Overview
Latin American CROs present significant advantages in clinical research, notably in cost-effectiveness, regulatory expertise, and access to diverse patient populations. These factors collectively enhance trial efficiency and data quality. Such strengths, exemplified by organizations like bioaccess®, position the region as an appealing destination for sponsors aiming to optimize their research strategies. This is particularly relevant for those looking to reduce costs and expedite processes, making collaboration with Latin American CROs a strategic choice in the evolving Medtech landscape.
Introduction
In the evolving landscape of clinical research, Latin American Contract Research Organizations (CROs) are emerging as vital players, particularly through the innovative contributions of bioaccess®. With a unique combination of cost-effectiveness, regulatory expertise, and access to diverse patient populations, these organizations are redefining the way clinical trials are conducted. This article delves into the competitive edge offered by Latin American CROs, highlighting their ability to:
- Accelerate timelines
- Enhance recruitment strategies
- Leverage advancements in infrastructure and technology
As the demand for efficient clinical trials grows, understanding the distinct advantages of partnering with Latin American CROs becomes essential for Medtech companies aiming to thrive in a global market.
Understanding the Competitive Edge of Latin American CROs
Latin American Contract Research Organizations (CROs) have established themselves as vital contributors to the global trials landscape, with bioaccess® leading the charge. The competitive advantages of partnering with bioaccess® underscore the strengths of Latin American CROs, including:
- Cost-effectiveness
- Regulatory expertise
- Access to a diverse patient population
Notably, in Brazil and Argentina, demographic stability is impressive, with a significant portion of the population over 65 years old benefiting from pension systems—93% and 85%, respectively—creating a reliable participant base for research studies.
This stability, coupled with low poverty rates (below 7% in Brazil and 3% in Argentina), provides a robust foundation for study recruitment. With over 15 years of experience in the Medtech sector, bioaccess® plays a pivotal role in advancing medical devices through its specialized expertise and tailored approach for Medtech startups. They offer essential services such as:
- Regulatory approval
- Site activation
- Participant recruitment
- Trial data management
These services enable clients to effectively navigate the complexities of trials. The region's commitment to enhancing research infrastructure has fostered advancements in technology and methodologies.
