Overview
The essential success factors for clinical trials in Latin America are pivotal to achieving success in this dynamic field. Understanding diverse regulatory frameworks, leveraging local healthcare structures, and fostering robust partnerships are crucial for enhancing patient recruitment and retention. Notably, Colombia presents competitive advantages, including cost efficiency and rapid regulatory approval. Tailored study designs that align with local practices further contribute significantly to the region's burgeoning prominence in global clinical research, establishing it as a key player in the Medtech landscape.
Introduction
In recent years, Latin America has emerged as a vibrant hub for clinical trials, driven by its diverse patient demographics, evolving regulatory frameworks, and strategic partnerships aimed at enhancing research capabilities. This evolution is exemplified by the collaboration between bioaccess™ and Caribbean Health Group, which positions Colombia as a key player in the global clinical research landscape.
With significant reductions in recruitment times and impressive retention rates, the region not only proves to be cost-effective but also offers high-quality healthcare services that attract international sponsors. As the demand for innovative medical solutions grows, it becomes essential for researchers and stakeholders alike to understand the nuances of conducting trials in this dynamic environment.
This article delves into the complexities and advantages of navigating the Latin American clinical trials landscape, highlighting the opportunities that lie ahead in this promising frontier of medical research.
Understanding the Latin American Clinical Trials Landscape
The region serves as a rich and dynamic setting for research studies, with success factors for Latin America trials shaped by diverse regulatory systems, patient populations, and healthcare structures. A notable advancement in this context is the collaboration between bioaccess™ and Caribbean Health Group, which aims to establish Barranquilla as a premier location for medical studies in Latin America, supported by Colombia's Minister of Health. This partnership not only enhances the region's appeal but also exemplifies the success factors for Latin America trials, highlighting Colombia's competitive advantages for initial human studies, including cost efficiency, regulatory speed, and high-quality healthcare.
With a remarkable reduction in recruitment time—over 50%—achieved through partnerships such as that of GlobalCare Clinical Trials with bioaccess™, alongside impressive retention rates of 95%, the success factors for Latin America trials position Colombia as an optimal location for research involving human subjects. Moreover, the streamlined process for obtaining research study approvals delineates several key steps as success factors for Latin America trials, including:
- IRB/EC approval
- INVIMA approval
- MinCIT import permits
These factors facilitate timely project initiation. As the area continues to evolve, the demographic complexity of this region presents unique opportunities for generating broadly applicable outcomes in global health contexts, which are vital success factors for Latin America trials.
