Overview
Post-Market Clinical Follow-Up (PMCF) studies are crucial for medical device manufacturers as they ensure ongoing safety assessment, enhance product performance, and foster trust with stakeholders. The article highlights that these studies not only comply with regulatory requirements but also provide valuable insights for product improvement and innovation, thereby reinforcing manufacturers' commitments to quality and patient safety.
Introduction
In the dynamic landscape of medical device development, the significance of Post-Market Clinical Follow-Up (PMCF) studies cannot be overstated. These studies serve as a vital mechanism for ensuring the ongoing safety and efficacy of medical devices after their market introduction.
With increasing scrutiny on device performance and safety, manufacturers are compelled to adopt proactive strategies that not only fulfill regulatory obligations but also foster innovation and enhance patient trust. By systematically collecting and analyzing real-world data, PMCF studies empower manufacturers to identify potential risks early, refine their products, and respond to stakeholder concerns effectively.
This article delves into the critical role of PMCF in safeguarding patient safety, meeting regulatory requirements, and driving continuous improvement in medical technology.
The Critical Role of Post-Market Clinical Follow-Up in Medical Device Safety
Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter, as they play a vital role in the lifecycle of medical instruments by serving as an important method for continuous safety assessment after market launch. When collaborating with bioaccess®, you utilize over 20 years of expertise in overseeing these evaluations, which extend beyond simple regulatory compliance; they are crucial in ensuring that instruments uphold their intended performance and do not introduce unexpected risks to patients. A recent analysis revealed that 12.1% of abstracts from various research highlighted device-related safety concerns, underscoring the need for continuous monitoring.
By systematically collecting data on equipment performance and patient outcomes, manufacturers can identify potential issues at an early stage and implement necessary corrective actions. This proactive approach not only enhances patient safety but also reinforces manufacturers' commitments to quality and regulatory compliance, ultimately cultivating trust among healthcare providers and patients.
Furthermore, the importance of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter lies in their ability to yield valuable insights that can inform future product iterations and innovations, thereby advancing medical technology. As noted by Dr. Joseph S. Ross, an expert in medical evaluation who has received grants from the Medical Innovation Consortium and the US National Institutes of Health, ongoing research and transparency in Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter are crucial for the integrity of health technology assessments. The importance of these investigations is further illustrated by a research analysis examining the relationship between Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter and the recall history of high-risk therapeutic instruments, which revealed that 10 of the instruments had been recalled at least once.
