Introduction
Panama has increasingly positioned itself as a pivotal player in the realm of medical device clinical trials, thanks to its strategic location, modern infrastructure, and supportive economic landscape. As the demand for innovative medical solutions rises globally, the nation offers a fertile ground for both local and international sponsors looking to conduct clinical research.
However, the journey is not without its challenges; regulatory complexities, language barriers, and fragmented resources often hinder collaboration, particularly with American trial sponsors. Noteworthy initiatives, such as partnerships between leading organizations, are actively working to overcome these obstacles and accelerate advancements in medical technology.
With a commitment to ethical standards and a burgeoning network of Clinical Research Organizations (CROs), Panama is not only enhancing its reputation but also making significant strides in ensuring that clinical trials are accessible, equitable, and aligned with international best practices.
As the landscape evolves, the future of clinical trials in Panama appears bright, promising to drive innovation and improve healthcare outcomes across the region.
Panama's Emergence as a Hub for Medical Device Clinical Trials
In recent years, Panama has emerged as a significant center for medical device testing, driven by its strategic geographic location, modern infrastructure, and favorable economic conditions. The country has actively sought to position itself as a center for innovation in the medical field, attracting both local and international sponsors. However, Medtech companies still encounter challenges such as regulatory hurdles, language barriers, and resource fragmentation that complicate collaboration with American research participants.
Significant collaborations, like the alliance between Greenlight Guru and bioaccess™, seek to tackle these challenges while speeding up Medtech advancements and research across Latin America. The creation of specialized study centers and collaborations with universities has further enhanced the country's reputation. Furthermore, the government has introduced several incentives to encourage innovation and development, making it an appealing location for conducting medical studies.
As emphasized by successful research, including PAVmed's first-in-human study in Colombia, the nation is increasingly acknowledged as a rising star in the global testing landscape, particularly for medical devices that require rigorous examination and validation before market entry.
Navigating Panama's Robust Regulatory Framework for Clinical Trials
