Overview
The article delineates seven essential strategies for successful clinical trial design, underscoring the necessity of clear research questions, appropriate endpoints, and adaptive methodologies to bolster participant engagement and ensure regulatory compliance. These strategies are substantiated by evidence demonstrating their efficacy in enhancing recruitment, retention, and overall study outcomes. This highlights the imperative for innovative approaches within the dynamic landscape of medical research, prompting stakeholders to consider how these strategies can be integrated into their practices.
Introduction
In the ever-evolving landscape of clinical research, the significance of effective trial design is paramount. As the medical field advances toward 2025, numerous factors—including regulatory compliance, patient-centric approaches, and technological innovations—are transforming the execution of clinical trials. Understanding the foundational elements that define a robust study, alongside innovative strategies that enhance participant engagement, is essential for achieving successful outcomes. Organizations like bioaccess® are at the forefront of advancing Medtech research, and this exploration delves into the critical components that underpin effective clinical trials. It highlights the trends and strategies poised to revolutionize the industry.
Understanding the Fundamentals of Clinical Trial Design
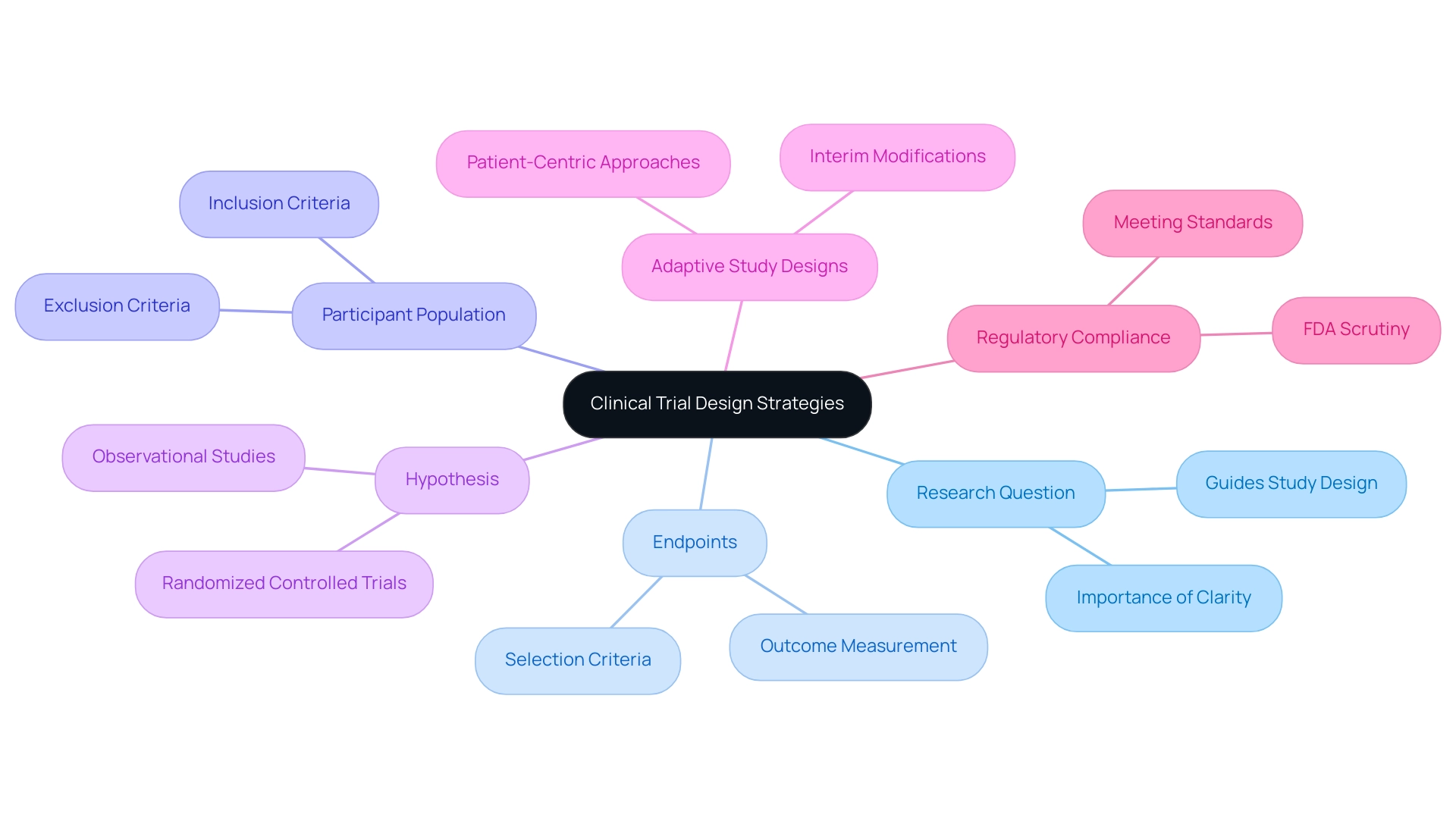
Clinical trial design strategies serve as the foundational framework for evaluating the safety and efficacy of medical interventions. Essential components of this design include the precise definition of the research question, the selection of appropriate endpoints, and the careful determination of the participant population. Mastering these fundamentals is crucial for developing a robust experiment that complies with regulatory standards and fulfills scientific objectives.
