Introduction
Colombia is increasingly recognized as a strategic hub for medical device research, thanks to its robust healthcare system and diverse patient population. The country is making significant strides in clinical trials, drawing global attention through partnerships and innovative projects that enhance Medtech developments. Notable collaborations, such as those between bioaccess™ and Caribbean Health Group, are transforming regions like Barranquilla into leading destinations for clinical research.
However, navigating the complexities of the regulatory landscape presents challenges for U.S. companies, including:
- Compliance with INVIMA's standards
- Overcoming language barriers
As Colombia positions itself as a leader in medical device innovation, the interplay of regulatory reforms, cost-effective trial environments, and collaborative opportunities offers a promising future for stakeholders in the medical device sector.
Why Colombia is a Strategic Hub for Medical Device Research
This country has emerged as a strategic hub for medical device research Colombia, bolstered by a robust healthcare system and a diverse patient population. This dynamic environment is increasingly drawing global interest, particularly as the nation enhances its investment in Medical Device Research Colombia. Recent collaborations, such as between Greenlight Guru and bioaccess™, demonstrate the commitment to accelerating Medtech innovations through Medical Device Research Colombia.
Furthermore, PAVmed's successful first-in-human implantations of its PortIO™ Intraosseous Infusion System in Colombia emphasize the positive results from Medical Device Research Colombia within the region. However, the healthcare landscape is not without its challenges; US Medtech companies face significant regulatory hurdles, language barriers, and fragmentation of resources that hinder effective collaboration with Latin American hospitals. Additionally, the US recorded 45,222 gun deaths in 2022, underscoring a broader human rights crisis that emphasizes the importance of effective healthcare solutions for violence-related injuries.
A well-established network of healthcare professionals and institutions actively participates in medical device research in Colombia, ensuring a supportive framework for innovation. Significantly, the Colombian administration has introduced various programs to encourage medical studies, including those related to medical device research Colombia, with tax benefits and simplified approval procedures for experimental procedures. As one expert noted, 'Colombia's commitment to improving healthcare access and its focus on medical device research Colombia positions it as a leader in the region.'
