Introduction
The realm of medical device clinical trials is a complex and dynamic landscape, shaped by regulatory requirements and the imperative to ensure patient safety and device efficacy. As these trials progress through various classifications—ranging from low-risk Class I devices to high-stakes Class III devices—the intricacies of their management become increasingly critical.
This article delves into the multifaceted stages of clinical trials, from preclinical studies to pivotal trials, and highlights the essential role of comprehensive trial management services. Furthermore, it examines the unique challenges faced in pilot studies, particularly in emerging markets like Latin America, where innovative partnerships are forging pathways to success.
By understanding these classifications and navigating the regulatory landscape, stakeholders can optimize their approach to clinical research, ultimately advancing the field of medical technology.
Understanding Medical Device Clinical Trials: Classifications and Stages
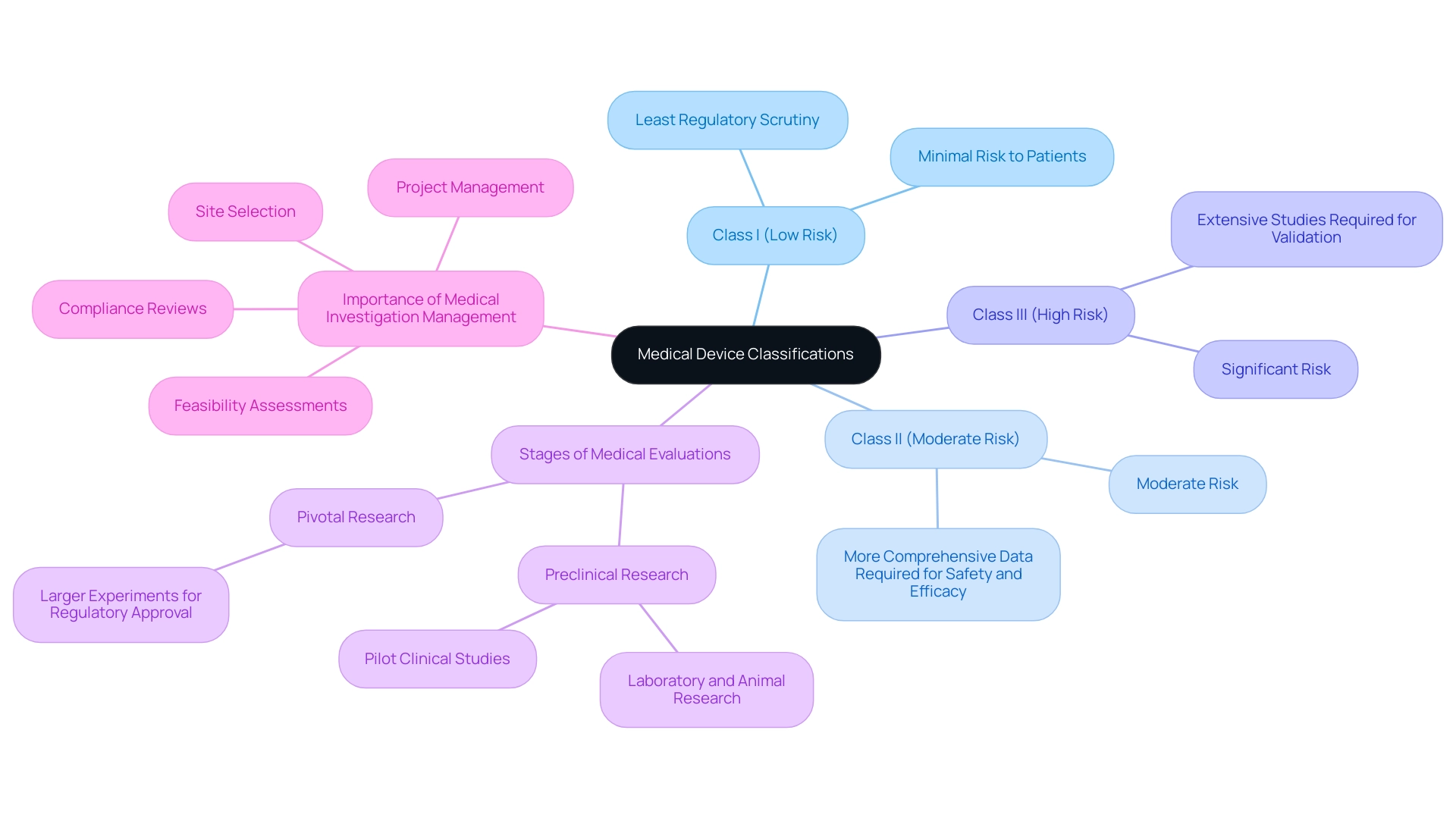
Medical equipment studies are classified according to the related risks of the instruments, which dictate the regulatory pathways and requirements for each study. The classifications are as follows:
- Class I (Low Risk): Devices that pose minimal risk to patients and generally require the least regulatory scrutiny.