Significantly, in 2023, the region constituted 2.1% of the global clinical research market, with projections indicating substantial growth potential, particularly in Colombia. Here, the success factors for Latin America trials encompass compliance with international standards and attractive R&D tax incentives—including:
- a 100% tax deduction for investments in science, technology, and innovation projects
- a 25% tax discount
- approximately $10 million in free government grants
For those interested in engaging with this evolving landscape, users can submit their research requirements through a contact form.
![]()
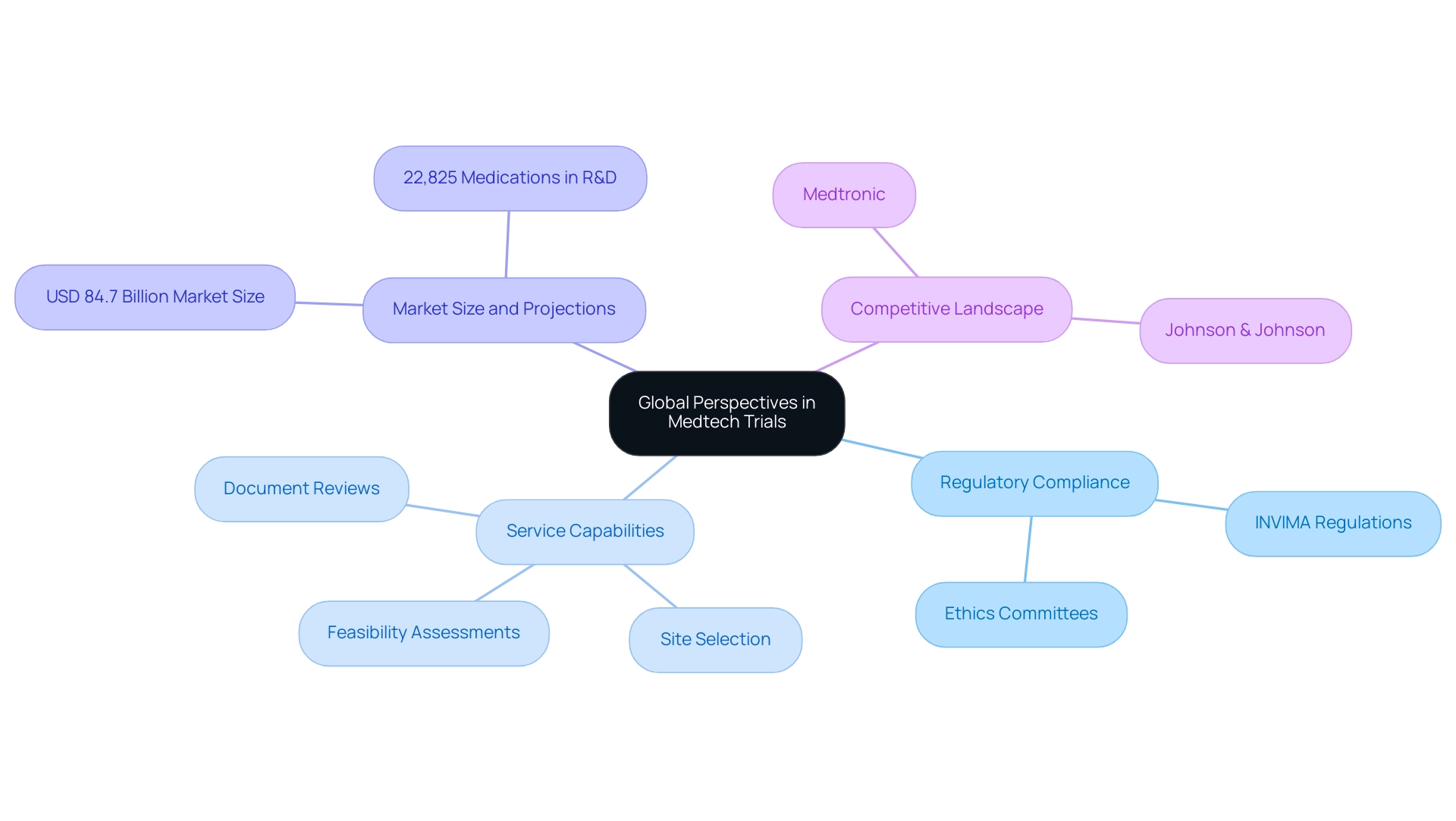
The Need for Global Perspectives in Medtech Trials
Conducting Medtech studies in Latin America necessitates a comprehensive global perspective that recognizes the unique success factors pertinent to trials in this region. It is imperative to customize research designs to align with local healthcare practices and patient expectations while ensuring strict compliance with regulatory frameworks, such as those established by INVIMA, the Colombia National Food and Drug Surveillance Institute, acknowledged as a Level 4 health authority by PAHO/WHO. Our service capabilities encompass:
- Feasibility assessments
- The selection of appropriate research sites and principal investigators (PIs)
- Thorough reviews and feedback on study documents to guarantee adherence to country-specific regulations
Additionally, we facilitate seamless setup and approval processes involving ethics committees and health ministries.
Furthermore, we assist with the import permit procedure and nationalization of investigational devices, which are critical for establishing studies in the region. As highlighted by a spokesperson from Johnson & Johnson, 'Two other medtech firms that complete the top three largest corporations – Medtronic and Johnson & Johnson – are both located in the United States,' illustrating the competitive landscape that influences study designs. By integrating insights from international methodologies, researchers can significantly enhance the relevance of their findings, ensuring that innovations effectively serve diverse populations.
The projected market size for Medtech in 2024, estimated at USD 84.7 billion, underscores the urgency of this approach. Additionally, with approximately 22,825 medications anticipated to be in the R&D pipeline worldwide by that same year, focusing on refining study designs not only aims to improve patient outcomes but also emphasizes the success factors for Latin America trials by fostering increased trust and collaboration among stakeholders. Such strategic adaptations, including robust project management and detailed reporting on study status and adverse events, are essential for navigating the complexities of the regional healthcare landscape while optimizing the impact of research initiatives and contributing to local economies through job creation and healthcare enhancements.