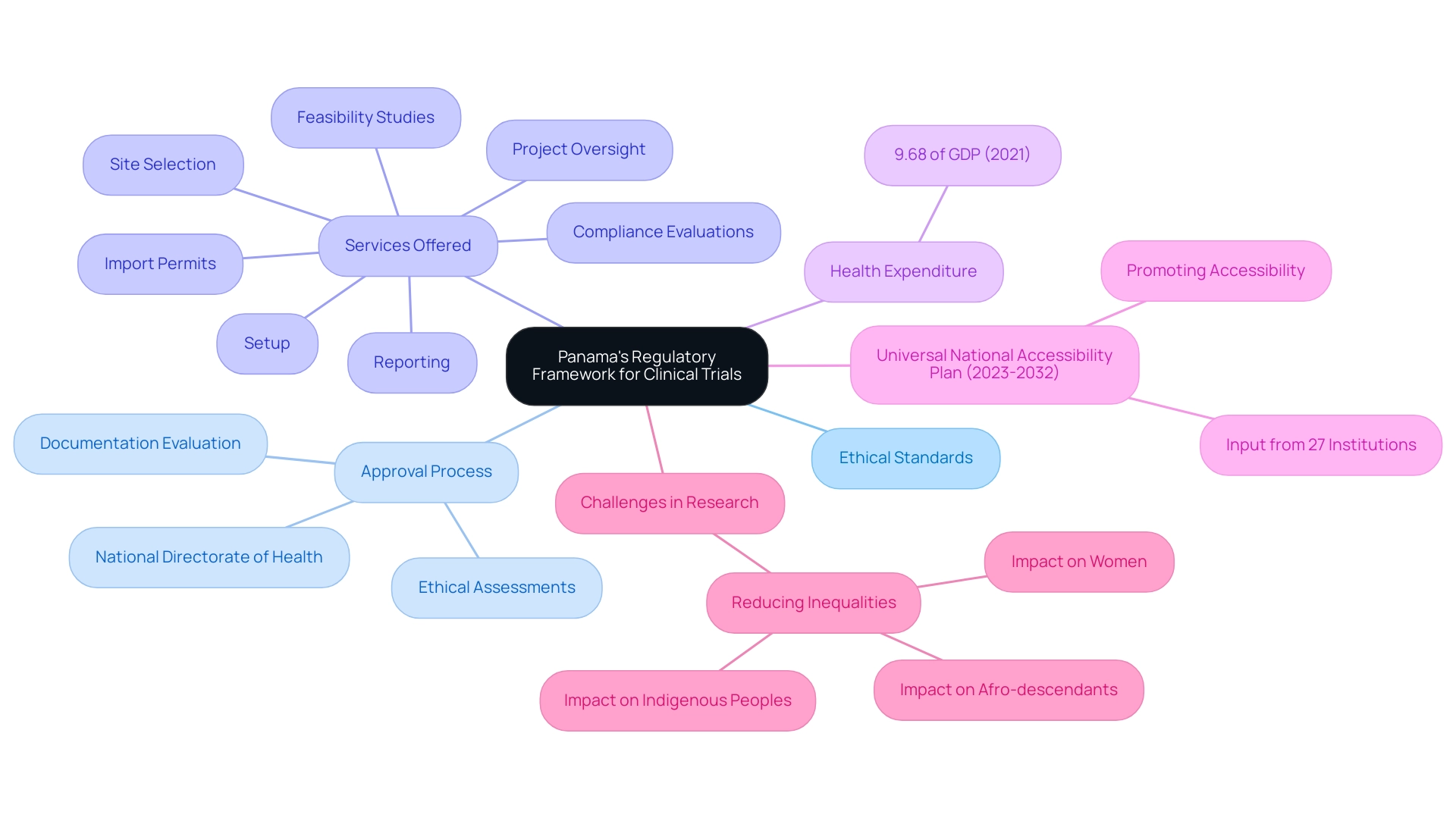
Panama's regulatory framework for medical research is designed to uphold ethical standards and international compliance, ensuring that studies are conducted with the utmost regard for participant safety and rights. The National Directorate of Health acts as the regulatory body, managing the approval procedure for research studies, which involves a thorough evaluation of documentation and ethical assessments prior to any investigation starting. This demonstrates the nation's dedication to ethical principles that are vital for thorough research management services, including:
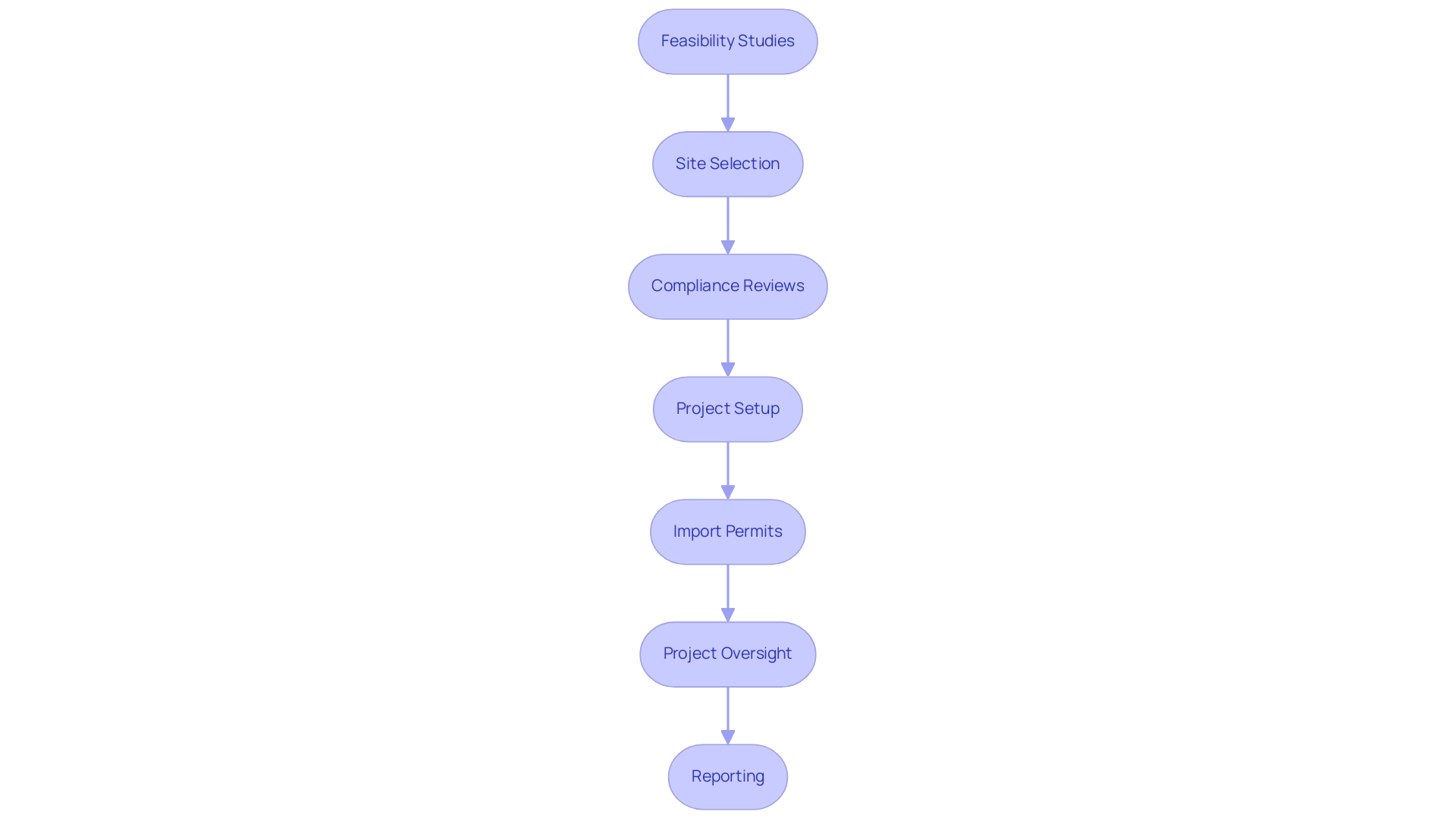
- Feasibility studies
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
As of 2021, health expenditure in Panama accounted for 9.68% of GDP, highlighting the significance of an effective healthcare system in supporting clinical studies. The nation's compliance with Good Clinical Practice (GCP) further boosts the credibility of studies conducted within its jurisdiction. Moreover, according to a statement from the World Bank, the main challenge is to reduce inequalities that especially affect women, Afro-descendants, and Indigenous peoples, highlighting the necessity for inclusive research practices.
The services we offer, including trial setup and project management, aim to address these challenges by ensuring that clinical trials are accessible and equitable. Additionally, the regulatory framework of the country aligns with the WHO Triple Billion Targets, which aim to improve health outcomes globally by ensuring that more people benefit from Universal Health Coverage (UHC) and are protected from health emergencies. The recent development of the first Universal National Accessibility Plan (2023-2032) in the country, created with input from 27 institutions, aims to promote access to healthcare for all societal groups, emphasizing the government's commitment to improving healthcare access.
By nurturing a strong, supportive regulatory framework and providing extensive research management services, the country is positioning itself to draw more research activities, ultimately aiding in advancements in medical knowledge, improved patient outcomes, and stimulating economic growth through job creation and global collaboration.
The Role of Clinical Research Organizations (CROs) in Panama