Notably, ongoing postmarket studies for these recalled items were comparable in size to those of non-recalled items, indicating that recall status did not significantly affect study enrollment. This systemic need for rigorous follow-up in all cases aligns with the principles of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter, safeguarding patient safety and enhancing medical outcomes. Furthermore, policy suggestions stress the significance of implementing penalties for not disclosing results and financing independent comparative effectiveness research, underscoring the necessity for a strong framework that facilitates the continuous assessment of medical technologies.
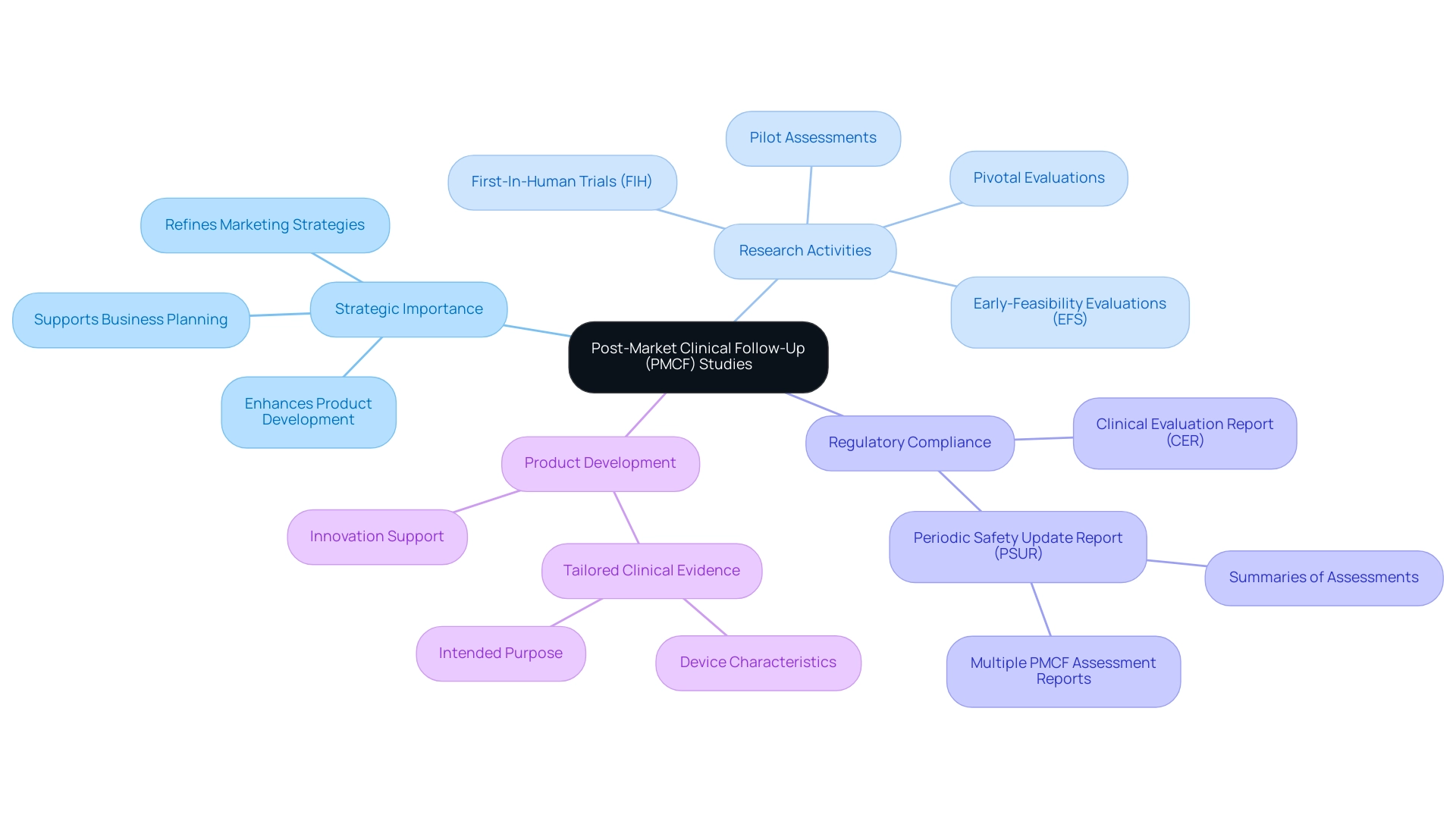
Moreover, findings suggest that pivotal premarket investigations were often small and lacked rigorous design features, while many postmarket evaluations focus on surrogate markers of disease and have short follow-up durations, further emphasizing the challenges in medical device assessments. At bioaccess®, we also manage Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pilot Studies, employing a customized approach to navigate your company towards acquisition, ensuring comprehensive support throughout the clinical trial process.
Understanding Regulatory Obligations for PMCF Studies
Under the EU Medical Device Regulation (MDR), manufacturers are required to conduct Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter as a vital component of their post-market surveillance obligations. At bioaccess®, we leverage over 20 years of experience in Medtech to ensure that our clients benefit from our comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These regulations require that manufacturers create a thorough plan for Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter, outlining methods for gathering and analyzing information on product performance and safety after market introduction.
The primary objective is to ensure an ongoing evaluation of the system's efficacy and safety in real-world conditions. Non-compliance with the guidelines outlined in Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter is not merely a regulatory oversight; it can lead to significant consequences, including hefty fines, product recalls, and potential withdrawal from the market. As expressed by regulatory experts, 'The PMS system should be proportionate to the risk class and suitable for the type of equipment.'
Furthermore, the MDCG 2019-3 rev.1 highlights the importance of clinical evaluation consultation procedure exemptions, which adds another layer of complexity for manufacturers. It is crucial for them to balance the need for high-quality Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter with resource constraints, as this can significantly impact their compliance efforts. Additionally, the recent changes in regulatory obligations, such as the UKCA marking requirements, underscore the evolving landscape manufacturers must navigate.
