Overview
Cost-efficient medtech trials in Paraguay can be accomplished by comprehensively understanding the local regulatory landscape, forging partnerships with regional organizations, implementing effective recruitment strategies, and managing trial logistics with precision. These steps are essential for navigating the complexities of clinical research in Paraguay. Local expertise, cultural sensitivity, and meticulous planning are paramount to enhancing operational efficiency and securing successful trial outcomes.
Introduction
In the rapidly evolving landscape of medical technology, Paraguay has emerged as a promising venue for conducting Medtech trials. As international interest grows, it is vital to understand the intricacies of the local regulatory environment, patient demographics, and effective recruitment strategies for success.
With the guidance of experienced partners like bioaccess®, organizations can navigate these complexities, ensuring compliance while optimizing trial logistics. This article delves into the essential steps for conducting successful Medtech trials in Paraguay, highlighting the importance of:
- Strategic planning
- Local partnerships
- Innovative recruitment approaches
These elements not only enhance trial efficiency but also contribute to the region's healthcare advancements.
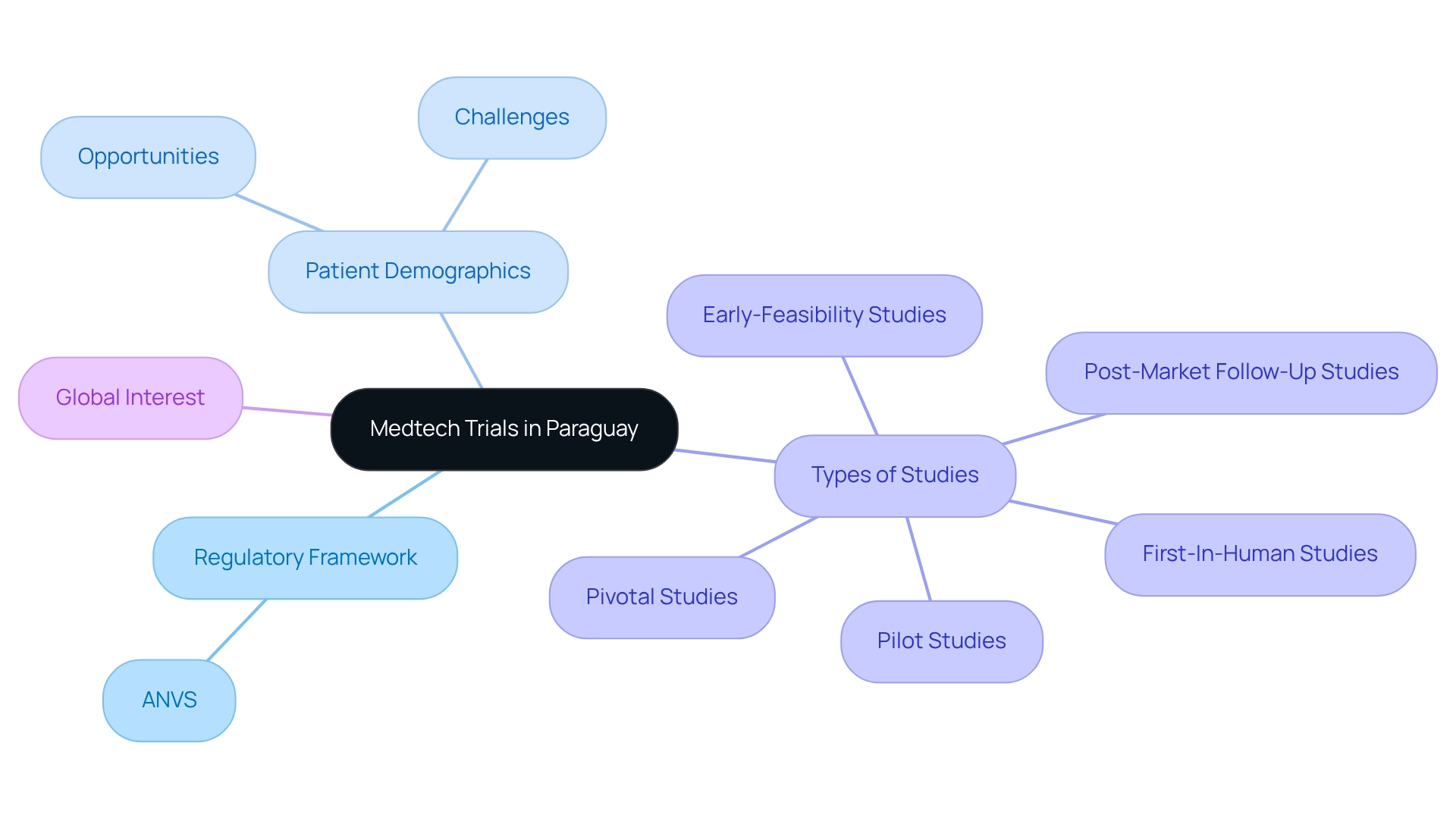
Understand the Landscape of Medtech Trials in Paraguay
To successfully conduct cost-efficient medtech trials in Paraguay, it is crucial to understand the local landscape. This entails grasping the regulatory framework established by the Agencia Nacional de Vigilancia Sanitaria (ANVS), which governs research studies. Furthermore, it is important to consider the patient demographics, as these can significantly influence recruitment strategies.
Paraguay's diverse population offers both opportunities and challenges, presenting a broad spectrum of potential participants while necessitating culturally sensitive approaches to recruitment and engagement.
With bioaccess®'s comprehensive management services for research—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—you can adeptly navigate these complexities.
Moreover, the increasing interest from global firms in conducting cost-efficient medtech trials in Paraguay is due to lower costs and expedited regulatory processes, which enhance study efficiency and support regional economic development through job creation and improved healthcare outcomes.
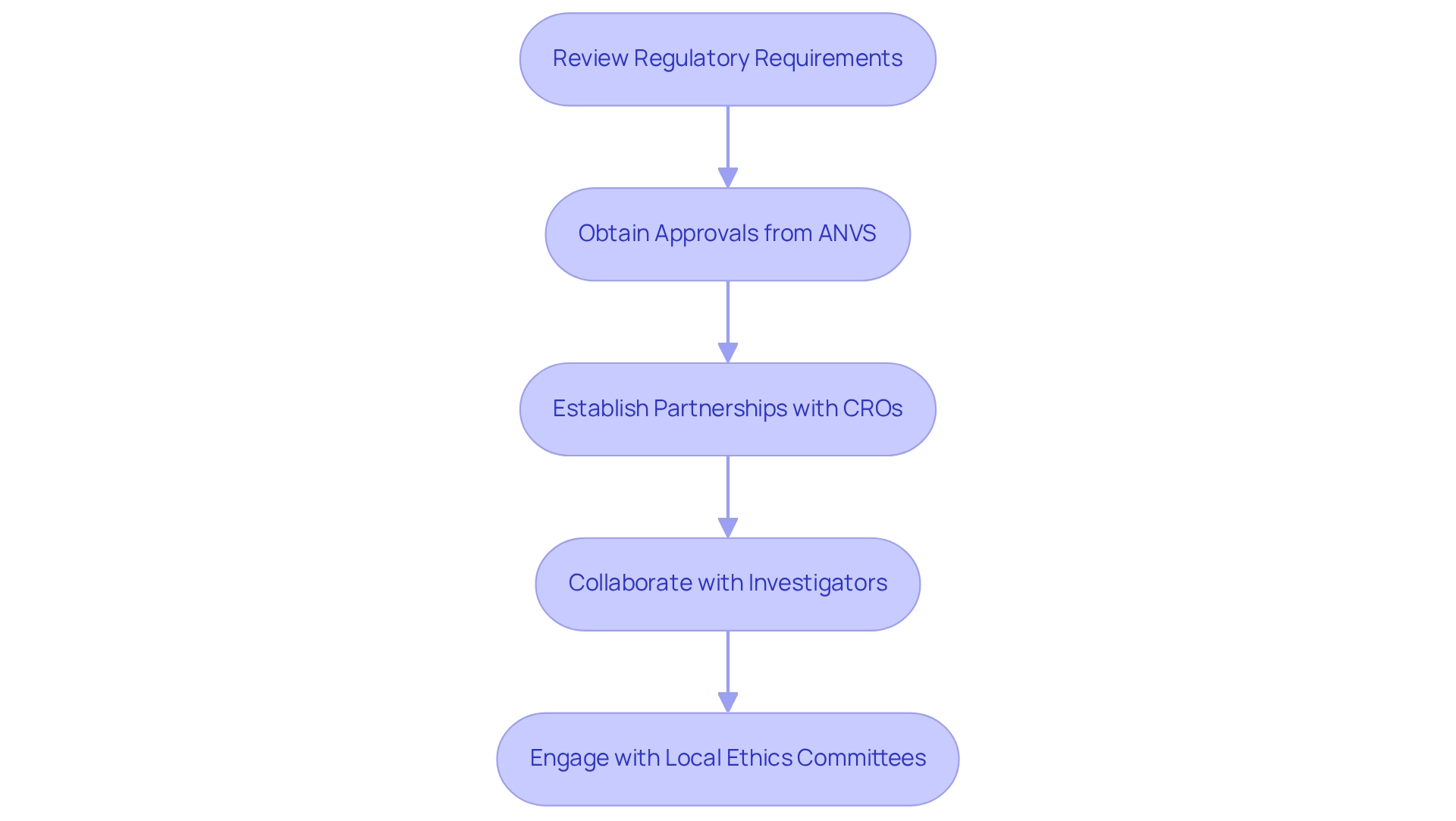
Establish Regulatory Compliance and Local Partnerships