A well-articulated hypothesis is instrumental in guiding the choice of study design, whether it involves randomized controlled experiments or observational studies. For instance, in 2025, the emphasis on defining appropriate outcomes has become increasingly critical, particularly in specialized fields such as psychedelic therapy, where meeting FDA scrutiny is paramount. Joseph Tucker from Enveric underscores this point, highlighting the significance of establishing suitable outcomes in these assessments to ensure regulatory compliance.
Moreover, clarity in inclusion and exclusion criteria is essential for recruiting the right participants, directly influencing the validity and reliability of study results. Recent advancements in medical study design have emphasized the significance of customizing these criteria to accurately represent the target population, ensuring that the findings are relevant and meaningful.
Current trends indicate a growing focus on clinical trial design strategies that utilize adaptive study designs, allowing for modifications based on interim results, thereby enhancing efficiency and responsiveness to emerging data. This approach not only accelerates the development process but also aligns with the industry's shift towards more patient-centric methodologies. As Max Baumann observes, "Entering 2025, we still observe biotech confronting essential business model obstacles as medical end-markets become increasingly saturated," highlighting the necessity for creative clinical trial design strategies.
In summary, the importance of defining research questions in the context of clinical trial design strategies cannot be overstated, as they lay the groundwork for all subsequent design decisions. By utilizing knowledge from successful medical studies and expert views, organizations like bioaccess®, with over 20 years of experience in the Medtech field, are well-equipped to manage the intricacies of research. bioaccess® focuses on extensive research study management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, ensuring that each study is meticulously planned and executed.
The partnership between bioaccess® and Caribbean Health Group to establish Barranquilla as a prominent location for medical studies in Latin America, backed by Colombia's Minister of Health, illustrates their dedication to promoting research in healthcare. The case analysis titled 'bioaccess®: Advancing Medtech Research in Healthcare' demonstrates how bioaccess® effectively assists Medtech startups and bridges the gap in research, ultimately promoting the timely progression of innovative medical devices.
Emphasizing Patient-Centricity in Trial Design
Incorporating patient-centric approaches in clinical trial design strategies is essential for fostering active patient engagement throughout the research process. This encompasses not only soliciting patient input on study protocols but also ensuring accessibility of research locations and maintaining clear, transparent communication regarding the study's purpose and procedures. For instance, studies that offer flexible participation options, such as telehealth visits, have demonstrated a significant increase in both patient recruitment and retention rates.
The importance of focusing on outcomes that matter to patients cannot be overstated. Developing studies centered on quality of life metrics and other significant endpoints can yield more impactful results and enhance patient satisfaction. Evidence from recent research indicates that when clinical trial recruitment companies effectively present trial information online, patient engagement markedly increases, highlighting the necessity for innovative communication strategies.
This aligns seamlessly with bioaccess®'s mission to advance medical devices sooner through its expertise in managing clinical trial design strategies, including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Trials (PMCF)
Ensuring that patient needs are prioritized in the design process. Furthermore, the resurgence of Medical Device Regulation (MDR) has necessitated a reevaluation of design and information management practices. Effective metadata management is now recognized as crucial for automating study builds and streamlining data collection processes, effectively addressing the challenges posed by traditional reliance on spreadsheets.
This transformation not only enhances the effectiveness of research studies but also aligns with the growing emphasis on patient-centeredness in clinical trial design strategies.
