Introduction
In the realm of clinical research, particularly within the unique landscape of Peru, understanding the regulatory framework is paramount for the successful execution of pilot studies. Researchers are tasked with navigating a complex set of guidelines established by the Peruvian Ministry of Health and the National Institute of Health, which encompass critical elements such as:
- The approval process
- Informed consent
- Data protection regulations
This comprehensive overview not only highlights the essential steps involved in conducting pilot clinical studies but also emphasizes the importance of:
- Stakeholder engagement
- Data management
- Quality assurance
By adhering to these regulations and employing a structured approach, researchers can ensure compliance while fostering trust and collaboration, ultimately leading to enhanced outcomes in medical research initiatives.
Understanding the Regulatory Framework for Clinical Studies in Peru
To effectively carry out Pilot Clinical Studies for Medtech in Peru, it is crucial for researchers to fully comprehend the regulatory framework set by the Peruvian Ministry of Health (Ministerio de Salud, MINSA) and the National Institute of Health (Instituto Nacional de Salud, INS). This framework encompasses several key components:
- Approval Process: Every clinical trial must obtain approval from MINSA and the relevant ethics committee. Researchers should compile a detailed study protocol that articulates the study's objectives, methodology, and potential risks involved, ensuring compliance with established guidelines. Recent updates from the WHO trial registration data set (PER-86) highlight the importance of adhering to these protocols in a global context. Leveraging bioaccess®'s 20+ years of expertise in managing clinical trials can streamline this process, ensuring that all necessary compliance reviews are conducted effectively.
- Informed Consent: It is essential that participants provide informed consent, which requires comprehensive communication regarding the project's purpose, procedures, risks, and benefits. As stated by HHS, "research involving human subject specimens is subject to informed consent requirements, if the specimens obtained may be classified as identifiable private information." Consent forms should be culturally sensitive and available in Spanish to accommodate the local population effectively.
- Reporting Obligations: Researchers are obligated to promptly report any adverse events or significant findings to MINSA throughout the study. Understanding these reporting requirements is vital for maintaining compliance and safeguarding participant welfare. The project management expertise offered by bioaccess® ensures that reporting is handled in a timely and thorough manner, which is crucial for maintaining regulatory compliance.
- Information Protection Regulations: Adherence to privacy laws is essential to safeguard participants' personal details. Researchers must familiarize themselves with the applicable guidelines on handling sensitive data in medical research, ensuring ethical standards are upheld.
In addition, the INS has initiated efforts to promote the use of its Virtual Submission Platform, which facilitates regulatory submissions and enhances stakeholder engagement. By acquiring a thorough understanding of these regulatory aspects and using the specialized services of bioaccess®, including trial setup, project management, and compliance reviews, researchers can skillfully navigate the complexities of conducting Pilot Clinical Studies for Medtech in Peru. This not only ensures compliance but also fosters trust with stakeholders, which is essential for the success of future research initiatives.
Step-by-Step Process for Conducting Pilot Clinical Studies
Carrying out Pilot Clinical Studies for Medtech in Peru requires an organized and methodical strategy to enhance the efficiency of the research. At bioaccess®, with over 20 years of experience in Medtech, our extensive clinical trial management services guarantee that every element of the preliminary research is meticulously managed, from feasibility assessments and site selection to compliance evaluations and project oversight. Here’s a comprehensive step-by-step guide:
- Define Research Objectives: Begin by clearly outlining the goals of your pilot research. Identifying what you aim to achieve—such as evaluating the safety, feasibility, or preliminary efficacy of a new medtech device—is essential. This clarity will direct the entire research.
- Develop a Research Protocol: Craft a detailed research protocol that encompasses methodology, participant selection criteria, data collection methods, and statistical analysis plans. This protocol must align with ethical standards and regulatory guidelines, ensuring that all aspects of the research are well thought out and compliant.
- Recruit Participants: Identify and recruit a suitable population for your research. Take into account factors such as age, health status, and willingness to participate. Leveraging local networks and community outreach can significantly enhance recruitment efforts, making it easier to find participants who meet your criteria.
- Obtain Necessary Approvals: Submit your research protocol to the relevant ethics committee and secure approval from Colombia's Ministry of Health (MINSA) and INVIMA. This process might involve revisions based on feedback from regulatory bodies, so be prepared for this iterative stage.
- Conduct the Study: Implement the study as per the approved protocol. Thorough documentation of all procedures, participant interactions, and collection efforts is essential to uphold the integrity of the research.