Panama Clinical Trials Services are provided by Clinical Research Organizations (CROs), which play a crucial role in the successful execution of clinical studies in Panama, offering a comprehensive suite of services that encompass study design, patient recruitment, data management, and regulatory compliance support. With a focus on advancing medical device studies, these organizations ensure a meticulous process that includes site feasibility assessments, investigator selection, and the navigation of complex regulatory requirements. However, medical device startups often face significant challenges, including competition from established players with greater brand recognition and financial constraints that limit their ability to conduct tests effectively.
By leveraging specialized knowledge and operational expertise, CROss streamline the clinical trial process, guaranteeing that studies are conducted with precision and in adherence to regulatory standards. The robust presence of esteemed Panama Clinical Trials Services in the region significantly enhances its appeal to international sponsors, providing the critical infrastructure and insights necessary for high-quality research. Notably, with a remarkable 95% renewal rate and access to 30,000 high growth opportunities, CROss in the region are poised to drive innovation within the medical device sector.
They also enable access to various local patient groups, a crucial element in the success of research studies, although attracting these patients can be difficult due to hesitance or ineligibility. As emphasized by Julio G. Martinez-Clark, a prominent LATAM Medtech CRO authority, "Overall, this country is a desirable site for medical device studies, taking into account the key aspects noted above." This positioning underscores the strategic importance of Panama Clinical Trials Services in fostering innovation and advancing medical technologies.
Furthermore, exploring Mexico's growing role in medical device studies offers a comparative viewpoint, highlighting Panama's competitive advantage in the area. It is noteworthy that North America is expected to dominate the CRO services market during the forecast period, with the US leading due to favorable government R&D investments, further contextualizing Panama's growing significance in the industry.
Patient Recruitment Strategies in Panama's Clinical Trials
Patient enrollment is among the most significant challenges in research studies. However, countries like Colombia are emerging as leaders in developing effective strategies to engage participants, notably through the partnership between bioaccess™ and GlobalCare Clinical Trials (GCCT). This collaboration, backed by Colombia's Minister of Health, has accomplished over a 50% decrease in recruitment time and upheld retention rates surpassing 95%, establishing a standard for trial efficiency.