Recent developments, such as Uruguay's implementation of fiscal rules for economic stability, further bolster the growth of the CRO sector, highlighting the necessity of a solid fiscal environment for research involving patients. While progress is evident in countries like Nicaragua and Guatemala, challenges persist concerning pension system coverage and poverty alleviation for the elderly. Nonetheless, these elements collectively improve trial efficiency and enhance data quality, showcasing the advantages of Latin American CROs.
As South American CROs, particularly bioaccess®, continue to innovate and adapt, they exemplify the benefits of Latin American CROs for sponsors seeking to refine their trial strategies in 2025 and beyond. Reach out to discover how we can accelerate your research initiatives in South America.
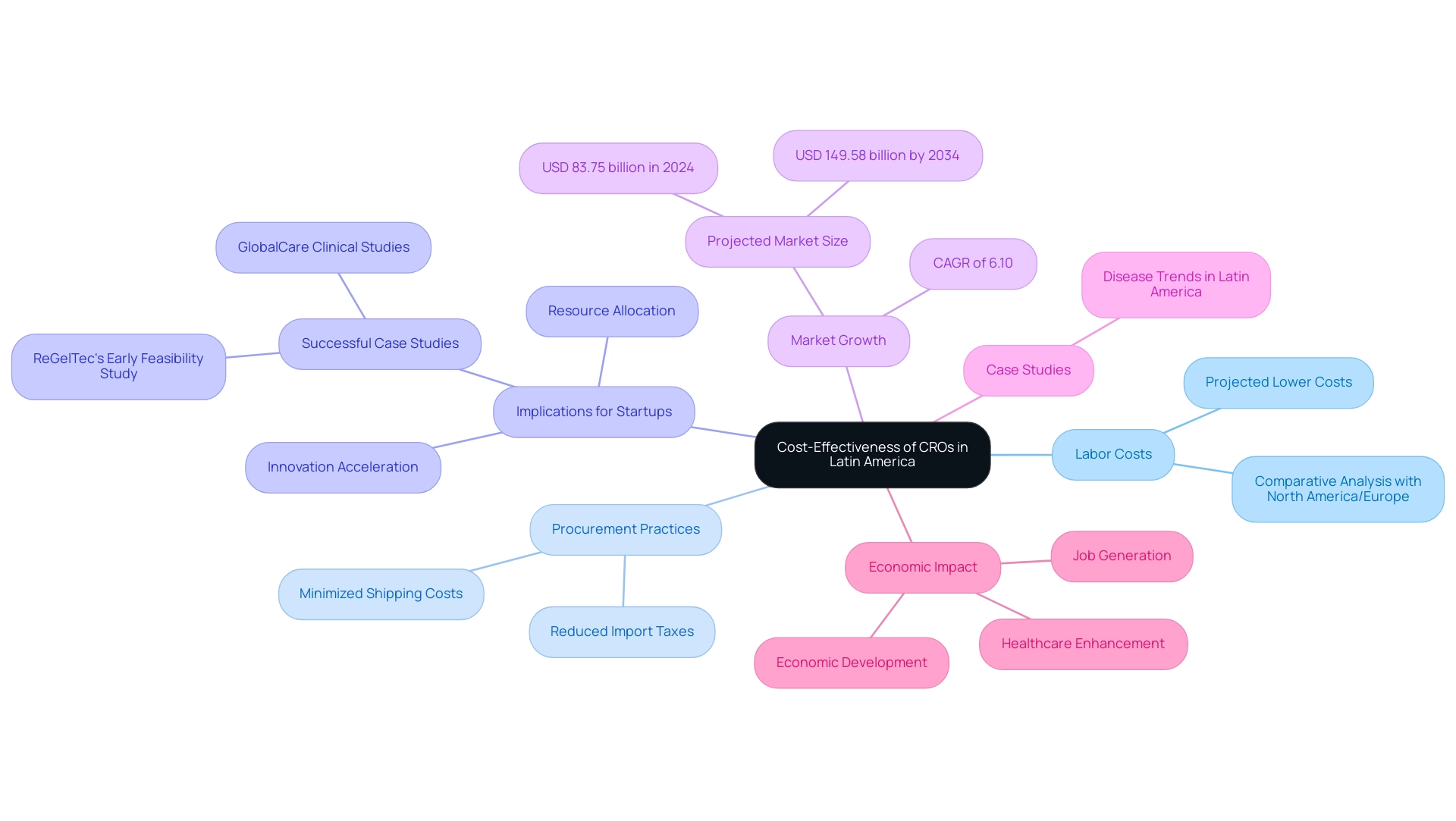
Cost-Effectiveness: A Major Advantage for CROs in Latin America
One of the most compelling advantages of Latin American CROs is their remarkable cost-effectiveness in conducting trials across South America. In 2025, labor costs in the region are projected to be significantly lower than those in North America and Europe, enabling sponsors to execute studies at a fraction of typical expenses. This financial advantage is further amplified by local procurement practices, which minimize shipping costs and reduce import taxes, thereby enhancing the overall viability of trials.
The implications of these savings are particularly beneficial for startups in the Medtech sector. By leveraging the cost-effective landscape of South American CROs, companies can allocate their resources more efficiently, accelerating the development of innovative medical technologies. For instance, ReGelTec's Early Feasibility Study in Colombia successfully treated eleven patients with chronic low back pain using HYDRAFIL™, showcasing how the partnership between bioaccess™ and Caribbean Health Group positions Barranquilla as a premier destination for research studies in South America, supported by Colombia's Minister of Health.
Recent studies suggest that the global research studies market, valued at USD 83.75 billion in 2024, is expected to reach USD 149.58 billion by 2034, growing at a CAGR of 6.10%. This growth underscores the rising acknowledgment of South America as a feasible choice for medical research, particularly due to the advantages of Latin American CROs for firms aiming to enhance their budgets without sacrificing quality. The market's expansion highlights the strategic significance of South America, as Medtech firms can capitalize on the benefits of Latin American CROs while contributing to the overall development of the research sector.
Case studies further illustrate this trend. For example, GlobalCare Clinical Studies has collaborated with bioaccess™ to enhance ambulatory services for studies in Colombia, achieving over a 50% decrease in recruitment time and 95% retention rates. Additionally, startups focusing on conditions such as cancer and diabetes have successfully leveraged the diverse disease landscape in South America.