- Data Analysis: Upon completing data collection, analyze the data using appropriate statistical methods. Ensure that your analysis aligns with the objectives outlined in the protocol to derive meaningful insights.
- Report Findings: Compile a comprehensive report that details the research's findings, highlighting both successes and challenges encountered. Sharing these insights with stakeholders is vital, and consider publication in relevant medical journals to contribute to the broader medtech field.
- Reflect and Adjust: Utilize insights from testing to make necessary modifications in design. As demonstrated in the case analysis titled 'Adjusting Learning Techniques,' insights obtained during the initial phase can result in improvements that boost data gathering and overall research effectiveness.
By following this organized method, researchers can effectively conduct pilot clinical studies for Medtech in Peru that provide valuable insights while ensuring adherence to regulatory standards. As Lauren Stewart aptly states, "Pilot Clinical Studies for Medtech in Peru provide feedback on what works and doesn't work in research," underscoring the significance of this preparatory phase in refining research methodologies. Moreover, materials from Sage, including CQ Library, Sage Data, and Sage Research Methods, can offer useful tools and information to assist the preliminary research process.
Identifying and Engaging Stakeholders
Identifying and engaging stakeholders is crucial for the success of Pilot Clinical Studies for Medtech in Peru. Here are essential steps to consider:
- Map Stakeholders: Begin by identifying all relevant stakeholders, such as healthcare providers, regulatory bodies, ethics committees, patient advocacy groups, and potential participants. Understanding their interests and concerns is vital for tailoring your engagement strategy effectively.
- Establish Communication: Develop a comprehensive communication plan that details how interactions with stakeholders will occur throughout the research. Regular updates and transparent communication are key to building respect and trust, as emphasized by Hinchcliff et al., who note that "building respect and trust through ongoing interaction" is essential for effective engagement.
- Involve Stakeholders Early: Engage stakeholders at the onset of the research design process. Their early input can yield valuable insights that enhance the project's relevance and feasibility, fostering a sense of ownership and collaboration among stakeholders. Significantly, 9% of participants in prior research indicated enhanced comprehension of research processes, highlighting the importance of early involvement.
- Address Concerns: Take a proactive approach in addressing any concerns stakeholders may have regarding the research. This should encompass clarifying research objectives, participant safety measures, and data handling protocols, ensuring that stakeholders feel informed and valued.
- Build Partnerships: Cultivating partnerships with key stakeholders can create a robust support network. Collaborating with local healthcare institutions and patient groups not only facilitates participant recruitment but also enhances the project's credibility and acceptance within the community.
Additionally, the influence of Pilot Clinical Studies for Medtech in Peru on local economies cannot be overlooked. These investigations create jobs, encourage economic development, enhance healthcare, and support international cooperation, which are vital for creating a sustainable research environment. The case analysis titled 'Literature Insights on Stakeholder Engagement' illustrates effective practices by emphasizing the need for clear roles, continuous involvement, and flexibility in research partnerships.
By efficiently recognizing and involving stakeholders, researchers can create a cooperative atmosphere that greatly aids the successful implementation of trial investigations. This approach aligns with the current trend towards greater stakeholder engagement in research, as evidenced by recent literature insights that underscore the effectiveness of sustained involvement and flexibility in research partnerships. Significantly, our vast experience of more than 20 years in overseeing a range of projects, including Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), and others, guarantees that we are well-prepared to handle the intricacies of research in Latin America.
Data Management and Quality Assurance
Effective information management and quality assurance are crucial for the success of pilot clinical studies for medtech in Peru, particularly within the framework of accelerated medical device clinical study services offered by bioaccess®. Here are essential steps to consider:
-
Develop a Comprehensive Information Management Plan: A well-structured information management plan is vital, detailing protocols for collection, storage, analysis, and sharing.
This plan should also prioritize information security and maintain participant confidentiality, ensuring ethical standards are upheld in compliance with local regulations.
-
Implement Electronic Information Capture (EDC) Systems: Leveraging electronic information capture systems enhances the efficiency of collection processes and minimizes the risk of errors.
These systems enhance information accuracy and facilitate real-time monitoring, resulting in prompt interventions when needed, and are essential to the success of pilot clinical studies for Medtech in Peru, overseen by skilled CROs like bioaccess®.
-
Incorporate SAS Programming: Utilizing SAS programming is essential in clinical trials for analyzing large datasets and drawing meaningful conclusions.
This programming tool assists in managing intricate information and enhances the overall quality of analysis, aligning with best practices in the field and supporting the rigorous standards bioaccess® upholds.