Statistics reveal that only 2% of nurses cite fear of losing patients to another healthcare provider as a reason for not making referrals, underscoring the potential for improved patient engagement strategies. As Ken Getz, a respected authority in the field, observes, the proactive role of healthcare providers in determining eligibility and promoting patient involvement has been largely overlooked, yet it is vital for successful study outcomes. By prioritizing patient-focused design, medical studies can achieve superior recruitment and retention statistics, ultimately leading to more successful and meaningful research outcomes.
With over 20 years of experience in Medtech, bioaccess® is exceptionally equipped to navigate the complexities of research activities in Latin America, addressing the demand for adaptability and specialized expertise in the process.
Strategic Planning: The Backbone of Successful Trials
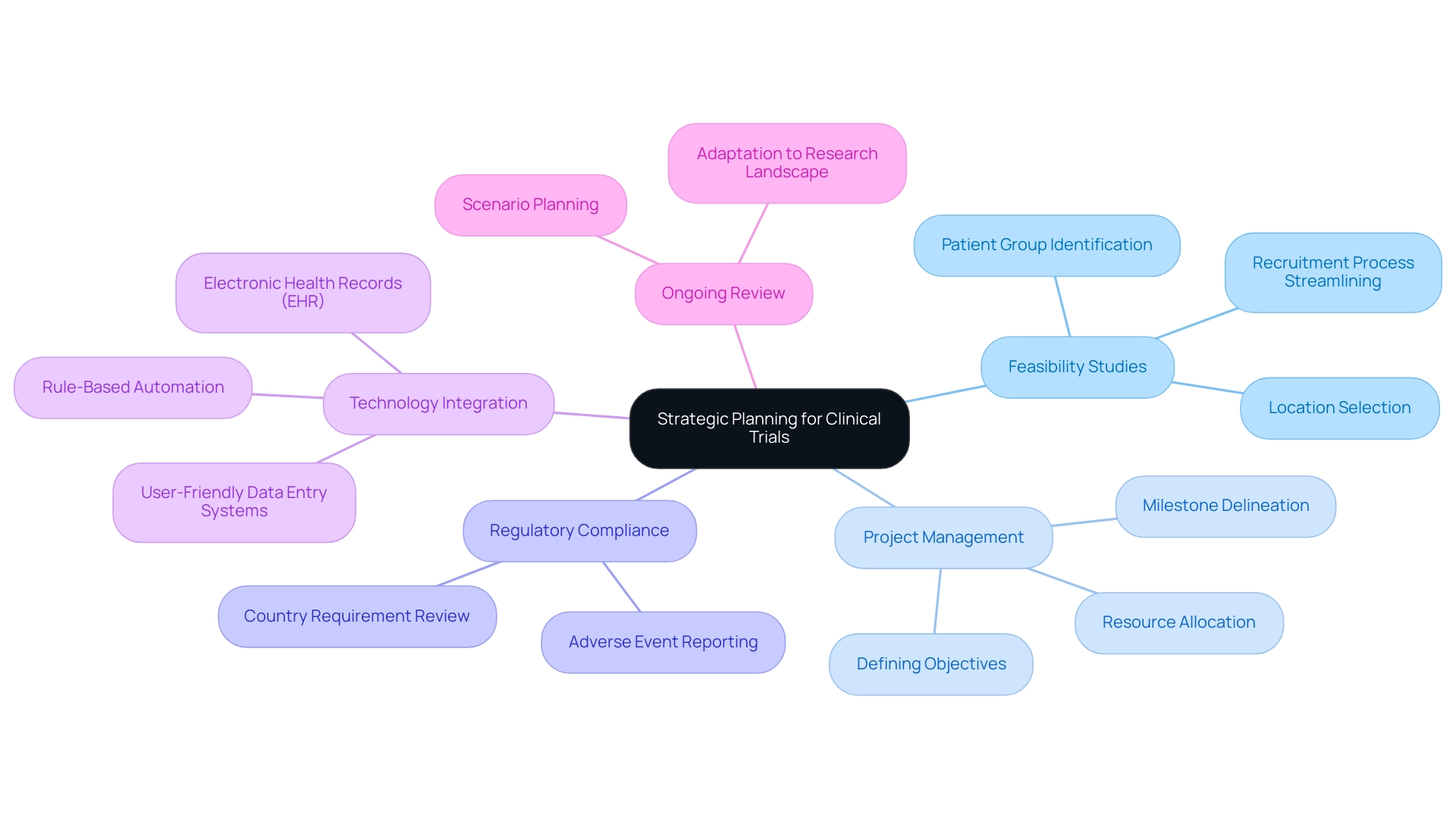
Effective strategic planning is crucial for the success of clinical studies, involving the implementation of clinical trial design strategies that encompass defining objectives, timelines, and resource allocation. A well-structured project plan should delineate key milestones and anticipate potential challenges. For instance, conducting a feasibility assessment prior to the experiment is essential; it not only identifies suitable locations and patient groups but also streamlines the recruitment process, ultimately enhancing the efficiency of the experiment.