These examples demonstrate how the distinctive opportunities offered by the region can lead to substantial financial benefits for Medtech startups, emphasizing the advantages of Latin American CROs.
bioaccess® provides extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, ensuring a streamlined process for sponsors. The economic impact of Medtech research studies on regional economies is also significant, as these evaluations contribute to job generation, economic development, and healthcare enhancement.
In summary, the cost-efficiency of research studies in South America not only supports financial viability for Medtech firms but also fosters a favorable environment for the rapid advancement of medical devices and technologies.
Regulatory Expertise: Navigating Compliance with Ease
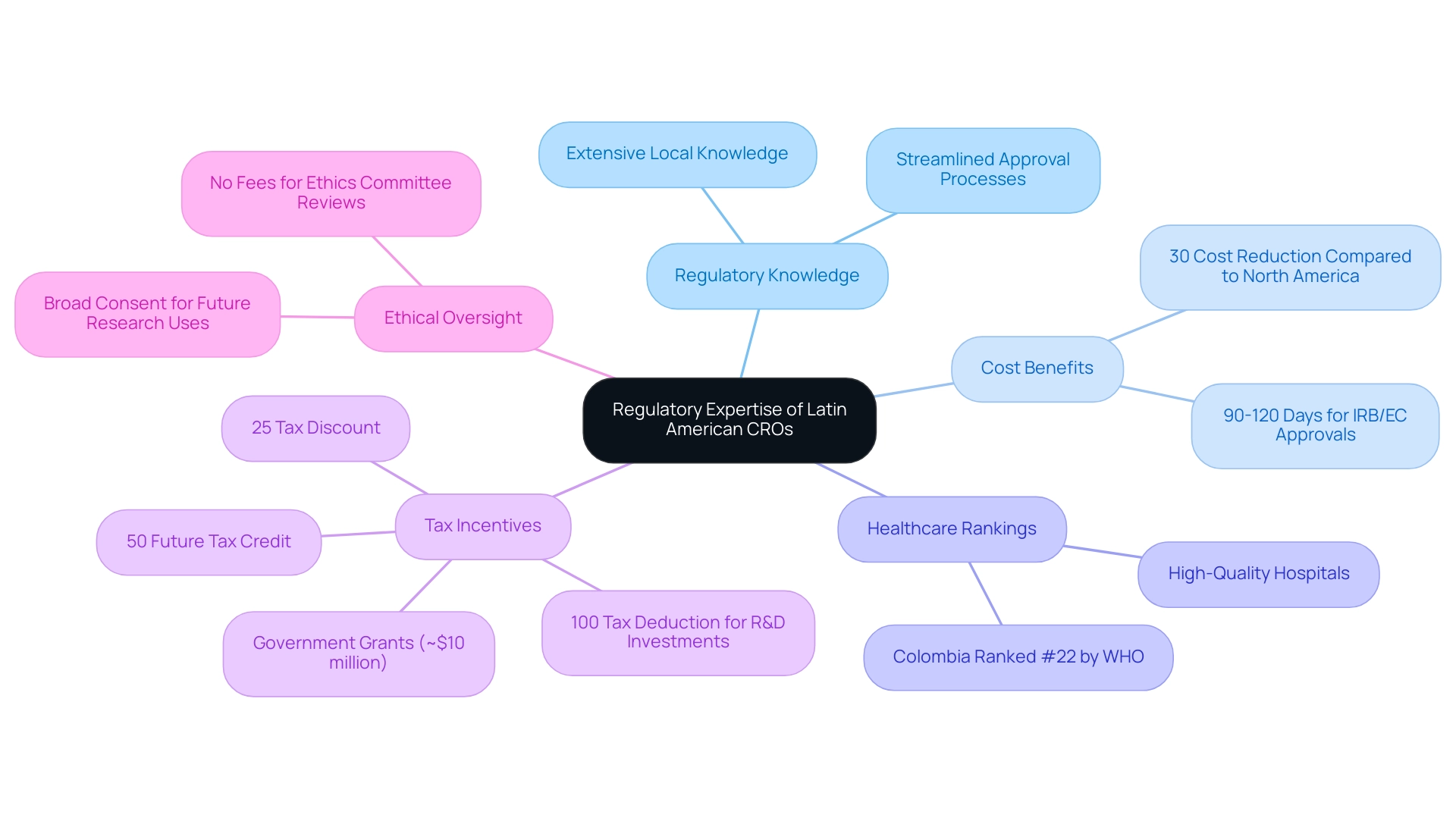
The advantages of Latin American CROs are underscored by their extensive regulatory knowledge, which is crucial for navigating the complex compliance environment in clinical trials. Their profound understanding of local regulations enables them to skillfully manage the approval procedures essential for research studies, significantly reducing the likelihood of setbacks. This expertise not only guarantees adherence to international standards but also instills confidence among sponsors and stakeholders, who can rely on a streamlined process that enhances the chances of successful study outcomes.