Furthermore, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research, backed by the Colombian Minister of Health. Furthermore, IDx Technologies' partnership with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection collaboration represents another significant step forward. GlobalCare Clinical Trials has also partnered with bioaccess™ to enhance its clinical trial ambulatory services in the region, achieving over a 50% reduction in recruitment time and a 95% retention rate.
Together, these advancements establish the country as an attractive location for global medical device firms aiming to perform Medical Device Research Colombia in a supportive and resource-abundant setting. With statistics indicating a continued rise in research activities, Medical Device Research Colombia positions the country to play a significant role in the advancement of medical device innovation.

Navigating the Regulatory Framework for Medical Devices in Colombia
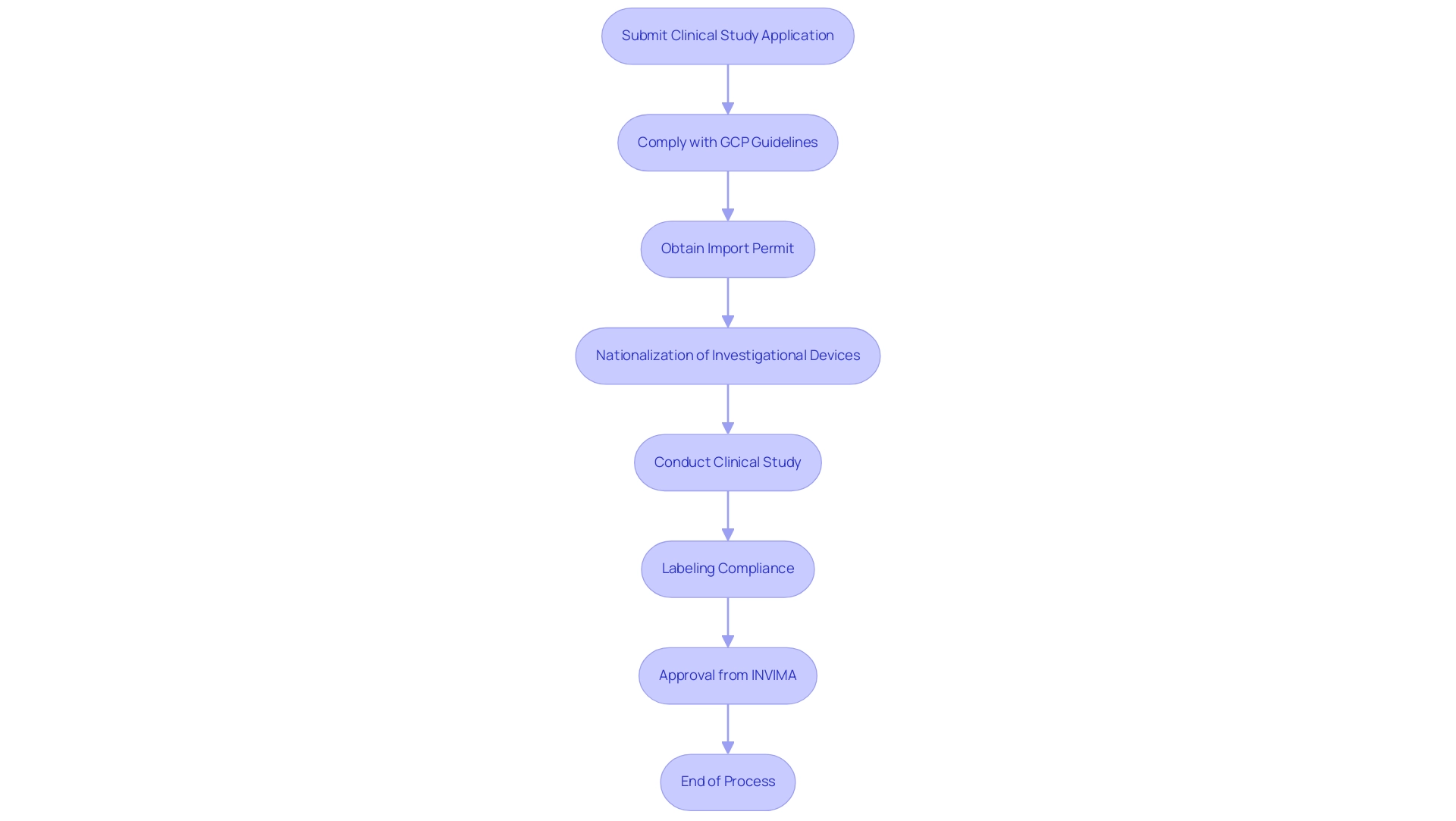
Colombia's regulatory framework for medical devices is chiefly governed by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which oversees the marketing authorization process and ensures compliance with health standards. Navigating this framework requires researchers to adhere to a structured series of steps, including the submission of clinical study applications and strict compliance with Good Clinical Practice (GCP) guidelines. These regulations are crucial, as they not only ensure that studies are conducted ethically and legally but also protect participant welfare and maintain the integrity of data.
Recent reforms have been implemented to streamline these processes and decrease approval timelines, making the country an increasingly appealing location for Medical Device Research Colombia. Importantly, INVIMA has been classified as a Level 4 health authority by PAHO/WHO, which underscores its competence and efficiency in health regulation. This classification further boosts the reliability of medical studies carried out in the country.
Notably, a key statistic underscores the importance of collaborative care: those who complete goals with support from their care teams are 250% more likely to achieve positive outcomes. This emphasizes the crucial intersection of regulatory adherence and efficient study management in promoting successful research settings. Moreover, as telehealth keeps gaining momentum, with a mid-2021 McKinsey survey showing that 58% of US-based physicians regard it more positively than prior to the pandemic, the consequences for medical trials in the country are substantial.