- Class II (Moderate Risk): Devices that present a moderate risk and typically necessitate more comprehensive data to demonstrate safety and efficacy.
- Class III (High Risk): Devices that pose significant risk and require extensive studies to validate their safety and effectiveness before regulatory approval can be obtained.
As of June 2022, there were numerous COVID-19 treatment vaccine research efforts worldwide, showcasing the pharmaceutical industry's rapid response to the pandemic. These assessments were categorized by phase, illustrating the extensive efforts made to ensure the safety and efficacy of new therapies.
The stages of medical evaluations for devices generally progress through the following sequence:
- Preclinical Research: These initial investigations involve laboratory and animal research to collect preliminary safety and efficacy data. Pilot Clinical Studies for Medtech in Panama are conducted on a smaller scale to evaluate the feasibility, time required, costs, and potential adverse events that may occur in larger medical experiments. In Latin America, Medtech firms, such as PAVmed, are overcoming obstacles like regulatory challenges and language barriers to effectively conduct pilot clinical studies for Medtech in Panama, as demonstrated by their successful first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia, which have showcased the region's potential for research. Furthermore, the multifaceted challenges faced by US Medtech companies in Latin America include professionalism, fragmentation of resources, and the lack of CRO corporate structures, which complicate collaboration with local hospitals.
- Pivotal Research: These are larger, more definitive experiments designed to provide the conclusive evidence needed for regulatory approval.
The significance of comprehensive medical investigation management services, including feasibility assessments, site selection, compliance reviews, research setup, import permits, and project management, cannot be overstated. Companies such as bioaccess® are at the forefront of offering expedited medical device research services in Latin America, ensuring that trials are carried out effectively and in accordance with regulatory standards. Their knowledge includes the complete process, from choosing research locations and lead investigators to overseeing project timelines and providing updates on research progress and negative occurrences.
Citing the survey period for the percentage of medical investigations with posted results globally as of November 8, 2024, highlights the continuous development and openness in medical research. Understanding the nuances of these classifications and stages is essential for effectively structuring pilot clinical studies for Medtech in Panama. It not only guarantees adherence to regulatory standards but also enhances the research process, ultimately aiding the success of trials in the medical product sector.