Recent regulatory reforms across various Latin American countries, particularly in Colombia, have further streamlined these processes, showcasing the benefits of Latin American CROs and rendering the region increasingly attractive for clinical research. As of 2025, Colombia offers substantial cost reductions of over 30% compared to studies in North America and Western Europe, with IRB/EC and INVIMA review times averaging just 90 to 120 days. The IRB/EC approval process entails a thorough evaluation of the ethical aspects of the study, while INVIMA oversees the safety and efficacy of the medical devices under investigation.
Furthermore, the World Health Organization ranks Colombia's healthcare system at #22 worldwide, highlighting the advantages of Latin American CROs, as its hospitals are recognized as some of the finest in South America.
In addition, the prohibition of charges for ethics committee evaluations ensures that financial barriers do not impede ethical oversight, fostering a conducive environment for inquiry. Julio G. Martinez-Clark, CEO of bioaccess, emphasizes that Colombia is actively pursuing initiatives to attract more trials, positioning itself as a knowledge economy by 2031. This proactive approach reflects a broader trend in the region, where regulatory expertise is becoming a cornerstone for CROs, further demonstrating the advantages of Latin American CROs in effectively navigating compliance challenges and enhancing the overall efficiency of clinical trials.
Moreover, with a population exceeding 50 million and nearly universal healthcare coverage, patient recruitment is facilitated, alongside generous R&D tax incentives that encompass:
- A 100% tax deduction for investments in science, technology, and innovation projects
- A 25% tax discount
- A 50% future tax credit
- Substantial government grants of approximately $10 million
The updated Common Rule permits extensive consent for the preservation and future utilization of identifiable private information and biospecimens, simplifying the study process while honoring participant rights, ultimately showcasing the adaptability and responsiveness of the regulatory environment in South America.
Access to Diverse Patient Populations for Enhanced Recruitment
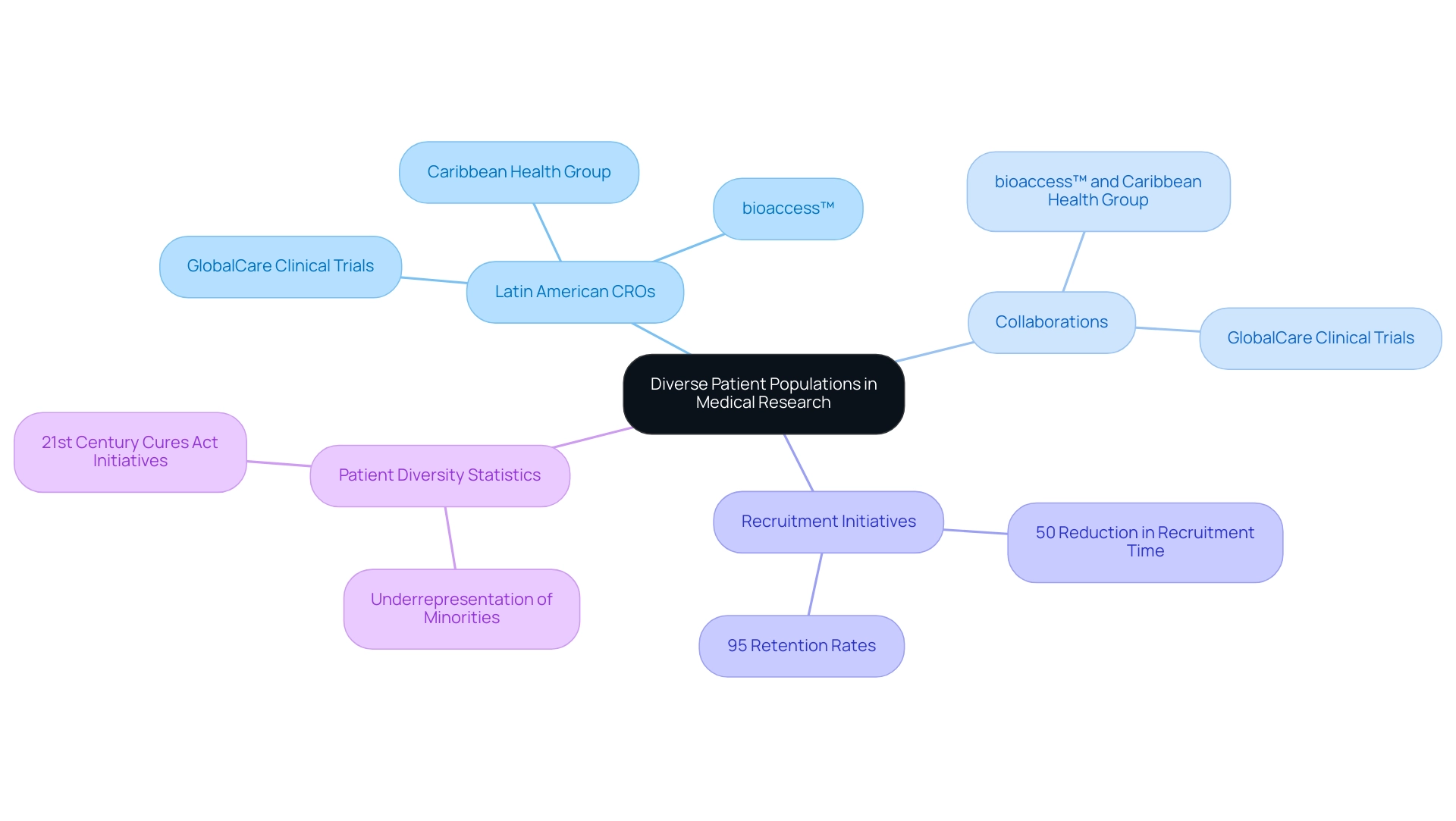
