Overview
Latin America leads in clinical trials due to its diverse patient populations, improved regulatory frameworks, and lower operational costs, which together enhance the representativeness and efficiency of research studies. The article highlights that countries like Brazil, Mexico, and Argentina not only provide a rich demographic for varied medical research but also benefit from streamlined processes and partnerships that facilitate effective study execution, thereby positioning the region as a vital hub for clinical research.
Introduction
The clinical trials landscape in Latin America is rapidly evolving, transforming the region into a pivotal hub for innovative medical research. With an influx of investments from pharmaceutical companies and an increasing recognition of its diverse patient demographics, countries like Brazil, Mexico, and Argentina are becoming focal points for clinical studies. This growth is not just about numbers; it reflects a deeper understanding of the importance of diverse genetic backgrounds in producing relevant and effective health solutions.
However, as the region embraces this opportunity, it faces a unique set of challenges, including:
- Regulatory hurdles
- Cultural barriers that can impact patient participation
As local partnerships emerge as a vital component in overcoming these obstacles, the future of clinical trials in Latin America looks promising, driven by:
- Strategic collaborations
- Technological advancements that aim to enhance the research landscape
With the pressing need for clinical trials in light of rising health concerns, particularly cancer, the momentum for clinical research in this vibrant region is more critical than ever.
The Evolving Landscape of Clinical Trials in Latin America
Latin America has solidly positioned itself as an essential participant in the worldwide research landscape, highlighting why Latin America leads in clinical trials, characterized by a dynamic range of medical investigation activities. During the last ten years, the area has experienced substantial expansion in the number of studies, driven by increasing funding from pharmaceutical firms and a rising awareness of the region's varied patient populations. Significantly, Brazil, Mexico, and Argentina have emerged as centers for research, with substantial populations and diverse genetic backgrounds enhancing the representativeness of study outcomes, which is essential for the global medical community.
Moreover, the development of regulatory frameworks in these nations has strengthened the research process, offering a streamlined and supportive atmosphere that attracts researchers and sponsors equally. However, Medtech companies face significant challenges, including language barriers, fragmentation of resources, and a lack of established contract research organization (CRO) corporate structures, which can hinder effective collaboration. The partnership between entities such as bioaccess™ and Caribbean Health Group establishes Barranquilla as a premier location for research studies in Latin America, backed by Colombia's Minister of Health.
This cooperative spirit is demonstrated by Flow-FX’s choice of Colombia for its first-in-human assessment of the Flow-Screw device for intraosseous antibiotic delivery, highlighting the region's ability to host innovative studies. To tackle these challenges, strategic alliances and creative solutions are crucial for improving the research landscape. This combination of factors not only emphasizes the current momentum but also indicates a strong future for medical research in the southern region of the continent.
With cancer occurrence in the region and the Caribbean expected to rise by 66%, surpassing 2.4 million new cases by 2040, the demand for research studies is more urgent than ever. Mirella Nardo from ICESP emphasizes this urgency, stating, 'Despite its large population, the disproportionately low availability of early-phase studies in the region underscores the immediate need for heightened research efforts.' Moreover, in 2023, the region accounted for 2.1% of the worldwide research market, demonstrating its potential for expansion in relation to other areas.
Key Advantages of Conducting Clinical Trials in Latin America
Carrying out medical studies in South America has considerable benefits, particularly in relation to why Latin America leads in clinical trials, due to the area's access to a varied patient demographic. This diversity is crucial for evaluating the efficacy and safety of treatments across varying demographics, ultimately facilitating the development of universally applicable medical solutions. Recent media discussions, particularly from Clinical Leader, have emphasized the crucial need for research diversity in the region, highlighting the significance of varied participation in attaining relevant health results.
As John Doe aptly states,
Increasing diversity in medical study participation will require deliberate efforts to earn the trust of non-White populations and overcome other social barriers that contribute to underrepresentation in medical research.
Furthermore, the regulatory landscape in Colombia, overseen by INVIMA (the National Food and Drug Surveillance Institute), has witnessed substantial improvements. As a Level 4 health authority recognized by PAHO/WHO, INVIMA's streamlined approval processes significantly reduce regulatory approval times, making the region an increasingly favorable option for researchers.
The lower operational expenses in Latin America compared to North America and Europe are part of why Latin America leads in clinical trials, increasing its appeal for research. The economic effect of Medtech research studies is substantial, contributing to job creation, economic growth, and healthcare enhancement in local communities. Together, these elements foster a flourishing setting for medical research, which illustrates why Latin America leads in clinical trials, facilitating quicker patient recruitment and resulting in more substantial study outcomes.