Navigating Challenges in Pilot Clinical Studies for Medtech
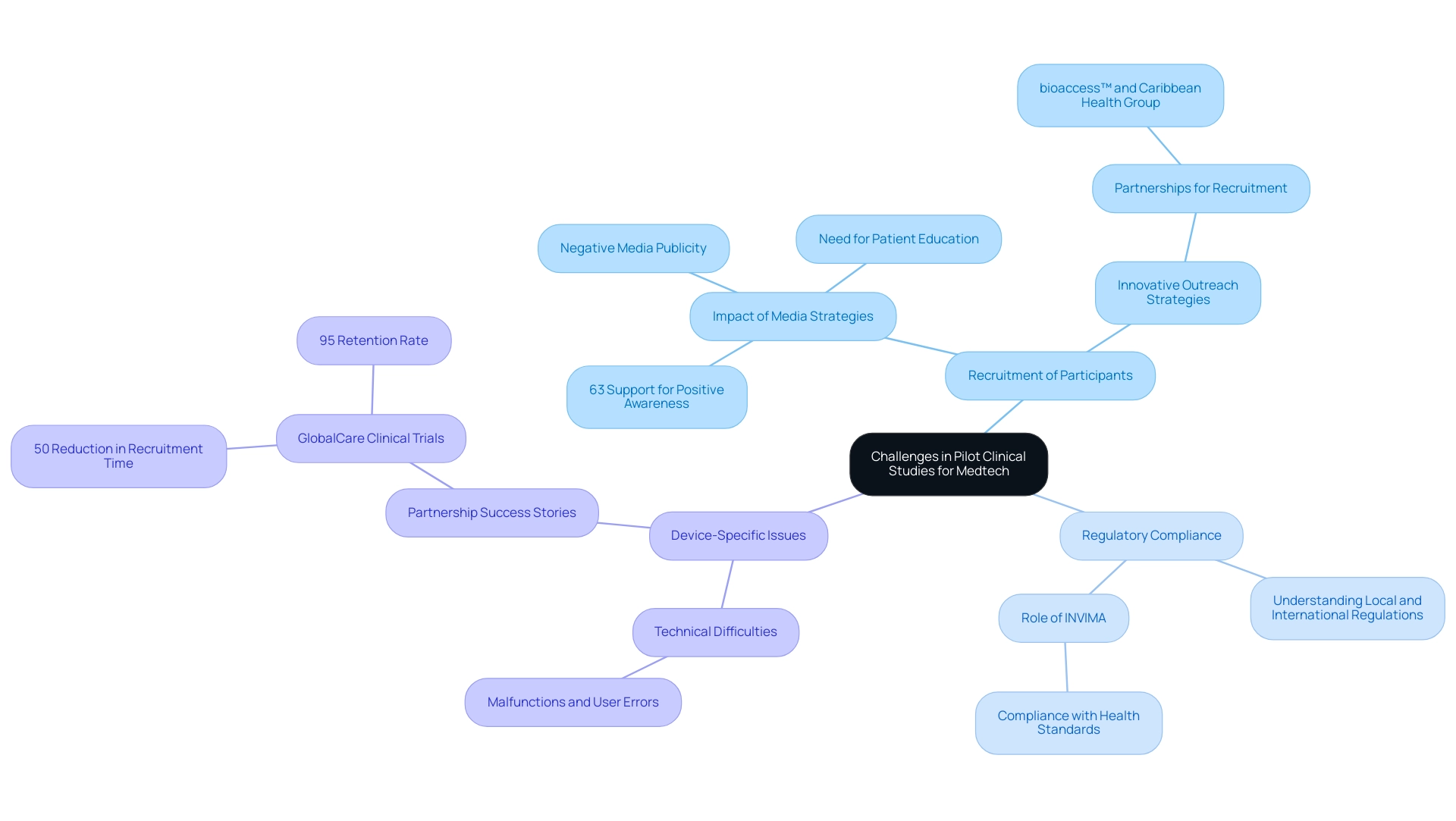
Pilot Clinical Studies for Medtech in Panama often face a variety of obstacles that can hinder success. Key issues include:
-
Recruitment of Participants: Identifying eligible participants can prove challenging, particularly when the device is aimed at a specialized demographic.
A recent survey indicated that 63% of participants strongly concurred that increasing public awareness about research studies through effective media strategies could significantly enhance recruitment efforts. However, negative media publicity and a lack of patient education also serve as substantial barriers to recruitment, emphasizing the need for innovative outreach strategies. Significantly, partnerships such as that of bioaccess™ and Caribbean Health Group in Barranquilla seek to convert the city into a leading location for pilot clinical studies for Medtech in Panama, backed by Colombia's Minister of Health, who has openly supported this initiative.
-
Regulatory Compliance: The regulatory landscape can be intricate. Achieving compliance necessitates a comprehensive understanding of both local and international regulations, which can vary greatly across jurisdictions. In Colombia, INVIMA plays a crucial role as a Level 4 health authority, overseeing the marketing and manufacturing of health products and ensuring compliance with health standards.
Familiarity with INVIMA's protocols is essential for successful management of the study.
-
Device-Specific Issues: Technical difficulties associated with the device, such as malfunctions or user errors, can adversely affect study outcomes and patient safety. 'GlobalCare Clinical Trials' partnership with bioaccess™ has already demonstrated significant improvements, achieving over a 50% reduction in recruitment time and a 95% retention rate, showcasing the effectiveness of strategic partnerships in overcoming common study challenges.