The region of America showcases a rich tapestry of ethnic and cultural diversity, illustrating the distinct advantages of Latin American CROs in providing unparalleled access to varied patient populations. This diversity significantly enhances recruitment initiatives, allowing for quicker enrollment and improved retention rates in research studies. The partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, at PROCOLOMBIA's office in Miami, exemplifies this potential, positioning Barranquilla as a key location for medical studies in Latin America, supported by Colombia's Minister of Health.
Moreover, GlobalCare Clinical Trials' collaboration with bioaccess™ has achieved over a 50% reduction in recruitment time alongside impressive 95% retention rates, further demonstrating the effectiveness of Latin American CROs. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, highlights that his experience with bioaccess® during its initial human study in Colombia underscores the potential of these collaborations to enhance medical study outcomes. Additionally, Dr. John B. Simpson's research in Cali, Colombia, emphasizes the advantages of Latin American CROs and the critical importance of collaborating with local experts to advance this goal.
As we look towards 2025, demographic diversity statistics reveal that racial and ethnic minority groups remain underrepresented in research studies, particularly among Black and Hispanic patients, underscoring the vital role of American CROs. The 21st Century Cures Act has ignited initiatives aimed at addressing these disparities, particularly emphasizing the necessity for diverse patient groups in medical studies. Mirella Nardo from ICESP notes, 'Despite its large population, the disproportionately low availability of early-phase studies in Latin America highlights the urgent need for enhanced investigation efforts.'
By engaging with the region's diverse patient groups, CROs can leverage the strengths of Latin American CROs to refine recruitment strategies and contribute to a more inclusive trial landscape. Furthermore, the NIH SGM research working group aims to deepen understanding of sexual and gender minority health, reinforcing ongoing efforts to enhance diversity and inclusion in medical research.
Accelerated Timelines: Speeding Up Clinical Trials
CROs from South America, particularly bioaccess®, have established a formidable reputation for their ability to conduct studies with remarkable speed and comprehensive management services for research. This efficiency is a result of lower regulatory hurdles, effective patient recruitment strategies, and streamlined operational processes, all contributing to expedited study initiation and completion. For example, recent legislative reforms in Brazil have significantly reduced approval times, allowing proceedings to commence in as little as 60 days.