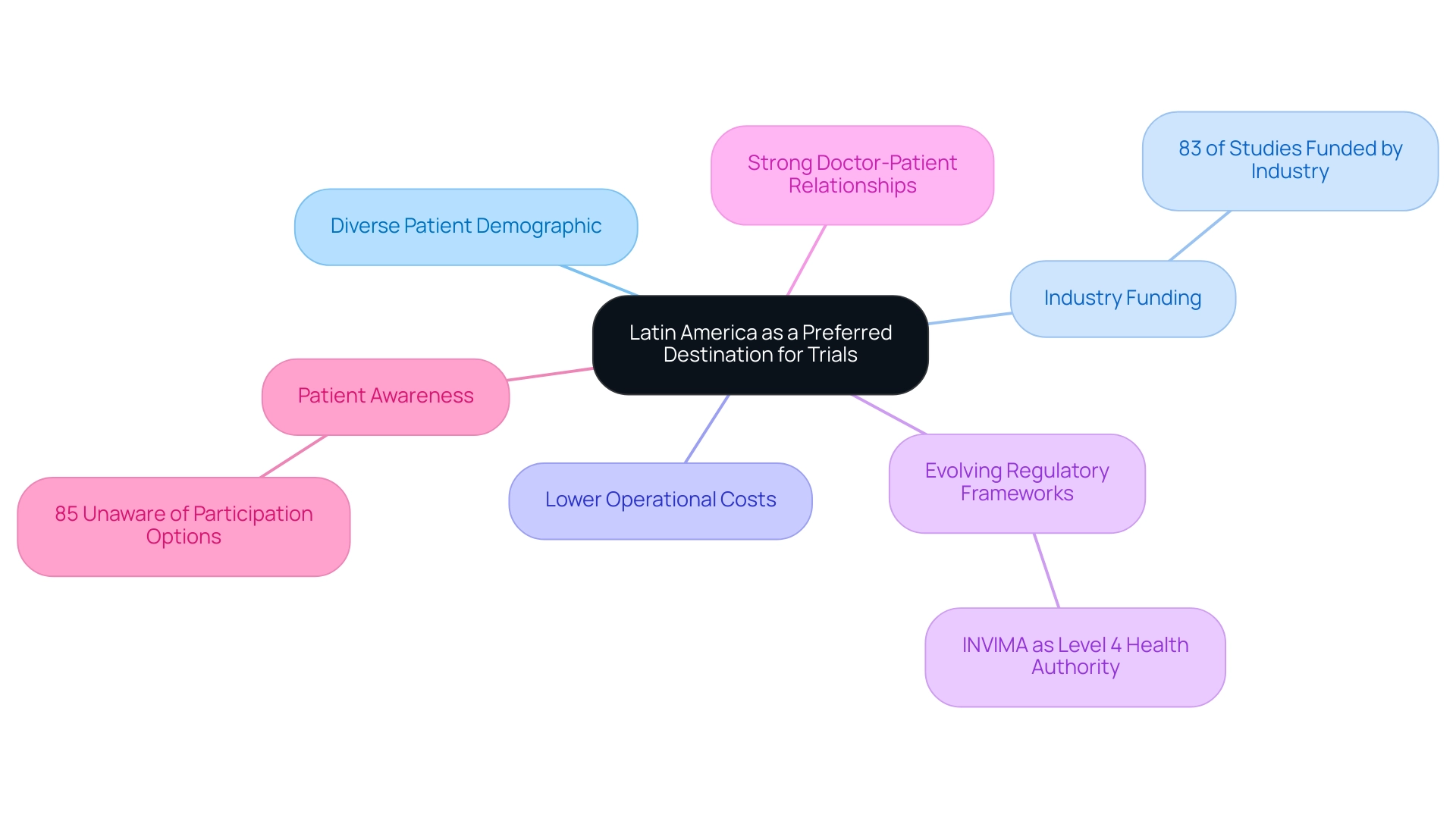
Why Latin America is Becoming a Preferred Destination for Trials
The region is increasingly recognized as a compelling location for research studies, shaped by a variety of success factors influencing Latin America trials. It boasts a substantial and diverse patient demographic, which is critical for the recruitment necessary for successful studies. Notably, an average of 83% of studies conducted in the region and the Caribbean receive funding from industry, underscoring the growing significance of this area in the research landscape.
Moreover, the operational expenses associated with conducting clinical studies in Latin America are significantly lower than those in North America and Europe, making it a cost-effective choice for sponsors. The evolving regulatory frameworks, particularly those established by INVIMA, facilitate expedited approval processes and enhance the region's attractiveness, as INVIMA is recognized as a Level 4 health authority by PAHO/WHO, overseeing the regulation of medical devices. Additionally, strong doctor-patient relationships, coupled with cultural diversity, not only support effective patient recruitment but also bolster retention rates throughout the study process.
However, awareness of research studies remains limited in the region, with 85% of patients unaware of their participation options at diagnosis. This gap presents a substantial opportunity for improved enrollment through targeted patient education. Collectively, these factors position the region as an optimal site for conducting efficient and impactful studies in 2024 and beyond, highlighting the success factors for Latin America trials. This is further supported by the comprehensive management services offered by bioaccess®, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
With over 20 years of experience in Medtech, bioaccess® is committed to ensuring successful outcomes for all stakeholders involved. By leveraging its expertise in feasibility studies, site selection, compliance reviews, and project management, bioaccess® contributes significantly to job creation and economic growth within local communities.