In 2025, statistics indicate that organizations prioritizing feasibility studies report a 30% increase in recruitment success rates compared to those that do not. Moreover, scenario planning plays a pivotal role in equipping research teams for unforeseen challenges, ensuring that clinical trial design strategies keep experiments on track despite potential setbacks. Consistently reviewing and modifying clinical trial design strategies during the study is vital, allowing teams to adapt to shifts in the research landscape. This dynamic approach is supported by recent advancements in medical studies, which focus on simplifying site experiences through quicker query responses and user-friendly data entry systems.
As a leading contract research organization in Latin America, bioaccess® specializes in clinical research services for the Medtech sector, providing valuable insights into effective strategic planning. Our comprehensive services encompass:
- Feasibility and selection of research locations
- Investigator selection
- Review and feedback on project documents to comply with country requirements
- Regulatory compliance
- Project management
- Reporting on project status and adverse events
These services ensure that research is conducted efficiently and in accordance with local regulations. Case studies, such as ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia, illustrate the impact of addressing site experiences by monitoring data entry speeds and simplifying query responses.
These efforts not only enhance the efficiency of medical studies but also improve patient care, demonstrating the tangible benefits of clinical trial design strategies in the realm of medical research. Furthermore, Colombia offers competitive advantages for conducting medical studies, including cost efficiency, regulatory speed, high-quality healthcare, and robust patient recruitment capabilities. Organizations like GSK are adopting rule-based automation for information cleaning to accelerate database lock times, underscoring the importance of integrating modern technologies into clinical trial design strategies. The Head of Clinical Information Engineering noted, "Traditionally, information management was outsourced to our CRO vendor partners."
Part of the initiative is to bring all our research in-house so that our internal teams can take a more active role. They can be more involved, allowing us to implement research internally, manage our information effectively, and provide high-quality care for our patients. This perspective highlights the essential function of implementing studies and efficient information management in achieving successful research outcomes. Furthermore, the incorporation of electronic health records (EHR) and other information sources is crucial for contemporary research studies, necessitating the application of clinical trial design strategies to address the challenges and factors that must be tackled in strategic planning.
Leveraging Technology for Enhanced Trial Efficiency
The integration of technology in clinical trial design strategies is pivotal for enhancing operational efficiency and ensuring successful outcomes. Electronic information capture (EDC) systems play a crucial role in streamlining collection and management, significantly minimizing the risk of errors. As we approach 2025, the reliance on EDC has become more pronounced, with studies indicating that organizations adopting these systems report a 30% reduction in discrepancies compared to traditional methods.
Artificial intelligence (AI) is revolutionizing patient recruitment by enabling faster and more accurate identification of suitable candidates. For instance, AI algorithms can sift through electronic health records to identify potential participants based on specific inclusion criteria, thereby accelerating the recruitment process. This technology not only enhances the speed of recruitment but also improves the quality of participant selection, which is essential for the integrity of clinical studies.
Moreover, remote monitoring technologies facilitate real-time information collection, allowing researchers to track patient progress and safety more effectively. This capability enhances clinical trial design strategies by enabling timely interventions when necessary. A recent case study highlighted the resurgence of Medical Device Regulation (MDR) and the emphasis on information standards, showcasing how companies are encouraged to transition from spreadsheets to more integrated solutions for metadata management.
This shift not only enhances the efficiency of medical data collection but also aligns with the evolving regulatory landscape.
As the medical market becomes increasingly crowded, the focus on optimizing clinical trial design strategies is paramount to achieve both approval-enabling endpoints and commercial success. Max Baumann, Head of Execution at Treehill Partners, emphasizes this by stating, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." This viewpoint highlights the importance of incorporating advanced technologies in clinical trial design strategies, which is not merely a trend but an essential requirement for organizations striving to succeed in this competitive landscape.