This swift turnaround is vital for companies aiming to maintain a competitive edge in the rapidly evolving Medtech landscape.
The data speaks volumes: participation in studies across South America surged from 6.04% in 2015 to 8.54% in 2021, reflecting an increasing recognition of the region’s potential for medical research. Furthermore, bioaccess® exemplifies this trend by advancing medical devices through its cost-effective, high-quality services, specializing in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With over 20 years of experience, bioaccess® bridges innovative Medtech firms with the untapped potential of medical investigations in South America, accelerating studies and facilitating the quicker introduction of medical devices to the market.
Expert insights underscore the impact of these regulatory changes on study speed. As Allison Kalloo, a founding partner and communications lead of Clinical Ambassador and iParticipate, notes, the current landscape is shaped by a demand for improved healthcare access and a commitment to diversity in research enrollment. This dedication is crucial, as diverse enrollment can yield more comprehensive data and better outcomes.
Strategies to enhance diversity, such as recruiting from independent disease registries, not only address representation but also bolster the efficiency of medical studies in the region.
In conclusion, the synergy of legislative support, increased participation rates, and a focus on diversity positions regional Contract Research Organizations, especially bioaccess®, as indispensable partners for Medtech companies. They highlight the benefits of Latin American CROs in expediting medical investigations while fostering economic development and healthcare improvements. Moreover, bioaccess®'s tailored approach to navigating regulatory complexities ensures that studies are conducted efficiently and in compliance with local regulations, further solidifying their value as a partner in the Medtech industry.
Advancements in Infrastructure and Technology Supporting Research
The medical study framework in Latin America has made remarkable strides in recent years, underscoring the strengths of Latin American CROs and positioning the region as a formidable player in the global medical assessment landscape. Modern research facilities have emerged, equipped with state-of-the-art technology that enhances the overall research environment. Advanced data management systems streamline data collection and analysis, ensuring that researchers can access and interpret information efficiently.
The integration of digital technologies has revolutionized communication among stakeholders, fostering collaboration and transparency throughout the research process. This interconnectedness not only accelerates decision-making but also enhances the quality of information gathered, ultimately leading to more reliable outcomes.
As a testament to the region's growing capabilities, the Horizon Databook serves as a comprehensive resource for the research technology and services market, highlighting the increasing demand for efficient experimentation processes and the evolving regulatory landscape in South America. With the pharmaceutical sector investing over USD 1 billion in nearly 700 research trials across the region in 2019, it is clear that global sponsors are recognizing the benefits of partnering with Latin American CROs.
bioaccess® distinguishes itself in this environment by offering expedited medical device research study services, specializing in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies. Our team boasts over 20 years of proven experience in Medtech, ensuring the delivery of high-quality results tailored to your specific needs. Furthermore, recent legislative changes, such as Brazil's Law 14.874/24, approved in May 2024, aim to streamline the evaluation process for research studies, reducing bureaucratic obstacles and enhancing predictability.
This law is pivotal as it strengthens the authority of the research infrastructure in Brazil, showcasing the advantages of Latin American CROs, like bioaccess®, which are adept at managing complex studies effectively. Consequently, the benefits of Latin American CROs present an attractive option for global sponsors seeking to conduct research in a dynamic and supportive environment. Additionally, the impact of medical studies transcends individual trials, contributing to job creation, economic growth, and healthcare enhancement in local economies, further solidifying the value of collaborating with bioaccess®.