Additionally, researchers must be aware that labeling for medical devices and pharmaceuticals in Colombia must include essential information in both Spanish and the local language, emphasizing the need for compliance in all aspects of the regulatory framework. With bioaccess® at the forefront of Medical Device Research Colombia and Medtech research across Latin America, its emphasis on innovation and regulatory excellence acts as an essential resource for researchers navigating these intricate landscapes. Bioaccess® provides extensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, study setup, and project oversight, ensuring that all elements of the research process are meticulously managed.
The import permit and nationalization of investigational devices are essential steps within this process, reinforcing the significance of following regulatory requirements to enable successful evaluations.
Leveraging Colombia's Clinical Research Infrastructure
This country boasts a strong infrastructure for medical device research in Colombia, marked by a network of experienced sites, specialized facilities, and a group of qualified professionals. As of 2024, the nation is expected to have a considerable number of study locations, demonstrating its dedication to promoting medical advancement. Many hospitals and clinics throughout the country are fully equipped to carry out research studies covering various therapeutic areas, broadening the range of research initiatives.
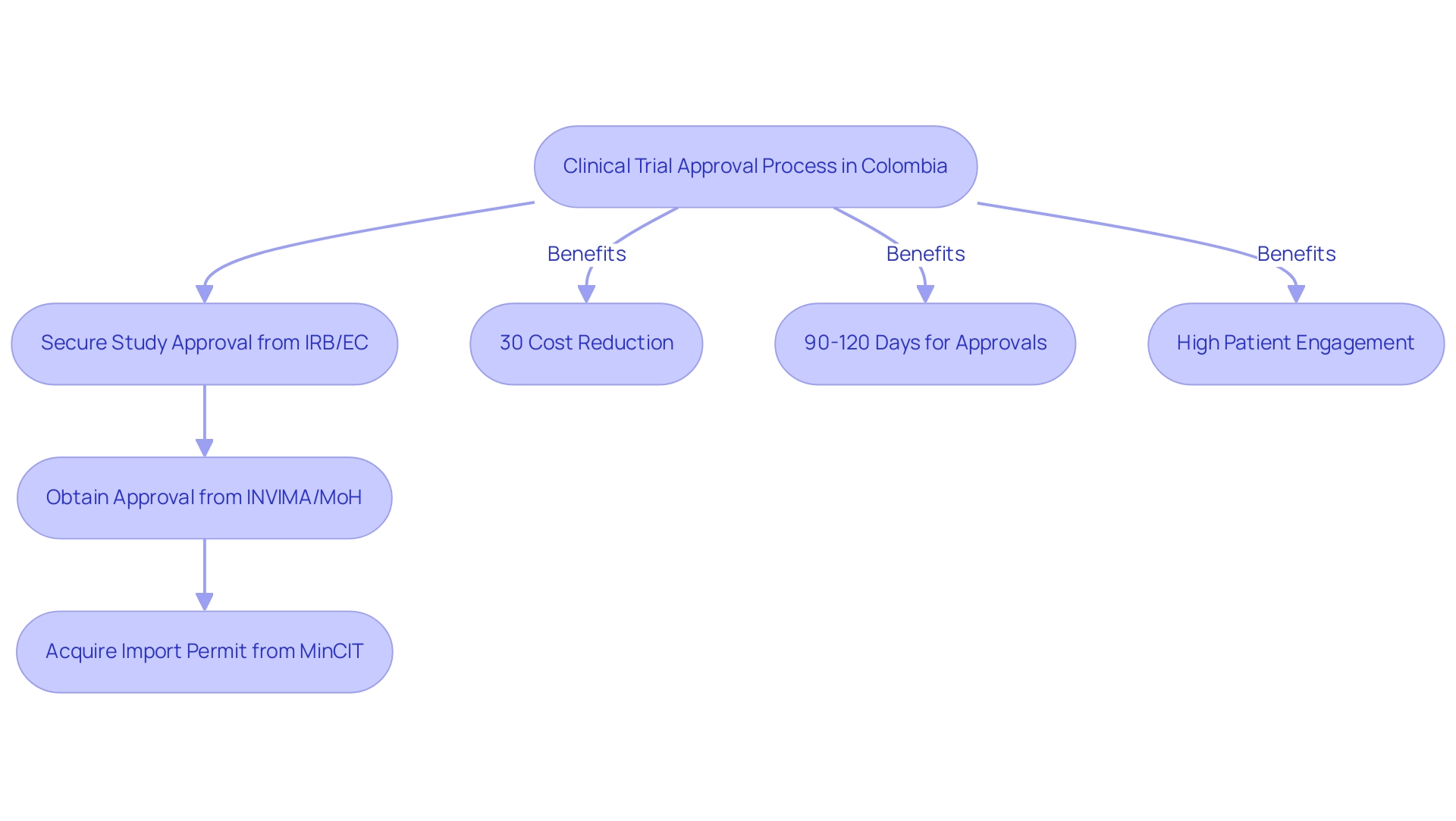
Moreover, the nation's competitive benefits, such as cost reductions exceeding 30% relative to North America and Western Europe, swift IRB/EC and INVIMA authorization processes lasting merely 90-120 days, and a top-tier healthcare system, establish it as a leading location for Medical Device Research Colombia. The country’s rapid subject recruitment capabilities—fueled by a diverse population of over 50 million and high patient engagement—support the effectiveness of Medical Device Research Colombia. Notable collaborations, such as bioaccess™'s partnership with Caribbean Health Group to make Barranquilla an appealing research destination and GlobalCare Clinical Trials' expansion of ambulatory services, have led to over a 50% decrease in recruitment time and a retention rate surpassing 95%.
Additionally, investments in science, technology, and innovation projects benefit from substantial R&D tax incentives, including a 100% tax deduction and various future tax credits. This infrastructure not only supports the implementation of Medical Device Research Colombia but also boosts quality, ensuring that findings effectively translate into better health outcomes for the population. The process for obtaining clinical trial approval in the country for medical device research Colombia involves several key steps:
- Securing study approval from your site's institutional review board (IRB)/ethics committee (EC)
- Obtaining approval from the regulatory agency (INVIMA)/Ministry of Health (MoH)
- Acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) to ship investigational devices to your site
In the context of regional health challenges, as noted by Kathleen Page, MD, 'The Venezuelan government should immediately acknowledge the public health crisis in the country and engage with the international community to facilitate an appropriately scaled response without delay.' This highlights the significance of merging new studies with current local health infrastructure, as such integration can enhance health systems and improve care delivery in the region. A pertinent case study titled 'Integrating Studies with Existing Infrastructure' illustrates how Medical Device Research Colombia can enhance healthcare delivery while conducting inquiries, ensuring that these endeavors do not detract from the primary goal of providing care to the population.
Cost-Competitive Environment for Medical Device Trials in Colombia
This country has emerged as a highly cost-effective location for Medical Device Research Colombia, primarily due to its significantly lower operational costs, which encompass labor and facility expenses. Recent reports suggest that operational expenses for Medical Device Research Colombia can be up to 40% lower than in North America, a financial benefit further enhanced by a favorable exchange rate, making research projects more economically feasible. Alongside these economic advantages, bioaccess® specializes in managing a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
This enhances the region's attractiveness for Medical Device Research Colombia.
Their expertise in extensive research services—including:
- feasibility studies
- site selection
- compliance evaluations
- setup
- import permits
- project management
- reporting
ensures that studies are conducted efficiently and effectively. Streamlined regulatory processes, governed by INVIMA, the National Food and Drug Surveillance Institute classified as a Level 4 health authority by PAHO/WHO, allow companies to initiate Medical Device Research Colombia studies more quickly, conserving valuable resources. The ongoing Venezuelan Refugee and Migrant Crisis, which has led to over 6.1 million refugees seeking healthcare access in neighboring countries, underscores the importance of efficient healthcare solutions in the region.