Furthermore, bioaccess® is set to assist Medtech startups in overcoming these obstacles, providing expedited research services that encompass regulatory approval, site activation, patient recruitment, and prompt information delivery throughout Latin America. The Japanese government's recent actions permitting Japanese locations to be part of global multi-center Phase III studies without prior Phase I Japanese patient information illustrate the changing regulatory environment, further highlighting the necessity for flexibility in research design.
Navigating Regulatory Compliance in Clinical Trials
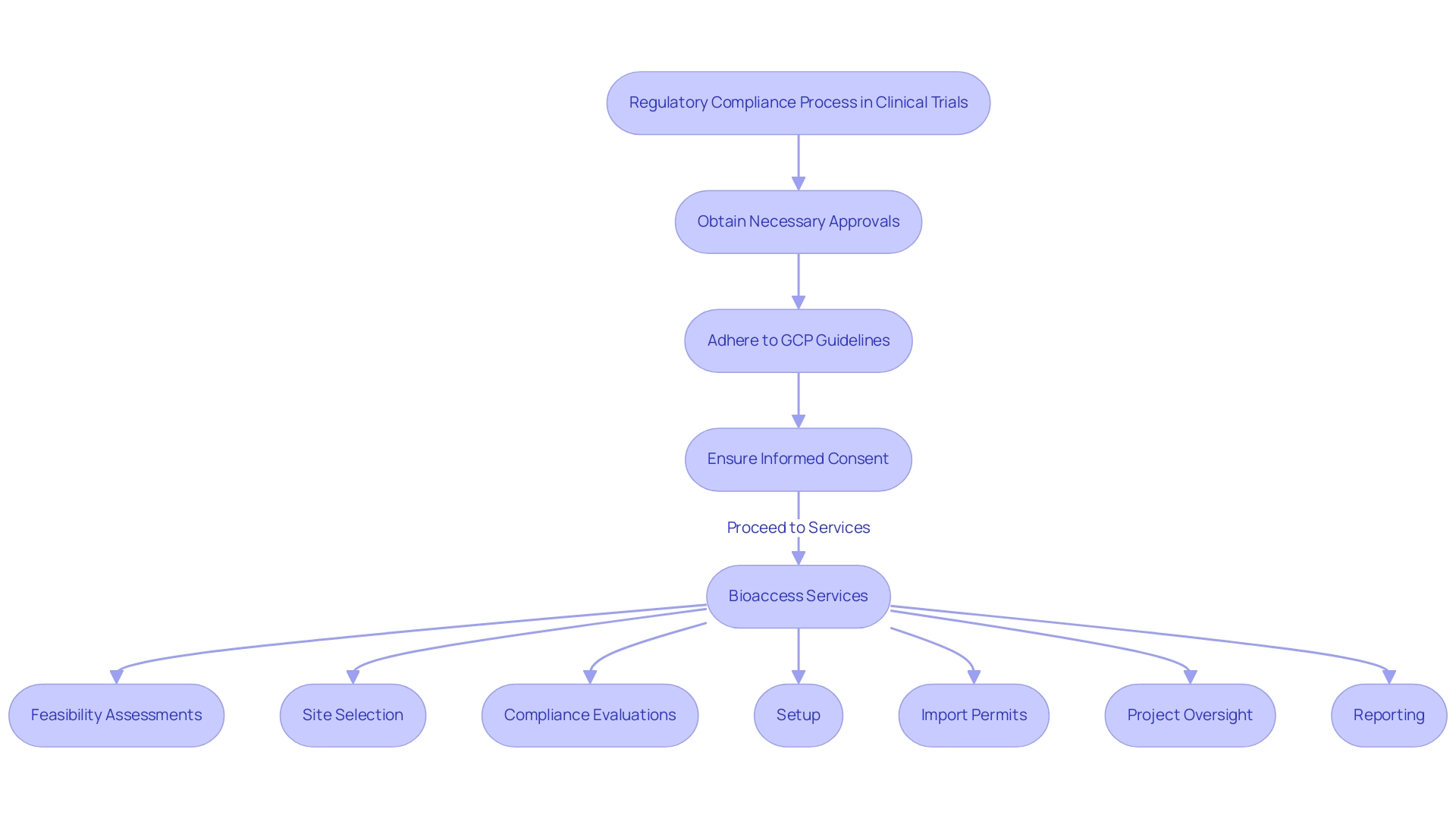
Navigating the regulatory environment is essential in research design, particularly in 2025, as regulatory bodies like the FDA and EMA continue to evolve their requirements. Key considerations include:
- Obtaining necessary approvals
- Adhering to Good Clinical Practice (GCP) guidelines
- Ensuring informed consent from participants
A well-organized regulatory approach that clearly outlines the submission process for research protocols is essential to avoid delays and ensure compliance with current regulations.
