vascular device in the region and PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia, both demonstrating successful medical outcomes.
How do partnerships improve clinical trial efficiency?
Partnerships, such as those managed by bioaccess®, enable joint research initiatives that include various study types, leading to enhanced clinical trial efficiency, faster outcomes, and reduced costs.
What challenges do healthcare technology startups in Latin America face?
Startups in Latin America encounter challenges such as regulatory hurdles, limited financial resources, and language barriers, which require a solution-driven approach to overcome.
How does the economic environment in Latin America affect medtech collaboration?
With countries like Chile and Guatemala achieving fiscal stability and returning to pre-pandemic conditions, the medical technology sector is positioned for growth, creating unique opportunities for US investment in innovative health solutions.
What future trends are anticipated in medical services delivery?
A significant share of clinicians believes that in the next decade, most medical services will be provided in patients' homes rather than traditional settings, highlighting the importance of medtech collaborations.
What is the impact of the shift toward direct-to-consumer markets in medtech?
The shift toward direct-to-consumer markets, such as the demand for continuous glucose monitors, allows MedTech companies to engage health-conscious consumers and generate new revenue streams through partnerships.