A recent study on participation in research involving 2,888 individuals uncovered significant demographic differences in invitations and involvement, especially among various racial/ethnic groups and educational attainment. The results showed that respondents identifying as non-Hispanic other had lower odds of responding 'A lot' regarding the influence of wanting to get better, indicating a significant gap in engagement. This highlights the necessity for targeted outreach and engagement strategies to enhance diversity in research.
Such proactive actions are vital to utilize the full capabilities of varied patient groups in research studies. Moreover, collaborating with a reliable CRO such as bioaccess® can further speed up the research process in the region, ensuring adherence and effectiveness while improving the overall research environment.
Challenges and Barriers in Latin American Clinical Trials
Carrying out medical studies in the southern continent offers a distinct array of challenges, regardless of the area's many benefits. One significant hurdle is the low level of awareness about trials among patients, as evidenced by a case study on patient recruitment that revealed many individuals remain uninformed about their options at the time of diagnosis. This awareness gap is compounded by the diverse healthcare systems across various countries, creating inconsistencies in patient access and experience.
However, as Steve Garchow observed in his discussions about the medtech landscape of the region, the area's regulatory frameworks have seen advancements, although delays and inconsistencies in approval processes persist. Cultural differences also play a crucial role, affecting patient willingness to participate and adhere to study protocols. Despite these challenges, the diverse disease landscape, including prevalent conditions such as cancer and diabetes, contributes to why Latin America leads in clinical trials.
The region is a 'world of opportunity' for research trials, illustrating why Latin America leads in clinical trials, thanks to its 'varied patient demographic' that enables wider inclusion and exclusion standards. Notably, with Argentina's urban populace accounting for 92% of its overall population, patient demographics are favorable for recruitment. Furthermore, the significantly lower dropout rates in Latin America compared to the U.S. and EU are part of why Latin America leads in clinical trials, making it a fertile ground for subject recruitment and retention.
The effect of medtech research studies on local economies is also significant, driving job creation, economic growth, healthcare improvement, and fostering international collaboration. To enhance the potential of research studies in the region, stakeholders must comprehend and tackle these obstacles. By proactively tackling these challenges, sponsors can significantly enhance the efficiency and effectiveness of their research initiatives.
Furthermore, utilizing organized marketing strategies and regional expertise is crucial for maneuvering through the intricacies of the American healthcare landscape, as emphasized in the insights from Steve Garchow. Interacting with local stakeholders and comprehending the life science innovation divide can further enhance the strategy for executing successful studies in the southern continent.
The Role of Local Partnerships in Advancing Clinical Research
Community collaborations are vital for the progress of medical research in Latin regions, highlighting why Latin America leads in clinical trials and producing many advantages that improve the overall effectiveness of studies. Collaborations among academic institutions, hospitals, and pharmaceutical companies are particularly valuable, as they facilitate knowledge sharing and optimize resources. As Dr. Darrell M. Gray, II states, 'Local partnerships are crucial in bridging the gap between research and community engagement.'
For example, when collaborations are established with local healthcare providers, they can greatly enhance community involvement and elevate awareness about ongoing studies, which is essential for increasing enrollment rates. This is supported by a recent webinar, where 74 participants (36%) highlighted the relevance of local partnerships in fostering community involvement. Furthermore, these collaborations ensure adherence to local regulations and ethical standards, thereby fostering trust among participants and stakeholders.
Given Colombia's favorable landscape, including over 30% cost savings compared to North America, an expedited IRB/EC and MoH review process of just 90-120 days, and a robust healthcare system ranked highly by the WHO, these elements contribute to understanding why Latin America leads in clinical trials, making local partnerships even more vital. They are instrumental in maximizing patient recruitment from a population of over 50 million, with nearly universal healthcare coverage. The comprehensive CRO research trial services available in Colombia, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
illustrate why Latin America leads in clinical trials.
The function of INVIMA as a Level 4 health authority, overseeing medical device regulation and ensuring adherence to international standards, is also crucial in upholding the integrity of research. A case study titled 'Allocative Efficiency in Healthcare' illustrates this well, assessing an integrated healthcare model's ability to address key challenges in Mexico and Colombia, showing high feasibility for Colombia but low for Mexico, indicating varying levels of support among stakeholders. By leveraging local expertise and networks, research initiatives in Latin America are not only strengthened but also positioned to achieve more successful outcomes.