To effectively navigate these hurdles, it is essential to formulate a well-defined recruitment strategy tailored to the target population. This should include innovative approaches, such as leveraging media outreach to dispel misconceptions and promote education about clinical research. Additionally, maintaining open lines of communication with regulatory bodies is vital to ensure compliance and address challenges proactively.
The necessity for expedited patient enrollment during the COVID-19 pandemic has underscored the importance of having a Recruitment Lead with specialized knowledge to create effective, site-specific recruitment strategies.
As shown in the case study named 'Rethinking Patient Recruitment Support,' utilizing a Recruitment Lead could greatly enhance enrollment rates and hasten the introduction of investigational products to the market.
Moreover, comprehensive training sessions for both staff and participants on the correct usage of the equipment can reduce user errors and improve the quality of study results. As emphasized by Premier Research, when studies are make-or-break for the sponsor or provide essential hope for patients in need, the necessity to speed up the timeline is even greater. Thus, addressing these challenges not only improves recruitment rates but also facilitates faster delivery of investigational products to the market.
Regulatory Considerations for Conducting Clinical Trials in Panama
In Panama, the implementation of pilot clinical studies for medtech is regulated by specific guidelines set forth by the Ministry of Health and relevant authorities. Comprehending these guidelines is essential for the successful execution of studies, particularly in a nation where the total inbound stock of foreign direct investment (FDI) was 85.7% of GDP in 2022, indicating a strong economic climate that fosters clinical research. Key considerations include:
-
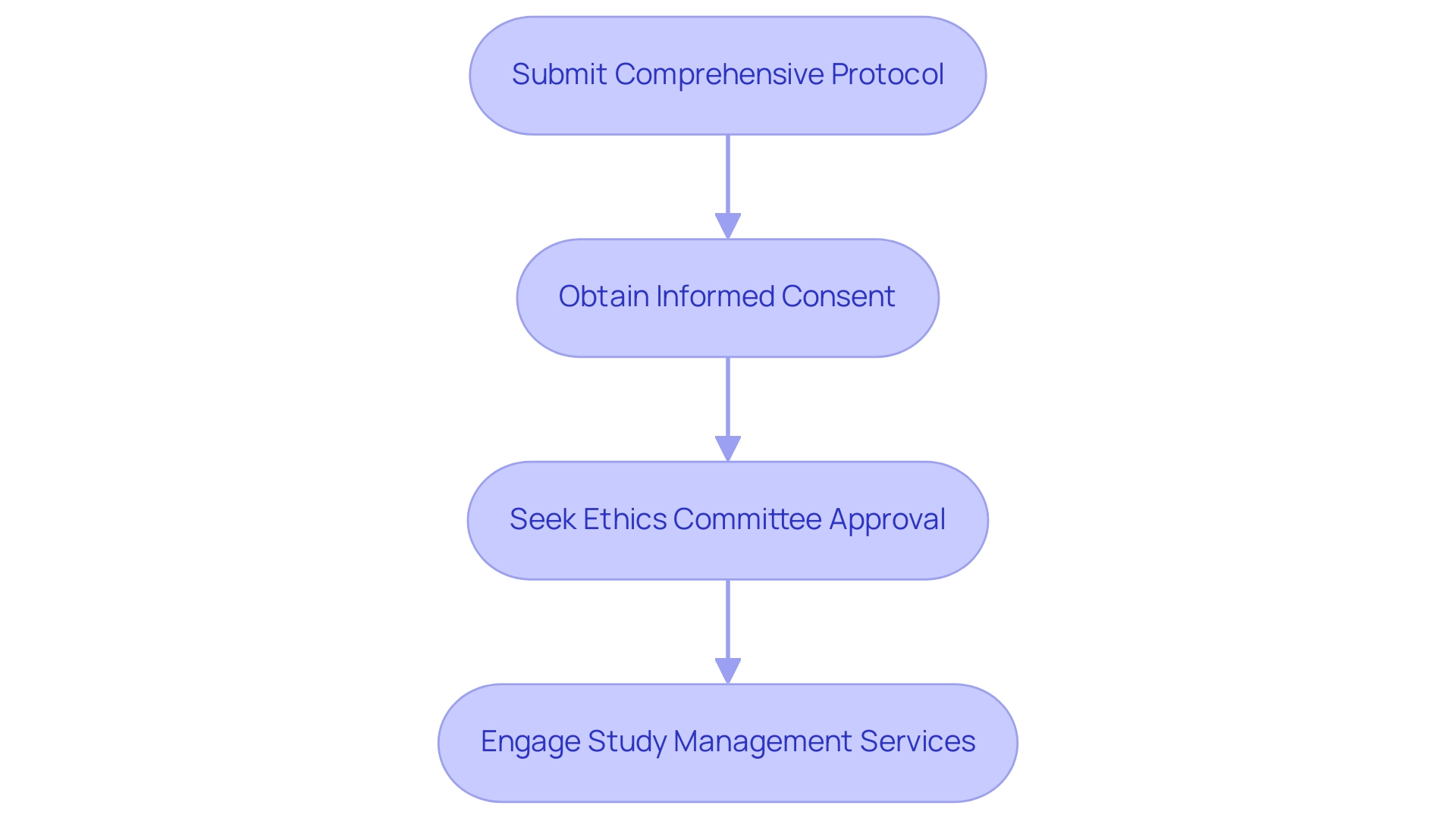