By fully understanding and meeting these regulatory obligations, manufacturers not only enhance patient safety but also protect their business interests and maintain a strong reputation within the industry. Guided by specialists such as Katherine Ruiz, who focuses on regulatory matters for medical equipment and in vitro diagnostics in Colombia, bioaccess® is prepared to assist you in navigating the intricacies of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter and other clinical research efficiently. Our expertise also extends to Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, and Pivotal Studies, ensuring a comprehensive approach to clinical trials.
Enhancing Clinical Evidence and Product Improvement
Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter in systematically gathering clinical data that enhances the performance and safety of medical devices throughout their life cycle. By analyzing real-world usage patterns and patient feedback, manufacturers can pinpoint areas for improvement and make data-driven decisions regarding product modifications. Notably, high-quality surveys constitute only a fraction of a clinical trial or registry budget, allowing manufacturers to efficiently allocate resources while conducting essential clinical programs.
This proactive approach not only mitigates emerging safety concerns but also stimulates innovation by enabling manufacturers to tailor their products more effectively to the needs of healthcare providers and patients. bioaccess® provides invaluable assistance in creating and executing post-market clinical follow-up surveys, ensuring adherence to regulatory compliance, which is essential for maintaining product safety and efficacy. With more than 20 years of expertise in Medtech, bioaccess® offers extensive clinical trial management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
The extensive clinical data gathered through post-market clinical follow-up activities is essential for backing future regulatory submissions and marketing approaches, ultimately improving the competitive advantage of the product in the healthcare sector. For example, numerous PMCF evaluations, especially Type 3 PMCF evaluations, function within the intended application of instruments without enforcing extra burdensome or invasive procedures. These research efforts, governed by national rules rather than the MDR, must be reported to the local ethics committee, highlighting the importance of local regulatory adherence.
As Dr. Johannes Goldmann succinctly puts it,
Legislators and manufacturers want to ensure that medical devices are safe, perform well, and are effective over the complete life cycle.
This highlights the essential role of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter in promoting product enhancement and ensuring continuous adherence to safety and efficacy standards, all supported by the expertise and adaptable, tailored approach of bioaccess®.
Building Trust with Stakeholders and Patients
The importance of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter lies in their ability to meet regulatory demands while providing manufacturers with a vital opportunity to demonstrate their commitment to safety and quality. By openly sharing results from post-market clinical follow-up studies with stakeholders—ranging from healthcare providers to patients—manufacturers can establish credibility and cultivate collaborative relationships. This transparency is vital for addressing any concerns regarding the safety and efficacy of medical devices.
Interacting with patients and healthcare professionals during the process not only builds trust but also provides invaluable insights that guide future product development and improve patient care. As highlighted by industry experts, returning health information and being transparent about the intent when sharing research data can significantly increase trust in research, especially among participants from marginalized communities. Additionally, it is essential to recognize that access to the full issue costs USD 707.00 for 30 days, which may affect stakeholders' choices concerning PMCF research.
Clarifying how this pricing impacts stakeholder engagement is essential, as research indicates that women are less likely to increase their trust if health information is shared with friends and family or health tech companies, emphasizing the nuanced dynamics of trust in this context. An analysis of the impact of privacy policy transparency on trust reveals that clear privacy policies enhance patients' cognitive trust in health information exchange systems, leading to greater willingness to share personal health information and participate in health initiatives. Ultimately, the trust built through effective communication and transparency can lead to stronger partnerships and bolster a manufacturer's reputation within the medical device industry.
With more than 20 years of experience in Medtech, bioaccess® demonstrates this dedication through recent initiatives that provide customized services for post-market clinical evaluations, improving strategies for regulatory compliance and stakeholder involvement. By managing a variety of research initiatives, including Early-Feasibility Investigations (EFS), First-In-Human Trials (FIH), Pilot Projects, and Pivotal Trials, bioaccess® showcases its leadership in Medtech clinical research in Latin America. Through such efforts, the influence of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter on stakeholder trust is not only evident but essential for the ongoing advancement of medical equipment safety in the region.
Strategic Implications for Medical Device Manufacturers
Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter in shaping the strategic direction of medical product manufacturers. Leveraging over 20 years of expertise in Medtech, bioaccess® systematically gathers and analyzes post-market data, enabling manufacturers to uncover trends and shifts within the healthcare landscape. This proactive approach not only enhances product development but also refines marketing strategies and business planning.