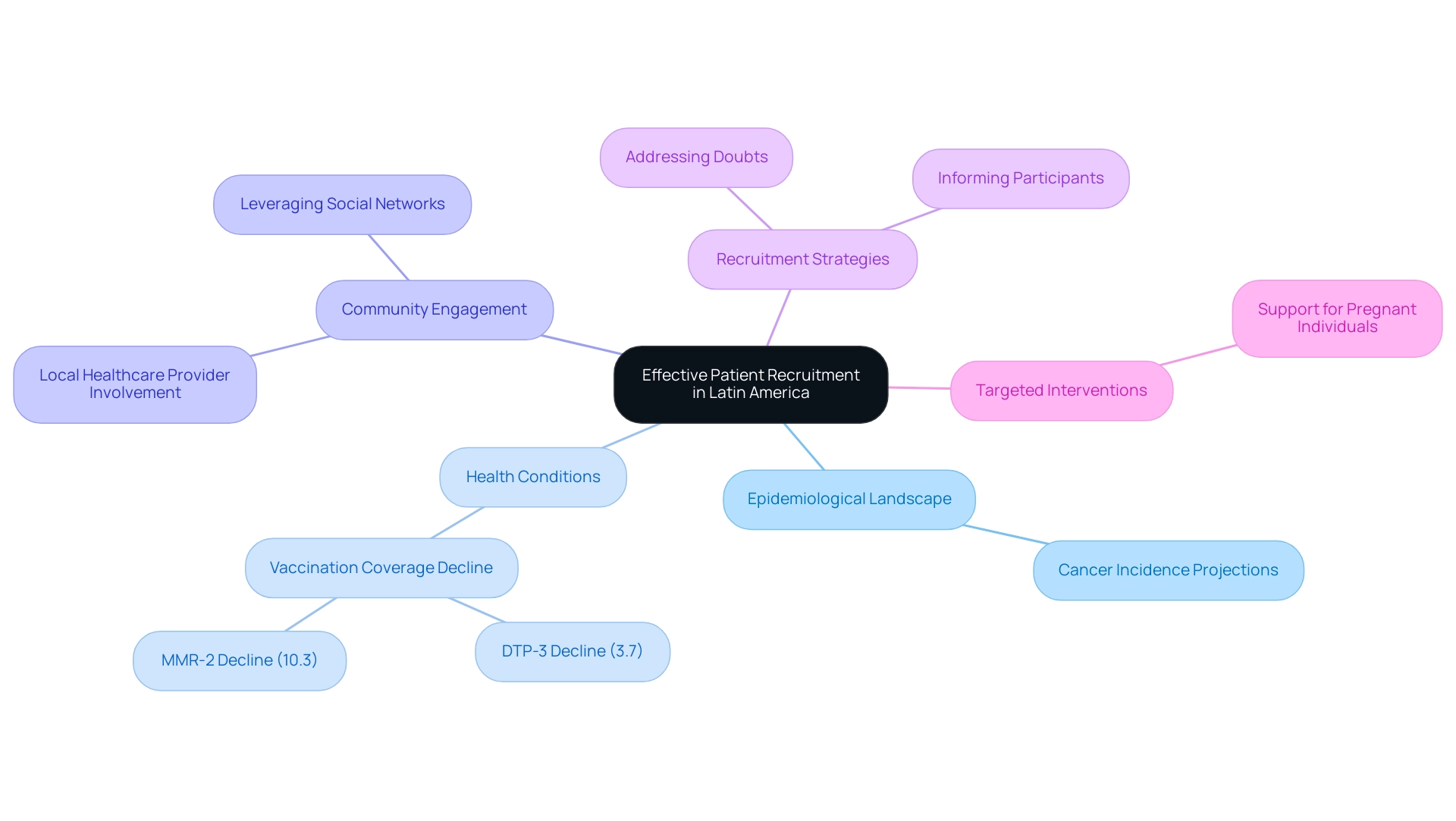
Effective Patient Recruitment and Understanding Epidemiology
Effective patient recruitment in Latin America necessitates a comprehensive understanding of the local epidemiological landscape, which serves as a critical success factor for clinical trials in the region. As the Medtech sector navigates unique challenges and opportunities for market access, it is essential to recognize the projections indicating a rise in cancer incidence across various subregions by 2040. Researchers must identify prevalent health conditions and tailor their recruitment strategies accordingly. Notably, routine childhood vaccination coverage has declined by 3.7% for DTP-3 and 10.3% for MMR-2 since 2020, underscoring the shifting health landscape that can significantly impact recruitment efforts.
Community-based approaches are pivotal; engaging local healthcare providers and leveraging social networks can substantially enhance recruitment initiatives. Moreover, informing prospective participants about the advantages and processes associated with research studies is crucial for alleviating doubt and improving enrollment rates. For instance, during the pandemic, pregnant individuals faced increased risks, necessitating targeted interventions to support this vulnerable group.