Approval Process: A comprehensive protocol must be submitted for review, detailing the study's objectives, methodology, and ethical considerations. This step is vital, as it ensures that the research is scientifically sound and aligns with national health strategies, which are adaptable to prioritize various health indicators as highlighted by the flexible approach to the Triple Billion targets.
- Informed Consent: Participants must provide informed consent, signifying their understanding of the associated risks and benefits of the study. The informed consent process has been a focal point of ethical discussions, with expert opinions emphasizing its role in safeguarding participants' rights and welfare.
-
Ethics Committee Approval: Prior approval from an ethics committee is mandatory to verify that the study meets established ethical standards. Recent developments indicate that while the Ministry of Labor's Board of Appeals and Conciliation resolves certain labor disputes, the designation of tribunal judges is still pending, which may impact the efficiency of this process.
Furthermore, Panama has established laws regarding labor rights and environmental protection, although enforcement can be inconsistent. This legal framework promotes compliance with international standards, which is essential for conducting research responsibly.
- Comprehensive Study Management: Engaging a partner with expertise in study management services, such as bioaccess®, is essential. They offer customized solutions that include feasibility studies, site selection, compliance reviews, setup for experiments, import permits, project management, and reporting on study status and adverse events. This knowledge is especially beneficial for maneuvering through the intricacies of research studies in Panama and guaranteeing successful results. Katherine Ruiz, a specialist in regulatory affairs, enhances the credibility of bioaccess®'s abilities in managing trials.
Understanding these regulatory requirements and the latest guidelines for 2024 is crucial for navigating the complexities of Pilot Clinical Studies for Medtech in Panama, especially considering the nation's dedication to maintaining labor and environmental standards. This ensures adherence to international norms while fostering a supportive environment for research.