As nations endeavor to tackle these challenges, entities seeking to enhance their funding allocations while maintaining strict quality standards will discover this South American nation an appealing choice for trials. This combination of economic efficiency, operational effectiveness, and the positive impact of Medtech clinical studies on local economies—such as job creation, economic growth, and improved healthcare—positions the country as a leader in Medical Device Research Colombia.

Opportunities for Collaboration in Colombia's Medical Device Sector
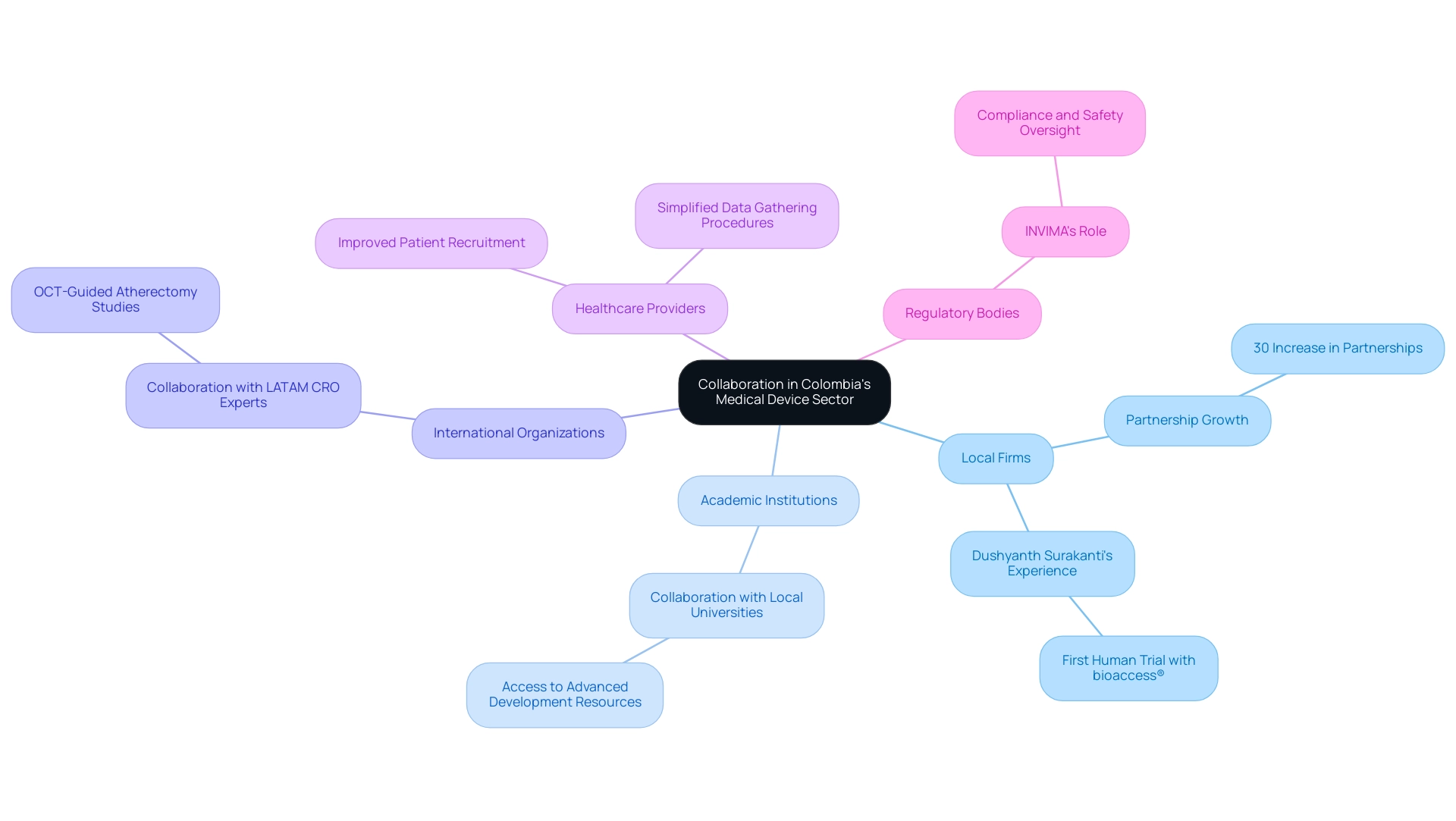
The nation's medical device sector is increasingly acknowledged for its vast collaboration potential in Medical Device Research Colombia, driven by an expanding network of local firms, academic institutions, and international organizations. Recent statistics reveal that partnerships between local businesses and academic institutions in the country have grown by over 30% in the past two years, highlighting the sector's commitment to innovation. Notably, Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shared his experience with bioaccess® during its first human trial in that country, illustrating the practical impact of these collaborations.