As emphasized by research expert Jennifer Mendoza, "Due to varying update cycles, statistics can display more up-to-date data than referenced in the text," which highlights the necessity for researchers to remain informed about dynamic health trends that directly influence recruitment strategies. By aligning recruitment efforts with relevant epidemiological data, researchers not only augment the relevance of their studies but also contribute to more impactful health outcomes tailored to the populations they serve. This alignment is a key success factor for clinical trials in Latin America, ultimately fostering economic growth and healthcare improvement in the region. Furthermore, employing comprehensive research management services—including study setup, project oversight, and compliance evaluations—ensures that investigations adhere to local regulations and ethical standards, thereby promoting successful patient recruitment and involvement.
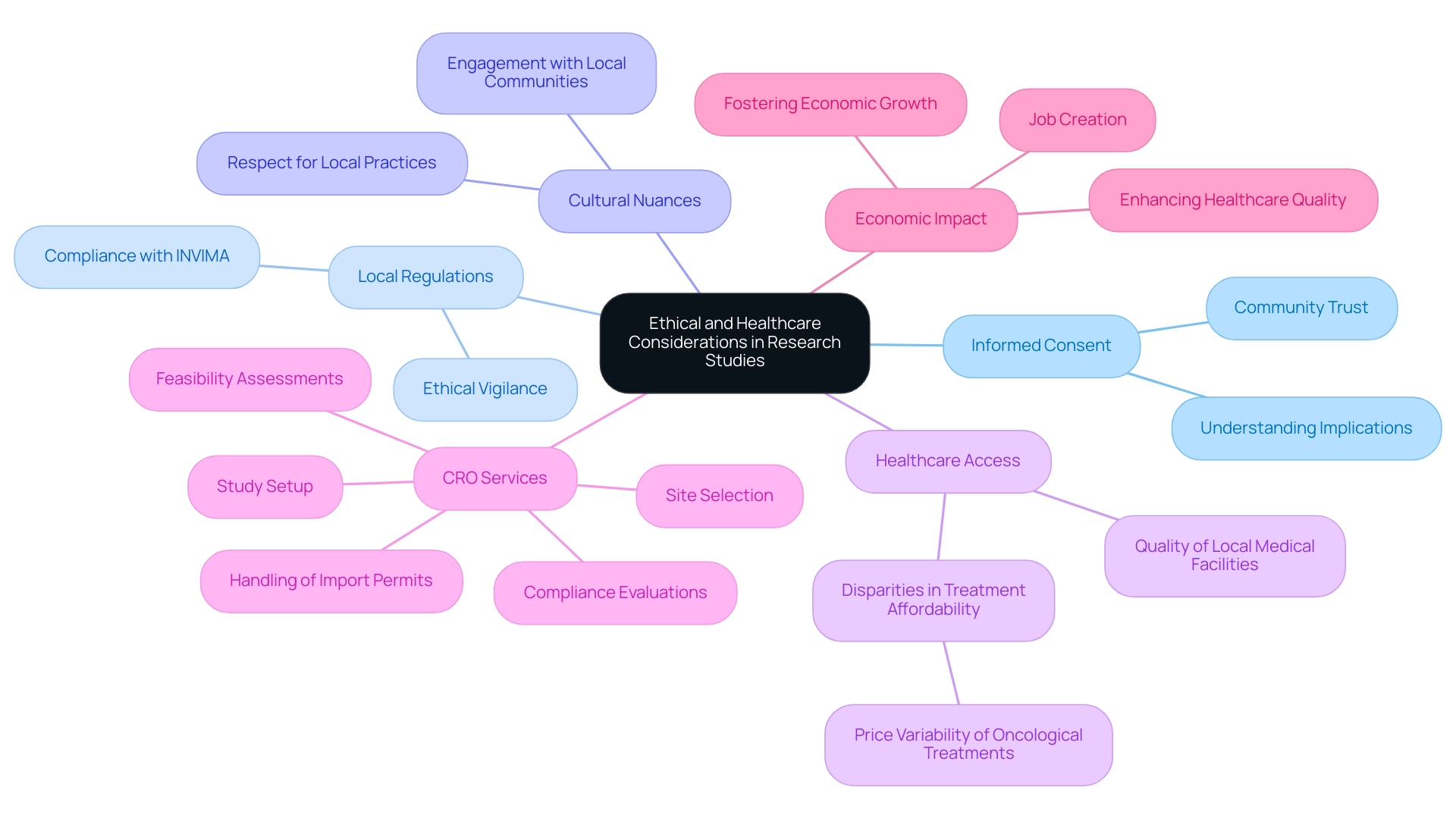
Navigating Ethical and Healthcare Considerations
Navigating the ethical landscape of research studies in Latin America necessitates a comprehensive grasp of the success factors for Latin America trials, including local regulations and cultural nuances. A cornerstone of this process is informed consent, requiring researchers to ensure that participants fully understand the implications of their involvement in clinical studies. Engaging with local ethics committees and healthcare providers is essential, as it not only facilitates smoother implementation but also fosters community trust—key success factors for Latin America trials.