The Role of Early Feasibility Studies in Pilot Trials
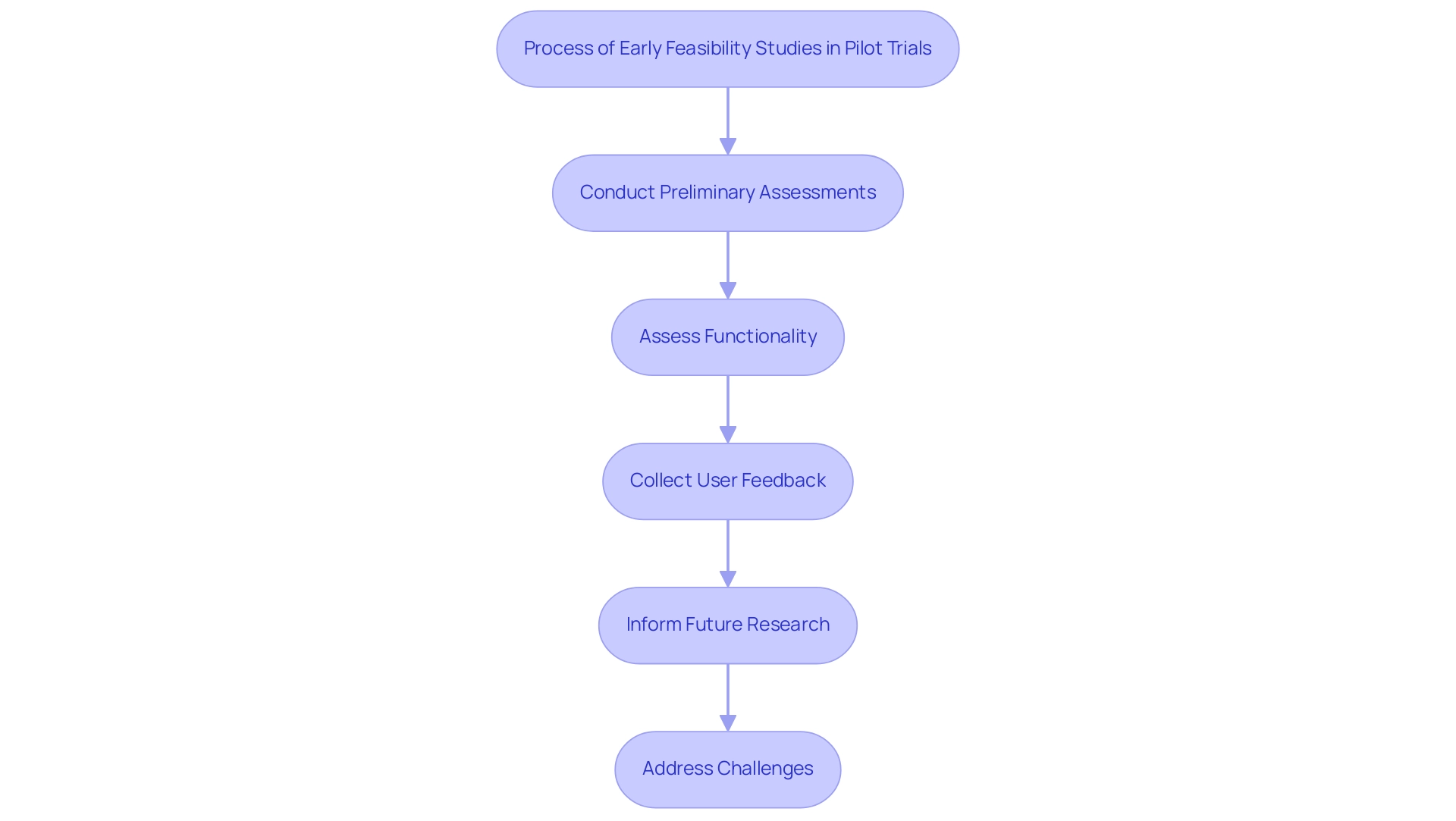
Preliminary assessments are essential for evaluating the initial performance of medical instruments in the context of Pilot Clinical Studies for Medtech in Panama. Carried out by bioaccess®, a pioneer in trial management services in Latin America, these examinations are essential for several key reasons:
- Assessing Functionality: They provide vital insights into whether the equipment operates as intended in real-world clinical environments.
- Identifying User Experience Issues: These investigations facilitate the collection of feedback from both participants and healthcare providers, which is essential for refining the device design and enhancing usability.
- Informing Future Research: Information collected from feasibility assessments serves as a basis for structuring and enhancing subsequent larger experiments, ensuring methodological rigor and relevance.
The significance of conducting early feasibility assessments cannot be understated; they are essential for preemptively addressing potential challenges that may arise in larger-scale experiments, thereby significantly increasing the probability of successful outcomes. Bioaccess® brings over 20 years of expertise in Medtech to manage evaluations, including Pilot Clinical Studies for Medtech in Panama, such as Early-Feasibility, First-In-Human, Pivotal, and Post-Market Follow-Up Trials, reflecting a commitment to excellence and adherence to regulatory standards.