Begin by thoroughly reviewing the regulatory requirements for clinical trials in Paraguay. This includes obtaining necessary approvals from the ANVS and ensuring compliance with ethical guidelines.
Establishing partnerships with regional Contract Research Organizations (CROs) like bioaccess provides invaluable assistance in navigating these regulations, including:
- Feasibility studies
- Selection of principal investigators
- Review and feedback on study documents
Collaborate with nearby investigators who understand the nuances of the regulatory environment and can expedite the approval process.
Furthermore, consider engaging with local ethics committees early in the planning stage to ensure that all ethical factors are addressed, while leveraging bioaccess's expertise in study setup, project management, and reporting on research status and adverse events.
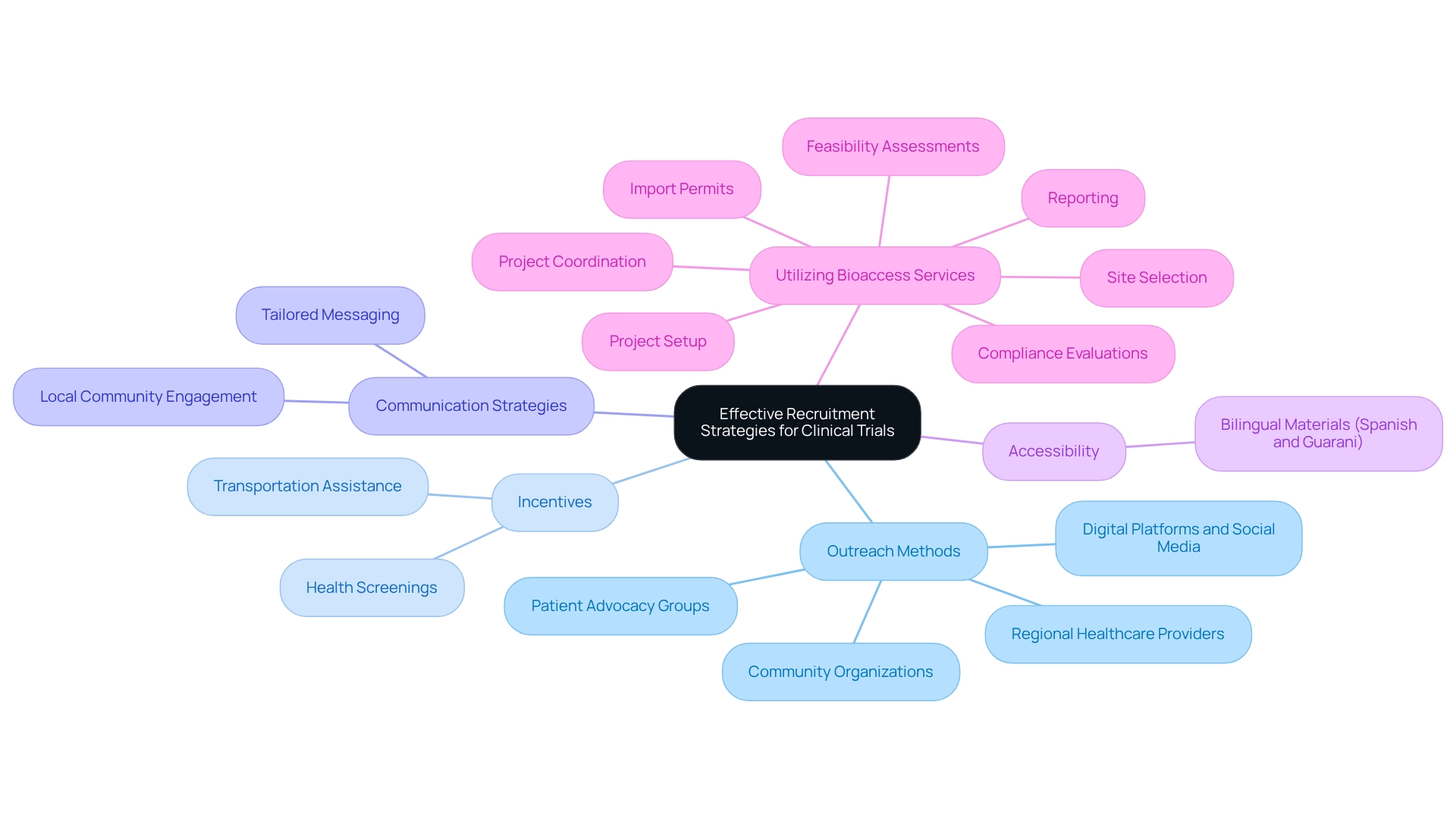
Implement Effective Recruitment Strategies for Clinical Trials
To effectively recruit participants, it is essential to develop a comprehensive recruitment plan that encompasses outreach to regional healthcare providers, community organizations, and patient advocacy groups. By leveraging digital platforms and social media, you can raise awareness about the assessment and its benefits.
Consider providing incentives for participation, such as transportation assistance or health screenings, to enhance engagement. Tailor your communication to resonate with the local community, emphasizing the potential advantages for both participants and the neighborhood.
Furthermore, ensure that your recruitment materials are accessible in both Spanish and Guarani to broaden your reach. Additionally, by utilizing bioaccess's extensive research project oversight services—ranging from feasibility assessments and site selection to compliance evaluations, project setup, import permits, project coordination, and reporting—you can significantly enhance the efficiency and effectiveness of your recruitment strategies.
Manage Trial Logistics and Resources Efficiently
Efficient study oversight is essential for the success of cost-efficient medtech trials in Paraguay, particularly in the medical research field. It begins with meticulous planning and coordination of logistical elements, including supply chain logistics, resource allocation, and communication with stakeholders. Developing a comprehensive project timeline that outlines key milestones and deadlines is crucial for maintaining momentum. Utilizing project management tools can significantly enhance tracking progress and ensuring accountability among team members, ultimately leading to improved operational efficiency.