-
Conduct Regular Audits: Setting a timetable for frequent reviews of the collection process is crucial to identify inconsistencies and ensure compliance with the protocol.
This proactive approach is essential in preserving information integrity and compliance, ensuring that Pilot Clinical Studies for Medtech in Peru produce valid results.
-
Invest in Staff Training: It is crucial that all team members engaged in information gathering and management receive adequate training in the protocols and quality assurance practices.
Ongoing education, as emphasized in the case analysis on 'Training and Development for Information Management Professionals,' ensures that management teams are prepared to tackle intricate challenges efficiently.
-
Monitor Information Quality: Establishing specific metrics for overseeing information quality throughout the research is crucial.
Regular assessments of information for completeness, accuracy, and consistency enable the swift identification and resolution of potential issues.
By prioritizing these aspects of data management and quality assurance, researchers can significantly enhance the reliability of their initial trials, contributing to more robust and impactful outcomes, particularly in the context of Pilot Clinical Studies for Medtech in Peru, when supported by the expertise of bioaccess® as a vetted CRO for U.S. medical device firms in Colombia.
Notably, bioaccess® specializes in Pilot Clinical Studies for Medtech in Peru, along with Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), backed by a team with over 20 years of experience in Medtech, ensuring compliance with local regulations and ethical standards.
Evaluating Pilot Study Outcomes and Next Steps
Assessing the results of preliminary clinical trials is essential for comprehending their impact and directing future research efforts. A search of PUBMED revealed that 24,423 investigations (16%) included 'trial' or 'feasibility' in their title or abstract, underscoring the importance of trial assessments in the research landscape. Recent case analyses, like ReGelTec's Early Feasibility Assessment on HYDRAFIL™ for addressing chronic low back pain in Colombia, showcase the successful implementation of preliminary trials in practical environments.
In this research, eleven patients with degenerative disc disease were treated using a patented hydrogel injected into the nucleus of the degenerated disc, showcasing innovative methods in practice. Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has led to more than a 50% decrease in recruitment time and an impressive 95% retention rate, emphasizing the operational benefits of organized preliminary research. This partnership utilizes bioaccess®'s expertise in comprehensive clinical trial management services, including feasibility assessments, site selection, and project management.
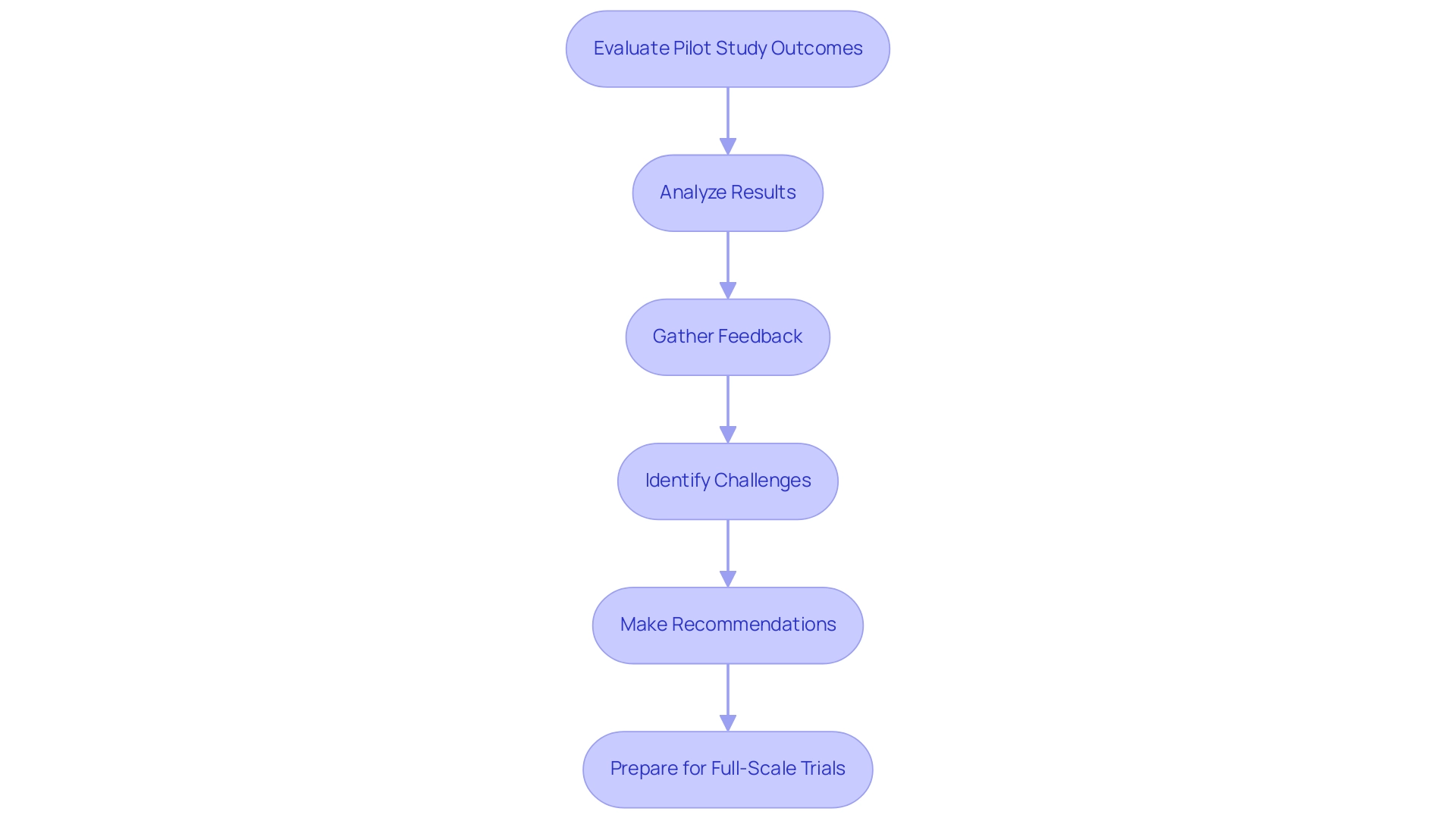
Here’s a structured approach to evaluating pilot studies:
- Analyze Results: Begin by thoroughly reviewing the information gathered during the pilot project to determine if the project objectives were achieved. Employ appropriate statistical methodologies to assess efficacy and safety outcomes, ensuring that feasibility outcomes report point estimates and their precision where applicable.