Collaboration Opportunities: Building Partnerships for Innovation
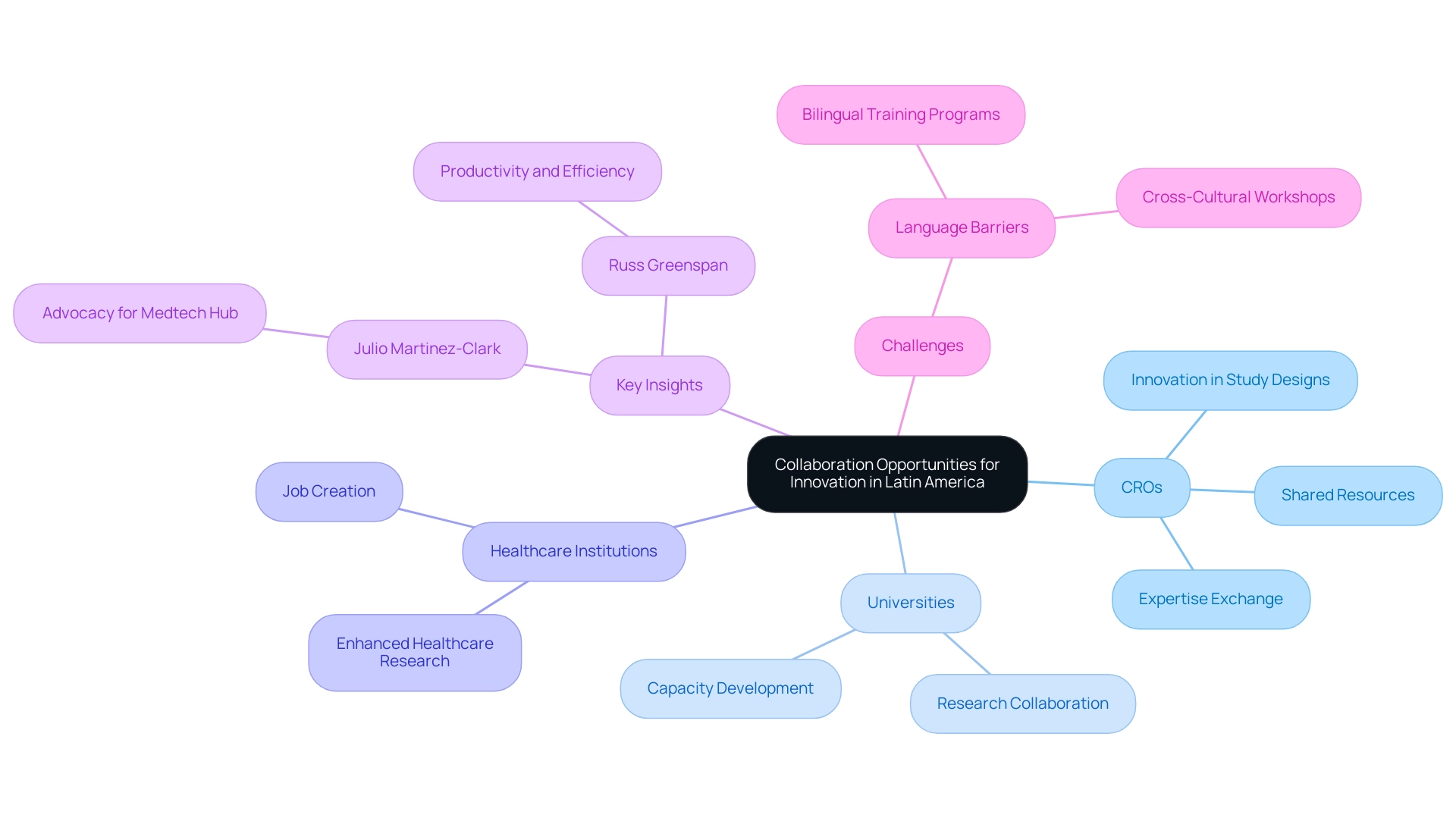
The advantages of Latin American CROs encompass a wealth of collaboration opportunities that can significantly enhance innovation within clinical trials. By forging strategic partnerships with local universities, healthcare institutions, and international sponsors, these organizations can leverage shared resources and expertise to refine study designs and methodologies. Such collaborations not only foster innovation but also play a crucial role in cultivating a robust environment for scholarly activity across the region. This is underscored by Julio Martinez-Clark, CEO of bioaccess, who advocates for the potential of this region as a hub for Medtech medical studies.
Julio's extensive experience in executing research studies and mentoring startups exemplifies his dedication to nurturing growth in this sector. Furthermore, these partnerships facilitate knowledge exchange and capacity development, thereby amplifying the benefits of Latin American CROs and ultimately enhancing the overall healthcare research landscape in Latin America. Media coverage from Clinical Leader reinforces these advantages, spotlighting the importance of collaboration in advancing trials, with articles detailing successful case studies and innovative practices that have emerged from these alliances.