In this context, bioaccess provides extensive management services for research projects, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These services include review and feedback on study documents to comply with country requirements and reporting on serious and non-serious adverse events. They are designed to streamline the clinical study process, ensuring that all aspects are meticulously managed to meet regulatory standards.
The FDA's Single IRB Requirement, expected to be implemented in 2025, emphasizes the necessity for streamlined processes in multi-site studies. This requirement aims to enhance efficiency and reduce redundancy in ethical reviews, ultimately benefiting patient care. Furthermore, adherence to GCP guidelines is not merely a regulatory obligation; it significantly impacts study outcomes.
Research shows that experiments demonstrating strict adherence to GCP are linked with enhanced information integrity and participant safety, which are essential for successful study outcomes. Regular training for the research team on the latest regulatory updates is crucial for maintaining compliance with evolving standards. This proactive approach fosters a culture of compliance and equips teams to navigate the complexities of regulatory requirements effectively. For example, statistics indicate that 45% of information in medical studies is recorded on the same day as the visit date, emphasizing the significance of effective data management procedures.
Additionally, Vivienne van der Walle, Founder and Medical Director, emphasizes that "anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." This perspective underscores the importance of enhancing site experiences.
In summary, comprehending and maneuvering through the regulatory environment is crucial for successful clinical trial design strategies. By prioritizing GCP adherence, ensuring that research teams are well-informed about regulatory changes, and emphasizing the selection of a principal investigator (PI), organizations can significantly enhance their study outcomes and contribute to the advancement of medical technologies.
Overcoming Enrollment Challenges in Clinical Trials
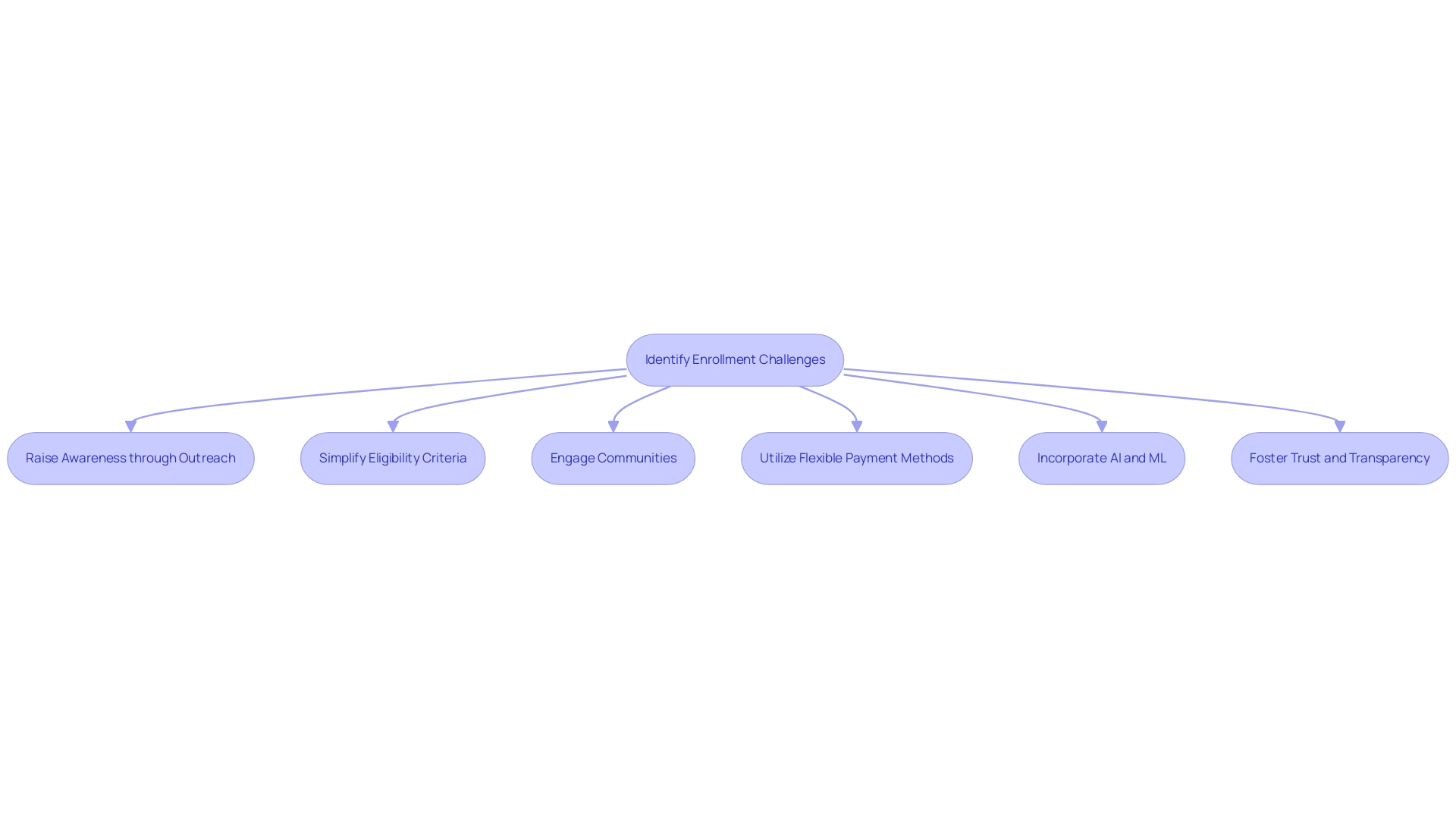
Enrollment challenges present substantial barriers to the timely advancement of clinical studies. Common obstacles include limited awareness of the study, stringent eligibility criteria, and logistical issues that complicate participation. To effectively address these challenges, researchers can implement targeted outreach campaigns designed to raise awareness about the study and its potential benefits. Simplifying eligibility criteria, when feasible, can also broaden the pool of potential participants, making studies more inclusive.
Community engagement strategies play a crucial role in connecting with underrepresented populations, which is essential for enhancing diversity in enrollment. For example, the World Health Organization and other regulatory agencies have highlighted the significance of including varied populations to guarantee that research outcomes are applicable across different demographics. In 2025, it is expected that incorporating real-world data and evidence will further enhance study design and patient recruitment, tackling the industry's persistent issues of high costs and extended timelines.