Likewise, Dr. John B. Simpson, CEO of Avinger, highlighted Avinger's satisfactory experience conducting OCT-guided atherectomy clinical studies in Cali, Colombia, demonstrating the effectiveness of collaborating with LATAM CRO Experts. These partnerships are crucial in enhancing exploration capabilities, fostering knowledge exchange, and driving innovation. Working together with local universities, for instance, provides access to advanced development resources that can significantly enhance product creation efforts.
Additionally, collaborations with healthcare providers not only improve patient recruitment but also simplify data gathering procedures, which are essential for successful research studies. Our comprehensive trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures a thorough approach to research. As Jeanne Williams, a seasoned expert in strategic frameworks and operational scaling, remarks, 'Developing effective collaborations is essential for navigating the complexities of medical device innovation.'
With over 20 years of experience in engineering and HR initiatives, including her role in managing AI and machine learning product and program management at Apple, Williams' insights underscore the importance of strategic partnerships. Moreover, understanding the regulatory landscape is crucial; INVIMA (Colombia National Food and Drug Surveillance Institute) plays a vital role in overseeing medical devices, ensuring compliance and safety as a Level 4 health authority recognized by PAHO/WHO. The impact of Medtech clinical studies on local economies is significant, creating jobs and promoting economic growth while improving healthcare outcomes.
By capitalizing on these collaborative opportunities and leveraging the resources and tools available to support decision-making and enhance productivity, stakeholders can expedite the development and commercialization of medical devices through medical device research Colombia, positioning themselves at the forefront of a dynamic industry poised for growth.
Conclusion
Colombia's emergence as a strategic hub for medical device research is underscored by its robust healthcare system, diverse patient population, and a commitment to innovation. The country's proactive regulatory reforms, spearheaded by INVIMA, have streamlined the clinical trial process, making it an increasingly attractive destination for international Medtech companies. These enhancements, coupled with significant cost advantages and a supportive research infrastructure, position Colombia as a leader in the advancement of medical device innovation.
The collaborative spirit within Colombia's medical device sector further amplifies its appeal. Partnerships between local companies, research institutions, and international organizations have seen substantial growth, fostering an environment ripe for innovation and knowledge exchange. The successful experiences shared by various stakeholders highlight the effectiveness of these collaborations in navigating regulatory complexities and enhancing research capabilities.
As Colombia continues to build its reputation in the global Medtech landscape, the interplay of regulatory excellence, cost-effectiveness, and collaborative opportunities paves the way for a promising future. Stakeholders who embrace these dynamics will not only contribute to the advancement of medical technology but also improve healthcare outcomes for the population, reinforcing Colombia's position as a key player in the medical device sector. The potential for growth in this arena is substantial, and as the country invests in its healthcare infrastructure, it stands ready to lead the way in medical device research and development.