As Russ Greenspan, CTO of PresenceLearning, notes, 'This alignment has been crucial in maintaining productivity and efficiency,' highlighting the significance of these partnerships. Moreover, recent statistics indicate a growing trend in collaboration between CROs and universities, further emphasizing the advantages of Latin American CROs in improving study outcomes and fostering economic growth. However, challenges such as language barriers, as illustrated in case studies, reveal the necessity for effective communication strategies to nurture these collaborations.
Julio's strategies for addressing these challenges, including bilingual training programs and cross-cultural workshops, can unlock additional avenues for innovation and success in clinical research. This ultimately leads to job creation and enhancements in healthcare within the region.
Conclusion
Latin American Contract Research Organizations (CROs) are swiftly establishing themselves as indispensable partners in the global clinical research landscape, particularly through their innovative methodologies exemplified by bioaccess®. The benefits of collaborating with these organizations are substantial, encompassing:
- Cost-effectiveness
- Regulatory expertise
- Access to a diverse array of patient populations
Notably, the capacity to conduct clinical trials at significantly lower costs compared to North America and Europe empowers Medtech companies to optimize their budgets while upholding high-quality standards.
Furthermore, the regulatory environment in Latin America has advanced, with nations such as Colombia refining approval processes to hasten trial initiation. This efficiency, coupled with the region's demographic diversity, enhances recruitment strategies, ensuring quicker patient enrollment and retention. The progress in infrastructure and technology further strengthens the capabilities of Latin American CROs, enabling them to adeptly manage complex clinical studies.
As the demand for expedited and efficient clinical trials continues to escalate, the strategic advantages presented by Latin American CROs become increasingly evident. By leveraging local expertise and resources, Medtech companies can not only accelerate their development timelines but also contribute to the economic growth and healthcare advancements within the region. The collaboration opportunities arising from these partnerships further solidify Latin America’s position as a crucial player in the future of clinical research.
In conclusion, engaging with Latin American CROs offers a compelling opportunity for Medtech companies aiming to enhance their clinical trial strategies. The distinctive combination of cost benefits, regulatory support, and access to diverse populations positions these organizations as invaluable allies in the pursuit of efficiently and effectively bringing innovative medical technologies to market.
Frequently Asked Questions
What is the role of Latin American Contract Research Organizations (CROs) in global trials?
Latin American CROs, particularly bioaccess®, are vital contributors to the global trials landscape, offering competitive advantages such as cost-effectiveness, regulatory expertise, and access to a diverse patient population.
What demographic factors make Brazil and Argentina attractive for clinical trials?
Brazil and Argentina have impressive demographic stability, with a significant portion of the population over 65 years old benefiting from pension systems (93% in Brazil and 85% in Argentina). Additionally, low poverty rates (below 7% in Brazil and 3% in Argentina) create a reliable participant base for research studies.
What services does bioaccess® provide for Medtech startups?
Bioaccess® offers essential services including regulatory approval, site activation, participant recruitment, and trial data management, helping clients navigate the complexities of clinical trials.
How does the cost-effectiveness of Latin American CROs benefit Medtech firms?
The cost-effectiveness of Latin American CROs allows Medtech firms to conduct trials at significantly lower labor costs compared to North America and Europe, enabling more efficient resource allocation and accelerating the development of innovative medical technologies.
Can you provide an example of a successful study conducted in collaboration with bioaccess®?
ReGelTec's Early Feasibility Study in Colombia successfully treated eleven patients with chronic low back pain using HYDRAFIL™, demonstrating the effective partnership between bioaccess™ and Caribbean Health Group.
What is the projected growth of the global research studies market?
The global research studies market, valued at USD 83.75 billion in 2024, is expected to reach USD 149.58 billion by 2034, growing at a CAGR of 6.10%.
How do Latin American CROs contribute to the economic development of the region?
Medtech research studies conducted by Latin American CROs contribute to job generation, economic development, and healthcare enhancement in the region, showcasing their significant economic impact.
What are the implications of recent developments in countries like Uruguay for the CRO sector?
Uruguay's implementation of fiscal rules for economic stability supports the growth of the CRO sector, highlighting the importance of a solid fiscal environment for research involving patients.
What are some challenges faced by Latin American countries regarding CROs?
Challenges persist in some countries, such as Nicaragua and Guatemala, particularly concerning pension system coverage and poverty alleviation for the elderly, which can impact trial recruitment and efficiency.