Moreover, addressing healthcare considerations, such as access to care and the quality of local medical facilities, remains critical for conducting studies ethically and effectively. As your trusted CRO, bioaccess® provides extensive management services for research studies that encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Handling of import permits
This ensures adherence to local regulations such as those supervised by INVIMA, the Colombia National Food and Drug Surveillance Institute. Notably, disparities in treatment affordability, as highlighted by the case study 'Price Variability of Oncological Treatments in Latin America,' reveal significant discrepancies in treatment costs, with Argentina often having the highest prices.
These disparities underscore the significance of incorporating ethical factors into study design, as success factors for Latin America trials involve prioritizing the rights and welfare of participants while also considering the broader societal impact of research. Additionally, the key point that judges often base decisions on individual patient needs rather than societal health priorities presents potential risks to healthcare system sustainability, further emphasizing the need for ethical vigilance. As indicated in documents from the USA, serious adverse events must be considered when assessing the ethical implications of medical studies, reinforcing the necessity of informed consent practices.
Furthermore, Medtech research studies contribute significantly to local economies by creating jobs, fostering economic growth, and enhancing healthcare quality. This reality emphasizes the importance of international collaboration in advancing medical research.
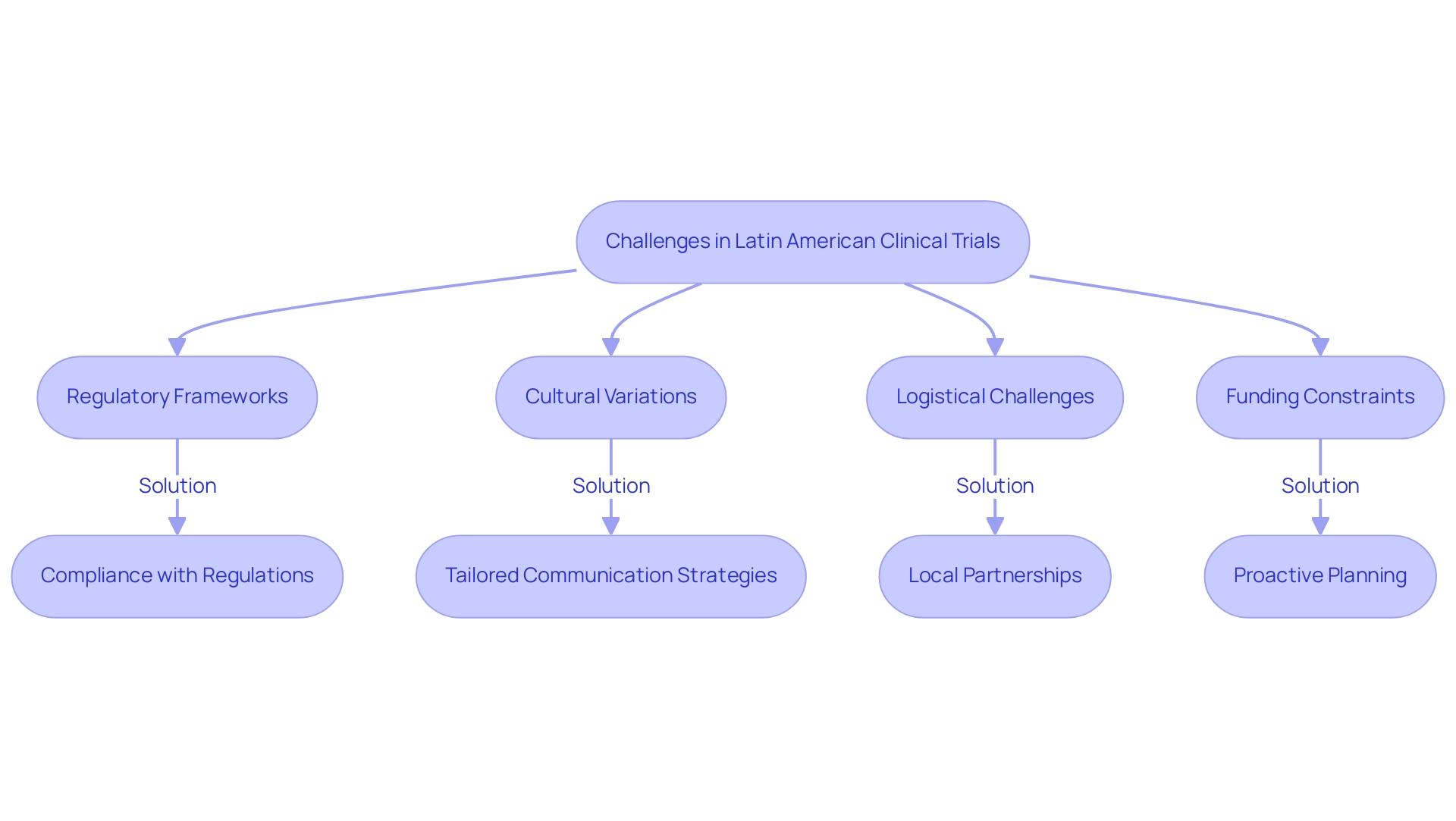
Overcoming Challenges in Latin American Clinical Trials
Carrying out research studies in Latin America necessitates a thorough understanding of the success factors pertinent to trials in this region, which encompass navigating intricate regulatory frameworks, cultural variations, and logistical challenges. With an impressive 83% of studies in the region sponsored by the industry, the pressure on sponsors to adeptly manage these complexities is significantly heightened. Notably, only 2% of studies receive financial backing from the National Institutes of Health and other US Federal Agencies, underscoring the constrained funding environment and its implications for research efforts in the area.