Our comprehensive service capabilities include feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and detailed reporting. As observed by Deepak L. Bhatt, Guest Editor-in-Chief, the insights acquired from such research reflect a consensus on the necessity of these evaluations, although they may not directly represent the practices and policies of regulatory bodies like the U.S. Food and Drug Administration. Furthermore, recent discussions surrounding improvements to the U.S. early feasibility assessment ecosystem highlight ongoing efforts to enhance the efficiency and effectiveness of these critical evaluations, ultimately supporting the advancement of medical device innovation.
Key resources, including guidance documents on Investigational Device Exemptions (IDEs) and structured feedback requests, are available to further assist in the execution of these projects. Moreover, strategies to address obstacles influencing the success of the program have been detailed, aiming to enhance the effect of early feasibility assessments and ensure their conformity with regulatory expectations.
Emerging Trends in Medtech and Their Impact on Pilot Studies
Emerging trends in medtech are profoundly shaping the landscape of pilot research studies, with key innovations including:
- Telemedicine Integration: The integration of telehealth technologies facilitates remote monitoring and data collection, significantly enhancing participant engagement and improving data accuracy. By enabling researchers to engage with participants in real-time, telemedicine can result in more robust study outcomes, as patients are more likely to follow protocols when they receive support and guidance remotely.
- Wearable Technology: The implementation of wearable technology in research studies offers a constant flow of real-time health information, which is essential for comprehending technology performance among varied populations. These devices not only empower patients to take charge of their health but also allow researchers to gather high-quality data in varied environments, thus enhancing the overall validity of the findings.
- Artificial Intelligence: The use of AI-driven analytics is transforming the research process by simplifying data gathering and examination. As outlined in the insights from bioaccess®, these advanced technologies can identify patterns and insights faster than traditional methods, leading to more efficient trial processes and potentially quicker pathways to regulatory approval.
In Latin America, the expertise of bioaccess® in managing pilot clinical studies for Medtech in Panama is underscored by their comprehensive service capabilities, which include feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. With more than 20 years of experience in Medtech, bioaccess® guarantees that these trends are effectively utilized to improve patient outcomes. Moreover, the impact of Medtech research studies on local economies cannot be overstated; these studies not only create jobs and promote economic growth but also advance healthcare and foster international collaboration.
By remaining attuned to these emerging trends, researchers can significantly enhance their pilot clinical studies for Medtech in Panama, ensuring they are not only relevant but also effective in a rapidly evolving medical landscape. Additionally, the upcoming launch for the Diagnostic Device Track on April 3 serves as a timely reminder of the importance of networking and knowledge exchange in the medtech field.
The projected growth in medtech for 2024, particularly in established markets like China, Japan, and the United States, underscores the need for innovative solutions to navigate challenges such as currency risks and increasing competition. As noted by industry experts, these strategic choices will be pivotal in leveraging the benefits of telemedicine and advanced technologies in clinical research.
![]()
Conclusion
Understanding the complexities of medical device clinical trials is essential for stakeholders aiming to navigate the intricacies of regulatory requirements and ensure patient safety and device efficacy. The classification of devices into Class I, II, and III delineates the varying levels of risk and regulatory scrutiny, guiding the design and execution of clinical trials. Comprehensive trial management services play a pivotal role in this process, from preclinical studies through pivotal trials, particularly in emerging markets like Latin America.
The challenges faced in pilot studies, such as participant recruitment, regulatory compliance, and device-specific issues, necessitate innovative strategies and partnerships. Efforts to enhance public awareness and streamline regulatory processes are crucial for successful trial outcomes. Additionally, the integration of early feasibility studies can provide invaluable insights that inform future research, thereby increasing the likelihood of successful trials.
As the medtech landscape continues to evolve, emerging trends such as telemedicine, wearable devices, and artificial intelligence are reshaping the way clinical trials are conducted. These innovations not only improve data collection and participant engagement but also facilitate faster pathways to regulatory approval. By embracing these advancements and understanding the regulatory frameworks in regions like Panama and Latin America, stakeholders can optimize their clinical research approaches and contribute to the advancement of medical technology.
In conclusion, the successful execution of medical device clinical trials hinges on a multifaceted approach that incorporates rigorous trial management, effective recruitment strategies, and the leveraging of emerging technologies. By fostering collaboration and maintaining compliance with regulatory standards, the medtech industry can pave the way for innovative solutions that enhance patient care and drive economic growth in the healthcare sector.