Statistics show that organizations utilizing adaptable payment methods can greatly improve research outcomes amidst increasing complexities. This flexibility is crucial as it allows for adjustments that can enhance participant recruitment and retention. Furthermore, the incorporation of artificial intelligence and machine learning technologies is anticipated to lower expenses by up to 20%, thus enabling more effective enrollment strategies.
Examples emphasize successful initiatives where community involvement has resulted in enhanced enrollment rates. For instance, the partnership between bioaccess™ and Caribbean Health Group intends to establish Barranquilla as a top destination for medical research in Latin America, backed by Colombia's Minister of Health. This initiative not only improves local healthcare capabilities through comprehensive services such as feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, but also aids in job creation and economic growth in the region.
Furthermore, a recent trend in medical studies has indicated that non-white individuals value interaction with the hospital or organization in charge of the research more than their white peers. This insight underscores the necessity of fostering trust and transparency in recruitment efforts. As Scott Mollan, Associate Director of Biostatistics, notes, a common rule of thumb is to take the 'optimal' (uniform) number of subjects per site and assume 3-5 times this value would be the maximum number of subjects allowed for a site. This perspective adds credibility to the discussion on enrollment strategies.
As we move through 2025, the focus on diversity and regulatory preparedness will be paramount. By utilizing community involvement and creative approaches, researchers can surmount enrollment obstacles, ultimately improving the efficiency of medical studies and accelerating the commercialization of health technologies through innovative clinical trial design strategies. The guidance issued by prominent regulatory organizations further highlights the significance of enrolling varied groups in research studies as a response to ongoing industry challenges.
Future Trends in Clinical Trial Design and Execution
As we approach 2025, several crucial trends in clinical trial design strategies are poised to transform study design and implementation. The emergence of decentralized clinical studies (DCTs) is leading the charge, offering participants unprecedented flexibility and accessibility by allowing them to engage in studies from the comfort of their homes. This shift not only enhances participant recruitment but also significantly improves retention rates, as individuals can partake in studies without the burden of travel.
Notably, the impact of DCTs on participant engagement statistics is becoming increasingly evident, as these studies adapt to the needs of participants. Moreover, the integration of artificial intelligence (AI) and machine learning is revolutionizing data analysis and patient recruitment processes. These technologies can reduce costs by up to 20%, streamlining operations and making the research process more adaptive and agile. AI-driven analytics are especially effective in tracking endpoints and managing patient engagement, ensuring that studies are not only efficient but also responsive to participant needs.