The recent review process underscored the importance of collaborative efforts, with multiple reviewers ensuring eligibility and data extraction through consensus, reflecting a commitment to rigorous standards in research practices. Moreover, the R&D tax incentives provided in Colombia, including a 100% tax deduction and various financial grants, significantly contribute to the economic advantages of conducting experiments here. Overall, the strategic integration of local partnerships, along with Colombia's competitive advantages, highlights why Latin America leads in clinical trials, paving the way for more effective and ethically sound studies across the region.
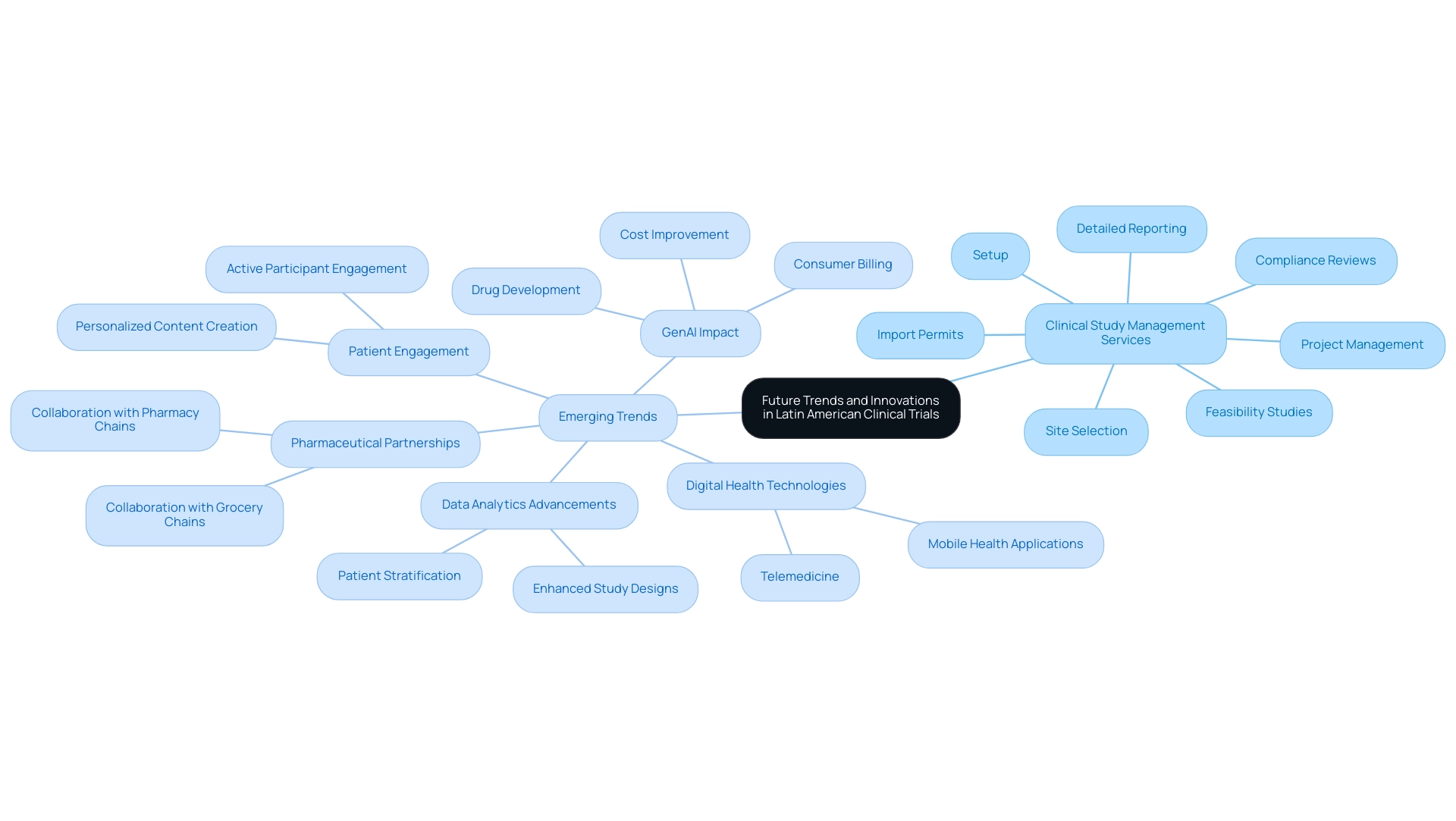
Future Trends and Innovations in Latin American Clinical Trials
The research landscape in Latin America is experiencing notable change, propelled by cutting-edge trends and technologies. Our comprehensive clinical study management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events
Compliance reviews ensure that all study documents meet country requirements, while the import permit process facilitates the nationalization of investigational devices.