Our comprehensive research study management services—encompassing feasibility assessments, site selection, compliance evaluations, study setup, import permits, and project oversight—are meticulously designed to address these challenges. Researchers must skillfully navigate the diverse regulatory frameworks across countries, which can lead to delays in study initiation. For instance, GlobalData reveals that a mere 1% of worldwide medical device clinical studies are conducted in the southern continent, accentuating the urgent need for enhanced regulatory alignment.
Cultural nuances play a pivotal role in patient engagement and retention, necessitating tailored communication strategies to cultivate trust and participation. To effectively tackle these challenges, the establishment of robust local partnerships emerges as a crucial strategy, complemented by our support in training research personnel to bolster study efficiency and effectiveness. Furthermore, the recent case study titled 'Investment Growth in Clinical Trials in South America' illustrates that investment in this sector has surged from $3-4 million to over $50 million annually in the Andean Region, despite the hurdles posed by linguistic, cultural, and socio-economic factors.
Proactive planning, adaptability, and a keen understanding of the local context are recognized as essential success factors for clinical trials in Latin America, crucial for effectively managing the obstacles that arise in this region, particularly as it anticipates continued growth and heightened investment in clinical research. Moreover, there exists a pressing need for improved funding and support for researchers to enhance cancer research and treatment outcomes. Specifically, our testing setup process emphasizes strict compliance with country requirements, including the nationalization of investigational devices, which is vital for securing regulatory approval.
Additionally, our reporting services ensure that study status, inventory management, and adverse event reporting are meticulously handled, facilitating successful management.
Implementing Best Practices for Successful Trials
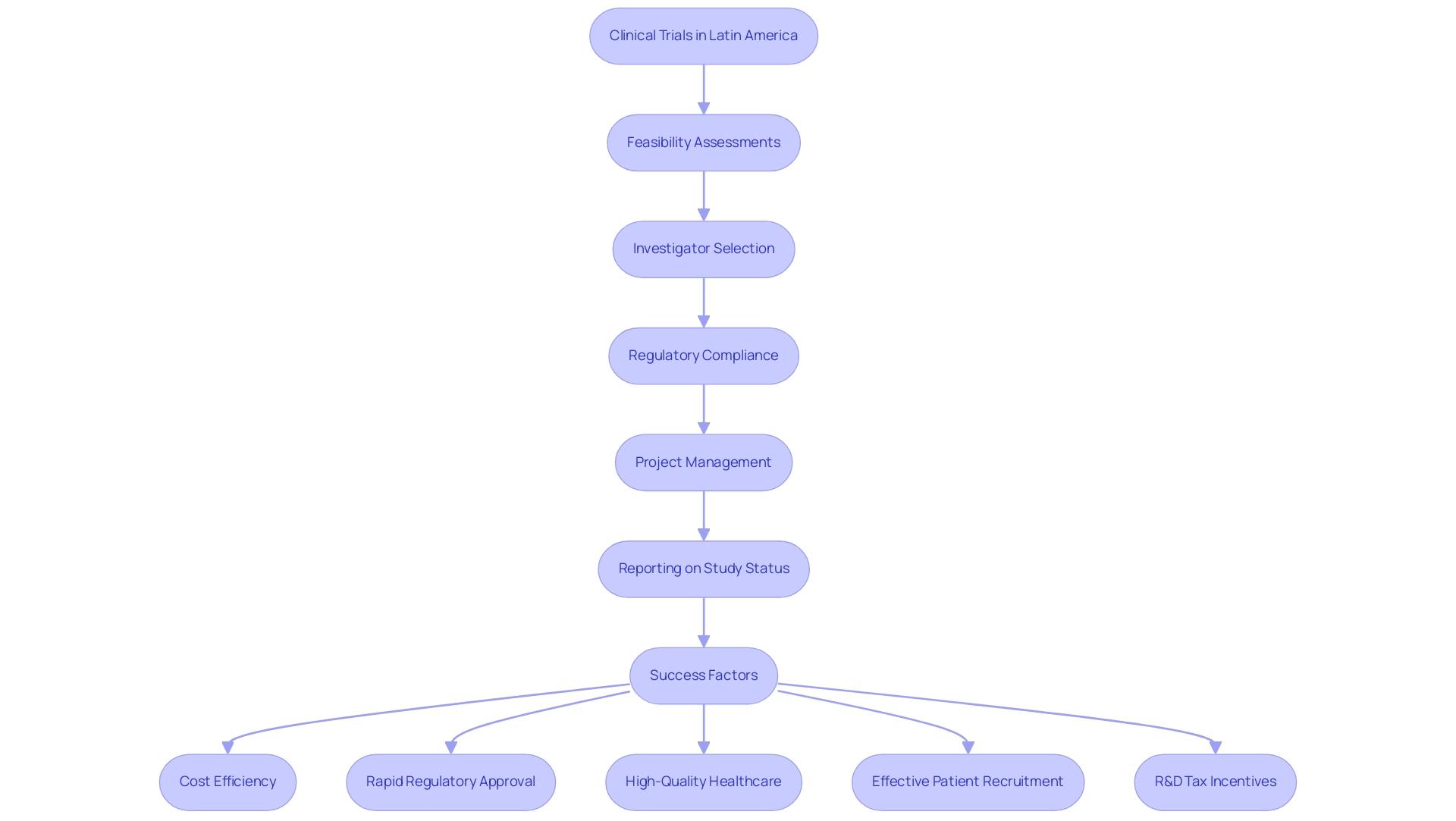
The success factors for clinical trials in Latin America hinge on the implementation of best practices that foster cooperation among all stakeholders, including sponsors, regulatory authorities, and research organizations. Particularly in Colombia, where the healthcare system ranks 22 out of 191 nations according to the World Health Organization, the region is increasingly positioned to enhance its research capabilities in health. The comprehensive process for advancing medical device studies encompasses critical steps such as:
- Feasibility assessments
- Investigator selection
- Regulatory compliance
- Project management
- Reporting on study status, inventory, and both serious and non-serious adverse events
Colombia offers competitive advantages for first-in-human clinical studies, including:
- Cost efficiency
- Rapid regulatory approval
- High-quality healthcare
- Effective patient recruitment
- R&D tax incentives
Clear communication is paramount; as Periyakoil observes, 'The sponsors did not contribute to the design, methods, analysis, or preparation of this manuscript,' underscoring the need for meaningful sponsor engagement throughout study processes. Furthermore, aligning local regulations with international standards, such as ICH E6 and E8, significantly enhances the quality and efficiency of studies.