Colombia's diverse population and extensive network of healthcare facilities provide a broad pool of potential participants, similar to the benefits offered by Panama Clinical Trials Services. Local healthcare providers in Colombia play a pivotal role in identifying eligible patients and facilitating their involvement in research studies. Awareness campaigns and educational initiatives further strengthen these efforts by informing the public about the advantages and significance of ongoing research studies.
As Maria Palombini, Global Practice Leader in Healthcare & Life Sciences, emphasizes, 'De-Centralized Clinical Trials Innovation and Technology Integration' is key to enhancing patient engagement. The case studies in Colombia highlight the influence of research project management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Project setup
- Import permits
- Project oversight
- Reporting
on local economies—promoting job creation, economic growth, and enhanced healthcare. Continuous collaboration between sponsors and CROs in various regions reflects the need for defined goals and measurement of results for effective vendor oversight.
As the medical study environment changes, these strategic priorities are essential for predicting new patient involvement approaches for 2024.
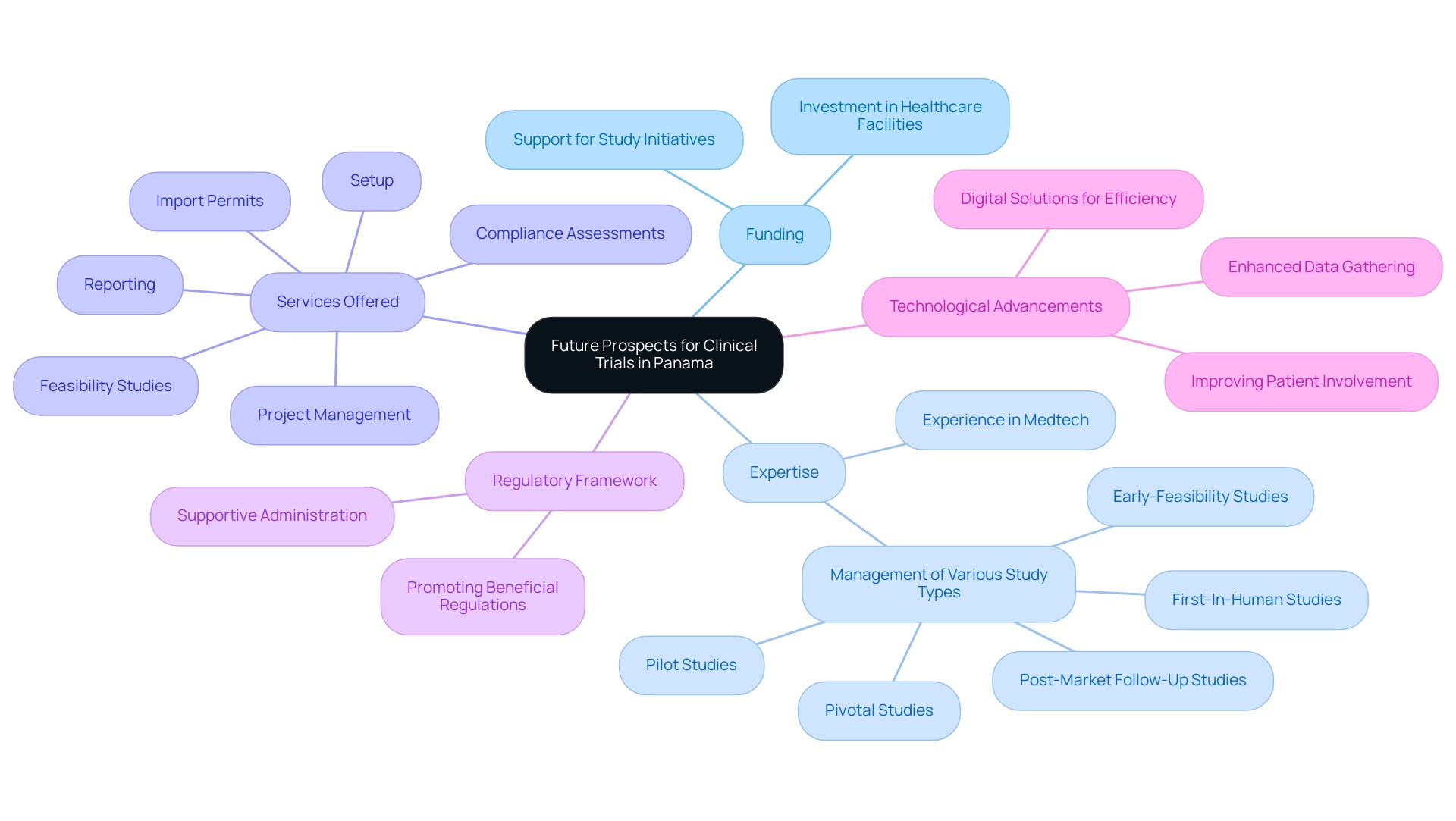
Future Prospects for Clinical Trials in Panama
The prospects for medical studies in the country appear encouraging, supported by funding in healthcare facilities and growing investigative capacities. With a rising worldwide demand for advanced medical devices and therapies, Panama is well-situated to draw more experimental activities. The expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, backed by over 20 years of experience in Medtech, will enhance the quality and compliance of studies conducted here.