- Gather Feedback: Actively solicit input from participants and stakeholders regarding their experiences throughout the research. This qualitative information is invaluable, offering insights that can highlight potential enhancements for future research. As mentioned by Halpern et al., "The overall aim of this preliminary research is to evaluate the feasibility of conducting a large investigation to [state primary objective of the main research]."
- Identify Challenges: Document any challenges encountered during the pilot project meticulously, analyzing their impact on outcomes. Comprehending these challenges is crucial, as it will guide needed modifications for future investigations and strengthen the robustness of later research phases.
- Make Recommendations: Based on your analysis, develop targeted recommendations for the next phases of research. This may involve refining research protocols, broadening participant populations, or adjusting intervention strategies to better meet objectives. Adhering to guidelines from the case analysis titled "Recommendations for Reporting Pilot Projects" can enhance the clarity and utility of your reports.
- Prepare for Full-Scale Trials: If the initial examination produces encouraging outcomes, begin arrangements for full-scale clinical trials. This process involves securing additional funding, enhancing stakeholder engagement, and addressing any regulatory requirements identified during the trial phase. The significance of evaluating outcomes from preliminary investigations cannot be overstated; they act as a crucial step that guides the planning and implementation of larger trials.
By rigorously evaluating pilot study outcomes, researchers position themselves to make informed decisions that significantly enhance the likelihood of success in future clinical research initiatives.
Conclusion
Understanding the regulatory framework surrounding clinical studies in Peru is crucial for researchers aiming to conduct pilot studies effectively. This involves navigating the approval process, ensuring informed consent, adhering to data protection regulations, and fulfilling reporting obligations. By engaging with stakeholders throughout the study and implementing robust data management and quality assurance practices, researchers can enhance compliance and foster trust, which is essential for successful medical research initiatives.
The structured approach to conducting pilot studies, as outlined in the article, emphasizes the importance of:
- Meticulous planning
- Participant recruitment
- Thorough evaluation of outcomes
By methodically defining study objectives, developing comprehensive protocols, and involving stakeholders early in the process, researchers can optimize their studies and generate valuable insights that inform future research.
Ultimately, pilot studies serve as a critical foundation for larger clinical trials, providing essential feedback that enhances study design and implementation. As demonstrated by various case studies, the successful execution of pilot studies not only contributes to medical knowledge but also promotes collaboration and economic growth within the local healthcare landscape. By adhering to the outlined best practices and leveraging the expertise available, researchers can navigate the complexities of clinical trials in Peru, leading to improved outcomes in medical research.