As highlighted by Dr. Nadine Jurrmann, "That level of clinical evidence shall be appropriate in view of the characteristics of the device and its intended purpose," underscoring the importance of tailored evidence to inform decision-making. Importantly, multiple post-market clinical follow-up assessment reports may be prepared during the Periodic Safety Update Report (PSUR) preparation period, and summaries of these assessments should be included in the PSUR. Furthermore, aligning project activities with overarching business objectives ensures compliance with regulatory standards, such as those outlined by INVIMA, while positioning companies for growth in a competitive market.
Bioaccess® also manages a range of research activities, including:
- Early-Feasibility Evaluations (EFS)
- First-In-Human Trials (FIH)
- Pilot Assessments
- Pivotal Evaluations
These activities collectively enhance the overall clinical trial management process. The cost-effectiveness of user surveys, which require fewer resources for design, recruitment, and data collection, enhances the efficiency of Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter, thereby reinforcing their strategic significance. Documentation of post-market clinical follow-up results is essential, as these findings must be reported in various formats, including the PSUR and Clinical Evaluation Report (CER), depending on the device classification.
This comprehensive approach to Post-Market Clinical Follow-Up (PMCF) Studies: Why They Matter not only supports ongoing compliance with regulatory requirements but also fosters innovation, making these studies a vital component of a strategic business framework.
Conclusion
Post-Market Clinical Follow-Up (PMCF) studies are indispensable to the medical device industry, ensuring that products continue to meet safety and efficacy standards long after their initial market introduction. These studies not only fulfill regulatory obligations but also serve as a proactive measure for manufacturers to identify potential risks, refine product performance, and enhance patient safety. By systematically collecting real-world data, PMCF studies empower manufacturers to make informed decisions that ultimately foster innovation and build trust with healthcare providers and patients alike.
The regulatory landscape surrounding PMCF is complex, necessitating a comprehensive understanding of obligations under frameworks like the EU Medical Device Regulation (MDR). Compliance not only protects patient interests but also safeguards manufacturers' reputations and business viability. As highlighted throughout the article, the insights gained from PMCF studies can inform future product iterations and marketing strategies, reinforcing the critical role these evaluations play in the lifecycle of medical devices.
By embracing transparency and actively engaging with stakeholders, manufacturers can cultivate trust and establish strong partnerships within the healthcare ecosystem. The strategic implications of PMCF extend beyond compliance; they are central to driving continuous improvement and innovation in medical technology. As the industry evolves, PMCF studies will remain vital in ensuring that medical devices not only meet regulatory standards but also adapt to the changing needs of patients and healthcare providers. In this way, the commitment to rigorous post-market evaluations will continue to enhance the safety, efficacy, and overall quality of medical devices in the years to come.
Frequently Asked Questions
What are Post-Market Clinical Follow-Up (PMCF) Studies?
PMCF Studies are evaluations conducted after medical instruments are launched in the market to continuously assess their safety and performance, ensuring they do not introduce unexpected risks to patients.
Why are PMCF Studies important?
They play a vital role in ongoing safety assessment, help identify potential issues early, enhance patient safety, and reinforce manufacturers' commitments to quality and regulatory compliance.
What percentage of research abstracts highlighted device-related safety concerns?
A recent analysis revealed that 12.1% of abstracts from various research highlighted device-related safety concerns.
How do PMCF Studies contribute to medical technology?
They yield valuable insights that can inform future product iterations and innovations, thereby advancing medical technology.
What are the regulatory requirements for PMCF Studies under the EU Medical Device Regulation (MDR)?
Manufacturers are required to conduct PMCF Studies as part of their post-market surveillance obligations, outlining methods for gathering and analyzing information on product performance and safety after market introduction.
What are the consequences of non-compliance with PMCF guidelines?
Non-compliance can lead to significant consequences, including hefty fines, product recalls, and potential withdrawal from the market.
What services does bioaccess® provide in relation to PMCF Studies?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What challenges do manufacturers face in conducting PMCF Studies?
Manufacturers must balance the need for high-quality studies with resource constraints, navigate evolving regulatory obligations, and ensure compliance with guidelines.
How can manufacturers benefit from working with bioaccess®?
By leveraging bioaccess®'s over 20 years of experience in Medtech, manufacturers can receive expert guidance in navigating the complexities of PMCF Studies and other clinical research.
What other types of studies does bioaccess® manage?
In addition to PMCF Studies, bioaccess® manages Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pilot Studies.