Our service abilities encompass:
- Feasibility and selection of study locations
- Compliance assessments
- Setup
- Import permits
- Project management
- Reporting
These are vital for successful medical studies. The administration's dedication to promoting a beneficial regulatory framework and backing study initiatives will likely enhance the nation's competitiveness in the medical testing market. Moreover, as technology keeps advancing, the country may utilize digital solutions to improve efficiency in assessments, patient involvement, and data gathering.
Overall, this combination of a supportive ecosystem and a proactive approach to clinical research will significantly contribute to Panama Clinical Trials Services, which will help Panama grow as a key player in the global clinical trials landscape, creating jobs, promoting economic growth, and improving healthcare outcomes.
Conclusion
Panama's emergence as a vital hub for medical device clinical trials is underscored by its strategic advantages, including modern infrastructure and a favorable regulatory environment. The nation's commitment to ethical standards and international compliance ensures that clinical trials prioritize participant safety and rights, fostering an atmosphere conducive to innovation. Despite challenges such as regulatory complexities and resource fragmentation, collaborative efforts among organizations are paving the way for enhanced research capabilities and streamlined processes.
The role of Clinical Research Organizations (CROs) is crucial in this landscape, as they provide the expertise necessary for navigating the intricacies of clinical trials. With a robust presence in Panama, these organizations facilitate patient recruitment and ensure adherence to regulatory standards, thus enhancing the attractiveness of the region for international sponsors. Initiatives aimed at reducing recruitment times and improving patient engagement are critical components of this evolving paradigm.
Looking ahead, Panama stands poised for significant growth in clinical research activities. The government's supportive policies, along with the potential for technological advancements, will further solidify the country's position in the global clinical trials market. As Panama continues to attract investment and foster collaborations, it is set to not only drive innovation but also improve healthcare outcomes across the region, reinforcing its reputation as a key player in the medical device sector.
Frequently Asked Questions
Why has Panama become a significant center for medical device testing?
Panama has emerged as a center for medical device testing due to its strategic geographic location, modern infrastructure, and favorable economic conditions, which attract both local and international sponsors.
What challenges do Medtech companies face in Panama?
Medtech companies encounter challenges such as regulatory hurdles, language barriers, and resource fragmentation, which complicate collaboration with American research participants.
How are collaborations helping to address these challenges in Panama?
Collaborations, such as the alliance between Greenlight Guru and bioaccess™, aim to tackle challenges and accelerate Medtech advancements and research across Latin America.
What role does the Panamanian government play in fostering medical research?
The government has introduced several incentives to encourage innovation and development in the medical field, making Panama an appealing location for conducting medical studies.
What is the regulatory framework for medical research in Panama?
Panama's regulatory framework is designed to uphold ethical standards and international compliance, managed by the National Directorate of Health, which evaluates documentation and conducts ethical assessments before research studies begin.
What services do Clinical Research Organizations (CROs) provide in Panama?
CROs in Panama offer a comprehensive suite of services including study design, patient recruitment, data management, and regulatory compliance support, essential for the successful execution of clinical studies.
What is the importance of patient enrollment in research studies?
Patient enrollment is critical for research studies, and effective strategies, like those developed in Colombia, aim to enhance participation and retention rates in trials.
How does Panama's healthcare expenditure reflect its commitment to clinical studies?
As of 2021, health expenditure in Panama accounted for 9.68% of GDP, indicating the significance of a robust healthcare system in supporting clinical studies.
What is the outlook for medical studies in Panama?
The prospects for medical studies in Panama are encouraging, supported by funding in healthcare facilities and growing investigative capacities, positioning the country to attract more experimental activities.
How does the regulatory framework in Panama align with global health goals?
Panama's regulatory framework aligns with the WHO Triple Billion Targets, aiming to improve health outcomes globally by ensuring broader access to healthcare and protection from health emergencies.