The integration of digital health technologies—including telemedicine and mobile health applications—is enhancing patient recruitment and monitoring processes. Significantly, pharmaceutical firms are increasingly partnering with grocery and pharmacy chains to reach potential research participants, which is vital for efficient recruitment strategies in various geographical areas. This shift enables more efficient remote testing, particularly advantageous in regions with diverse access to healthcare.
Moreover, the expected effect of GenAI is transforming drug development, consumer billing, and cost enhancement in the healthcare sector, further affecting research studies. The increasing reliance on real-world evidence fosters a more patient-centric approach, encouraging researchers to engage actively with participants to ensure studies align with patient needs. Furthermore, advancements in data analytics and artificial intelligence are facilitating more efficient study designs and enhanced patient stratification.
As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, articulates, 'I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.' These innovations not only enhance the quality of medical research but also illustrate why Latin America leads in clinical trials.
A recent report on provider-focused digital health tools illustrates this point, noting that such technologies are improving outcomes for patients with chronic diseases through better monitoring and decision-making by healthcare providers. Additionally, with 153 partners using the purpose of creating profiles to personalize content, the industry is moving towards more tailored patient engagement strategies. As the industry moves forward, these developments promise an exciting future for clinical research in the region.
Conclusion
The clinical trials landscape in Latin America is rapidly evolving, positioning the region as a key player in innovative medical research. Countries like Brazil, Mexico, and Argentina are enhancing the relevance of clinical trial outcomes through their diverse patient demographics and improved regulatory frameworks. However, challenges such as regulatory hurdles, cultural barriers, and limited patient awareness must be addressed to maximize this potential.
Local partnerships are essential for overcoming these challenges, enabling collaboration between academic institutions, hospitals, and pharmaceutical companies. Such alliances enhance community engagement and awareness of clinical trials while ensuring adherence to local regulations, fostering trust among participants. Furthermore, the integration of digital health technologies streamlines patient recruitment and monitoring, accommodating diverse populations.
With the demand for clinical trials increasing—particularly due to rising health concerns like cancer—the importance of Latin America is underscored. The region represented only 2.1% of the global clinical trials market in 2023, highlighting significant growth potential. By capitalizing on its strengths, including access to diverse patient populations and strategic collaborations, Latin America is poised to make a substantial impact in global clinical research. Continued innovation and partnerships will be crucial in meeting the healthcare landscape's pressing needs and advancing the future of clinical trials.
Frequently Asked Questions
Why is Latin America a leader in clinical trials?
Latin America has positioned itself as a leader in clinical trials due to its diverse patient populations, increasing funding from pharmaceutical companies, and substantial growth in the number of studies over the last decade.
Which countries in Latin America are recognized as centers for research?
Brazil, Mexico, and Argentina are recognized as major centers for research due to their large populations and diverse genetic backgrounds.
What role do regulatory frameworks play in research in Latin America?
The development of regulatory frameworks in countries like Colombia has strengthened the research process, providing a supportive environment that attracts researchers and sponsors.
What challenges do Medtech companies face in Latin America?
Medtech companies face challenges such as language barriers, fragmentation of resources, and a lack of established contract research organization (CRO) structures, which can hinder collaboration.
How is collaboration being promoted in Latin America for research studies?
Partnerships, such as that between bioaccess™ and Caribbean Health Group, are establishing locations like Barranquilla as premier sites for research studies in Latin America.
What is the significance of cancer research in Latin America?
With cancer cases expected to rise significantly by 2040, there is an urgent demand for research studies, highlighting the need for increased research efforts in the region.
How does the operational cost in Latin America compare to other regions for clinical trials?
The lower operational costs in Latin America compared to North America and Europe make it a more appealing location for conducting clinical trials.
What impact do Medtech research studies have on local communities?
Medtech research studies contribute to job creation, economic growth, and healthcare improvement in local communities.
What recent findings highlight the need for diversity in medical research participation?
A study indicated significant demographic differences in research participation, showing that non-Hispanic individuals had lower engagement, underscoring the need for targeted outreach to enhance diversity.
How can collaboration with CROs improve the research environment in Latin America?
Collaborating with reliable CROs, such as bioaccess®, can expedite the research process, ensuring adherence to protocols and improving overall research effectiveness.