The assessment underscores that regular updates to local regulations are vital for LATAM nations to refine their research frameworks and expedite patient access to new medications. Influential figures such as Julio Martinez-Clark, CEO of bioaccess, advocate for the implementation of research studies while guiding new businesses and promoting local opportunities. Additionally, Monica Mora, Chief Operating Officer, specializes in operations, logistics, and regulatory strategies for medical device companies in Latin America, ensuring that processes remain both efficient and compliant.
Her expertise encompasses streamlining processes to facilitate regulatory approvals and optimizing patient recruitment strategies. The nation has witnessed a marked increase in medical device evaluations, highlighting its growing role in medical research. By embracing technology in clinical study management, organizations can streamline data handling and enhance patient engagement—key components for success in today’s research landscape.
Continuous training and education for research staff on the latest methodologies and ethical standards are essential. By adopting a proactive, collaborative approach, researchers can significantly improve trial outcomes, which are among the success factors for trials in Latin America, leading to more efficient pathways for patient access to innovative treatments.
BOOK A MEETING.
Conclusion
Latin America is swiftly establishing itself as a premier destination for clinical trials, marked by its diverse patient demographics, evolving regulatory environments, and strategic partnerships that enhance research capabilities. The collaboration between bioaccess™ and Caribbean Health Group exemplifies this growth, positioning Colombia as a pivotal player in the global clinical research landscape. With significant reductions in recruitment times and impressive retention rates, the region not only offers cost-effective solutions but also high-quality healthcare services, attracting international sponsors eager to explore its potential.
Understanding the unique complexities of conducting trials in Latin America is essential for researchers and stakeholders. By tailoring trial designs to local healthcare practices and engaging effectively with patients, researchers can enhance recruitment and retention rates while ensuring compliance with regulatory frameworks. The region's cultural diversity and strong doctor-patient relationships further bolster its appeal, creating opportunities for innovative medical solutions that cater to a broad spectrum of populations.
As the demand for clinical research continues to grow, embracing best practices and fostering collaboration among stakeholders will be crucial. By navigating the regulatory landscape and addressing ethical considerations, researchers can optimize trial outcomes and contribute to local economies through job creation and improved healthcare. The future of clinical trials in Latin America is promising, and those who seize the opportunities presented by this dynamic environment will play a vital role in advancing medical research and delivering innovative solutions to global health challenges.