Establishing clear communication channels with all stakeholders—including investigators, sponsors, and regulatory bodies—fosters collaboration and transparency. This is vital for navigating the complexities of clinical studies, especially in diverse settings like Paraguay, where cost-efficient medtech trials require high-quality data to form trustworthy conclusions and ensure patient safety, making it essential to concentrate on data integrity throughout the study process. Supply chain logistics play a pivotal role in the success of experiments. Ensuring that all necessary equipment and medications are readily available when needed minimizes disruptions. Additionally, closely monitoring resource allocation helps prevent overallocation or shortages, which can lead to costly delays.
A significant illustration of efficient study oversight is the collaboration between GlobalCare Clinical Trials and bioaccess™, which has successfully broadened research ambulatory services in Colombia. This collaboration has resulted in over a 50% reduction in recruitment time and a remarkable retention rate of over 95%, showcasing the potential for enhanced operational efficiency in the region.
A case study titled 'Measuring Operational Efficiency in Clinical Trials' highlights the importance of identifying and tracking key performance indicators (KPIs) such as participant enrollment rates and data quality metrics. These insights are invaluable for assessing the effectiveness of operational processes and making informed adjustments, particularly for cost-efficient medtech trials in Paraguay. By collaborating with entities such as bioaccess®, Medtech firms can effectively manage research challenges and enhance resources for their objectives. Moreover, by applying these best practices and utilizing project management tools, Medtech companies can elevate their clinical research logistics, ultimately promoting innovation and efficiency in their research endeavors. As noted by Prime Source, 'We are more than just a service provider; we are your strategic partner in driving innovation and efficiency,' emphasizing the value of collaboration in achieving successful trial outcomes.
![]()
Conclusion
Navigating the complexities of Medtech trials in Paraguay necessitates a thorough comprehension of the local landscape, encompassing regulatory compliance, patient demographics, and effective recruitment strategies. A deep familiarity with the regulatory framework established by the Agencia Nacional de Vigilancia Sanitaria (ANVS) is paramount, as is the forging of robust local partnerships. Collaborating with established organizations like bioaccess® can significantly streamline the process, ensuring adherence to all ethical guidelines while enhancing the overall efficiency of clinical trials.
Successful recruitment strategies are vital for the triumph of these trials. Engaging with local healthcare providers and employing innovative outreach methods can effectively attract a diverse participant pool. Tailoring messaging to resonate with the local population and offering incentives for participation can further bolster recruitment efforts. By leveraging local knowledge and resources, Medtech companies can ensure their trials not only meet compliance standards but also address the specific needs of the community.
Ultimately, the success of Medtech trials in Paraguay rests on strategic planning and resource management. By implementing best practices in trial logistics and maintaining transparent communication among all stakeholders, organizations can optimize their operations and achieve meaningful outcomes. As international interest in Paraguay continues to escalate, embracing these strategies will not only facilitate successful trials but also contribute to the advancement of healthcare in the region. The potential for innovation and improved patient outcomes positions Paraguay as a promising destination for Medtech trials, and with the right approach, organizations can lead the way in this evolving landscape.
Frequently Asked Questions
What is essential for conducting cost-efficient medtech trials in Paraguay?
It is crucial to understand the local landscape, including the regulatory framework established by the Agencia Nacional de Vigilancia Sanitaria (ANVS) and the patient demographics that can influence recruitment strategies.
What challenges are associated with the patient demographics in Paraguay?
Paraguay's diverse population presents both opportunities and challenges, necessitating culturally sensitive approaches to recruitment and engagement.
What services does bioaccess® offer for managing research in Paraguay?
Bioaccess® provides comprehensive management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
Why is there increasing interest from global firms in conducting medtech trials in Paraguay?
The increasing interest is due to lower costs and expedited regulatory processes, which enhance study efficiency and contribute to regional economic development through job creation and improved healthcare outcomes.