Alongside these technological innovations, there is an increasing focus on real-world evidence, which is anticipated to influence trial designs by concentrating on outcomes that reflect daily medical practice. This approach aligns with the industry's shift towards optimizing development journeys for assets, ultimately aiming for commercial success. As Max Baumann, Head of Execution at Treehill Partners, points out, "Going into 2025, we continue to see biotech facing fundamental business model challenges as end-markets become ever more crowded."
As the medical research landscape evolves, it is crucial for researchers to stay informed about these trends. The shift towards insourced information management models, for instance, allows sponsors to maintain better control over their information, enhancing operational efficiency and transparency. This trend demonstrates how the changing environment is affecting data management in medical studies.
By embracing these innovations, researchers can optimize their clinical trial design strategies to significantly enhance patient outcomes. At bioaccess, our extensive trial management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Nationalization of investigational devices
- Project oversight
- Reporting on research status, including serious and non-serious adverse events
By leveraging these capabilities, we not only facilitate effective study planning but also contribute to local economies through job creation and healthcare improvement. Our commitment to driving global health improvement through international collaboration and innovation in Medtech positions us as a key partner in navigating the complexities of clinical research in 2025.
Conclusion
The landscape of clinical trials is undergoing a transformative shift as organizations embrace innovative strategies to enhance trial design and execution. Key elements such as the precise definition of research questions, strategic planning, and the integration of technology are essential for navigating the complexities of clinical research. The importance of patient-centric approaches cannot be overstated; engaging patients effectively not only improves recruitment and retention rates but also leads to more meaningful outcomes.
Looking ahead to 2025, the emergence of decentralized clinical trials and advancements in artificial intelligence are set to redefine how trials are conducted. These trends enhance accessibility and flexibility for participants while streamlining operations, enabling researchers to adapt swiftly to changing conditions. Furthermore, the emphasis on regulatory compliance and real-world evidence underscores the necessity of aligning trial designs with the needs of diverse populations, thereby fostering broader applicability of results.
Organizations like bioaccess® play a pivotal role in this evolving landscape by providing comprehensive clinical trial management services that ensure adherence to regulatory standards while prioritizing patient engagement. By leveraging their expertise, the Medtech sector can navigate the challenges posed by an increasingly crowded market, ultimately facilitating the timely advancement of innovative medical devices.
As the clinical research field advances, embracing these trends and strategies will be vital for achieving successful trial outcomes. The commitment to patient-centricity, strategic planning, and technological integration will enhance the efficiency of clinical trials and contribute to the overall improvement of healthcare and the commercialization of medical technologies.
Frequently Asked Questions
What are the essential components of clinical trial design strategies?
The essential components include the precise definition of the research question, selection of appropriate endpoints, and careful determination of the participant population.
Why is a well-articulated hypothesis important in clinical trial design?
A well-articulated hypothesis guides the choice of study design, whether it involves randomized controlled experiments or observational studies.
What is the significance of defining appropriate outcomes in clinical trials?
Defining appropriate outcomes is critical for ensuring regulatory compliance, especially in specialized fields like psychedelic therapy, where meeting FDA scrutiny is paramount.
How do inclusion and exclusion criteria impact clinical trials?
Clarity in inclusion and exclusion criteria is essential for recruiting the right participants, which directly influences the validity and reliability of study results.
What recent trends are emerging in clinical trial design strategies?
There is a growing focus on adaptive study designs that allow modifications based on interim results, enhancing efficiency and responsiveness to emerging data.
How does bioaccess® contribute to clinical trial design?
Bioaccess® offers extensive research study management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, ensuring meticulous planning and execution.
What role does patient engagement play in clinical trial design?
Incorporating patient-centric approaches fosters active patient engagement, which includes soliciting input on study protocols and ensuring accessibility and clear communication regarding the study.
How can clinical trials improve patient recruitment and retention?
Studies that offer flexible participation options, such as telehealth visits, have demonstrated significant increases in both patient recruitment and retention rates.
What are some key types of trials managed by bioaccess®?
Bioaccess® manages various trial types, including Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF).
What is the impact of Medical Device Regulation (MDR) on clinical trial design?
The resurgence of MDR has necessitated a reevaluation of design and information management practices, emphasizing effective metadata management for automating study builds and streamlining data collection processes.

