Introduction
In the rapidly evolving landscape of medical technology, early feasibility studies play a pivotal role in determining the potential success of new devices, particularly in regions like Paraguay. These preliminary assessments are crucial for navigating the complex regulatory environment and ensuring compliance with both local laws and international standards. By evaluating technical specifications, understanding patient needs, and assessing clinical practices, stakeholders can gain valuable insights that inform design modifications and strategic partnerships.
As Medtech companies in Latin America grapple with unique challenges, including language barriers and resource fragmentation, collaborations such as that between Greenlight Guru and bioaccess™ offer essential support. This partnership not only enhances clinical trial management but also accelerates the pathway for innovative medical devices to reach the market, reflecting a broader commitment to advancing healthcare solutions across the region.
Understanding Early Feasibility Studies for Medical Devices in Paraguay
Initial feasibility assessments for medical products act as preliminary evaluations that assist in identifying the potential success of a product in the market. In Paraguay, this research is vital due to the unique regulatory environment and the need for compliance with local laws and international standards. They usually include:
- Analyzing the technical features of the device
- Comprehending patient requirements
- Evaluating medical practices
Carrying out such research early on can offer essential insights that guide design adjustments and assist in obtaining funding or collaborations for further development. Furthermore, Medtech companies in Latin America face challenges such as language barriers and fragmented resources that can hinder effective communication and collaboration.
Through the partnership between Greenlight Guru and bioaccess™, Medtech firms can utilize extensive trial management services that encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
This collaboration seeks to close gaps in clinical research and innovation, as demonstrated by PAVmed's successful first-in-human trial in Colombia, ultimately speeding up the journey to market for new medical products in Latin America.
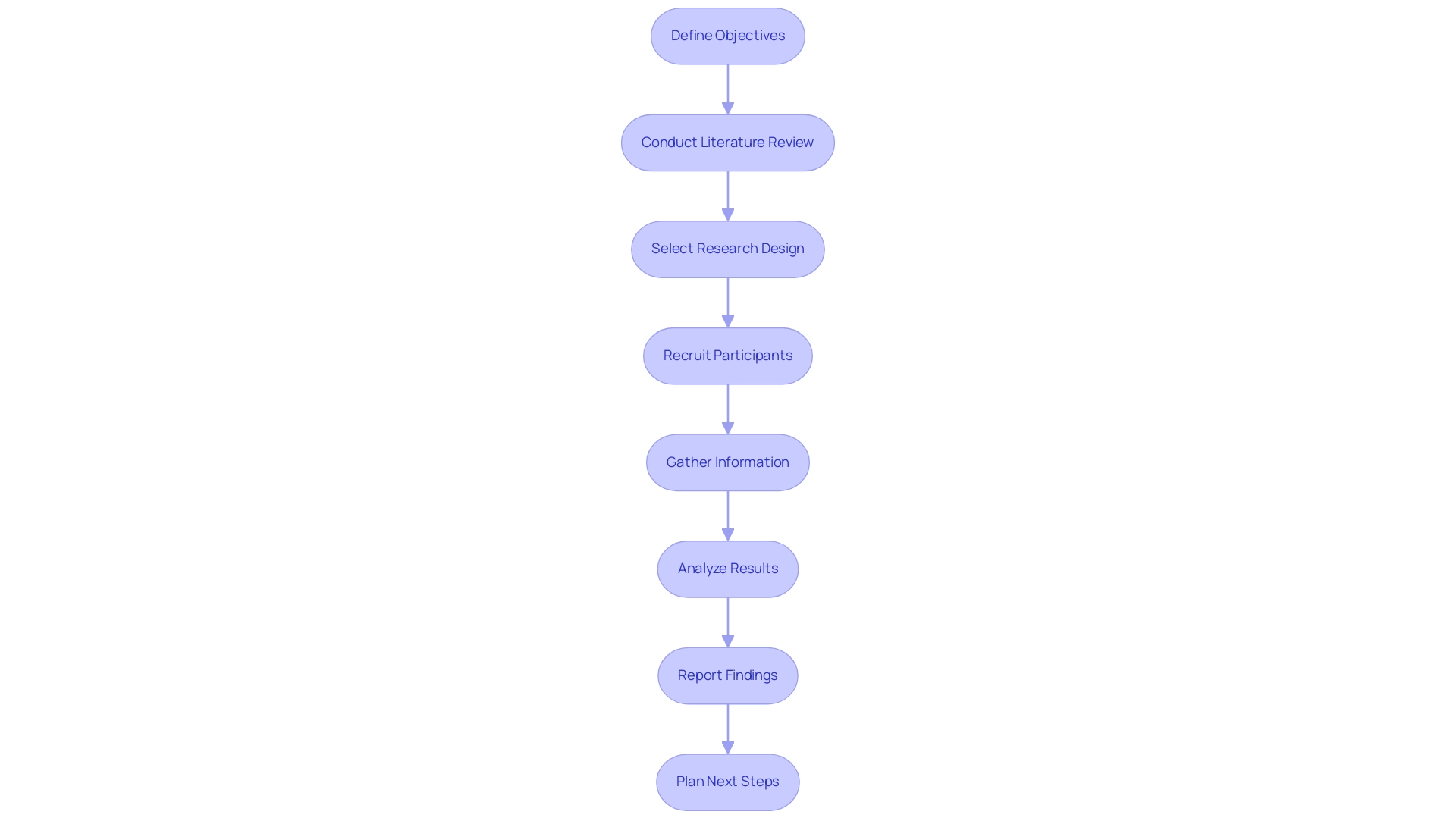
Step-by-Step Process for Conducting Feasibility Studies
- Define Objectives: Clearly outline the goals of the feasibility assessment, including specific questions you aim to answer regarding the medical device's design and market potential.
- Conduct Literature Review: Research existing research, market trends, and regulatory requirements in Paraguay, including insights from the Colombia National Food and Drug Surveillance Institute (INVIMA) to inform your approach and identify gaps in knowledge.
- Select Research Design: Choose an appropriate research framework, such as qualitative interviews with healthcare professionals or quantitative surveys targeting potential users, drawing on bioaccess®'s expertise in managing Early-Feasibility and First-In-Human investigations.
- Recruit Participants: Identify and enlist a varied group of stakeholders, including clinicians, patients, and regulatory experts, to gather comprehensive feedback, leveraging the local knowledge that bioaccess® has developed through its extensive trial networks.
- Gather Information: Implement the research design and collect data systematically, ensuring adherence to ethical standards and regulatory guidelines, particularly those set by INVIMA, which is recognized as a Level 4 health authority by PAHO/WHO.
- Analyze Results: Examine the data to identify trends, insights, and potential obstacles that may influence the product’s development and market entry, considering the economic impact of medical research on local job creation and healthcare enhancement.
- Report Findings: Compile a detailed report summarizing the project's objectives, methods, results, and suggestions for further development or adjustments to the apparatus, reflecting the comprehensive project management services of bioaccess®, including setup, compliance reviews, and ongoing monitoring.
- Plan Next Steps: Based on the findings, determine the feasibility of proceeding with further trials or product development, and outline any necessary adjustments to the device or research approach, utilizing bioaccess®'s expertise in navigating regulatory environments and conducting successful trials. Additionally, consider insights from key team members like Oswaldo Amaya and Juan Cuya, whose extensive backgrounds in clinical research and Regulatory Affairs can further inform the project's direction.
Key Regulatory Considerations
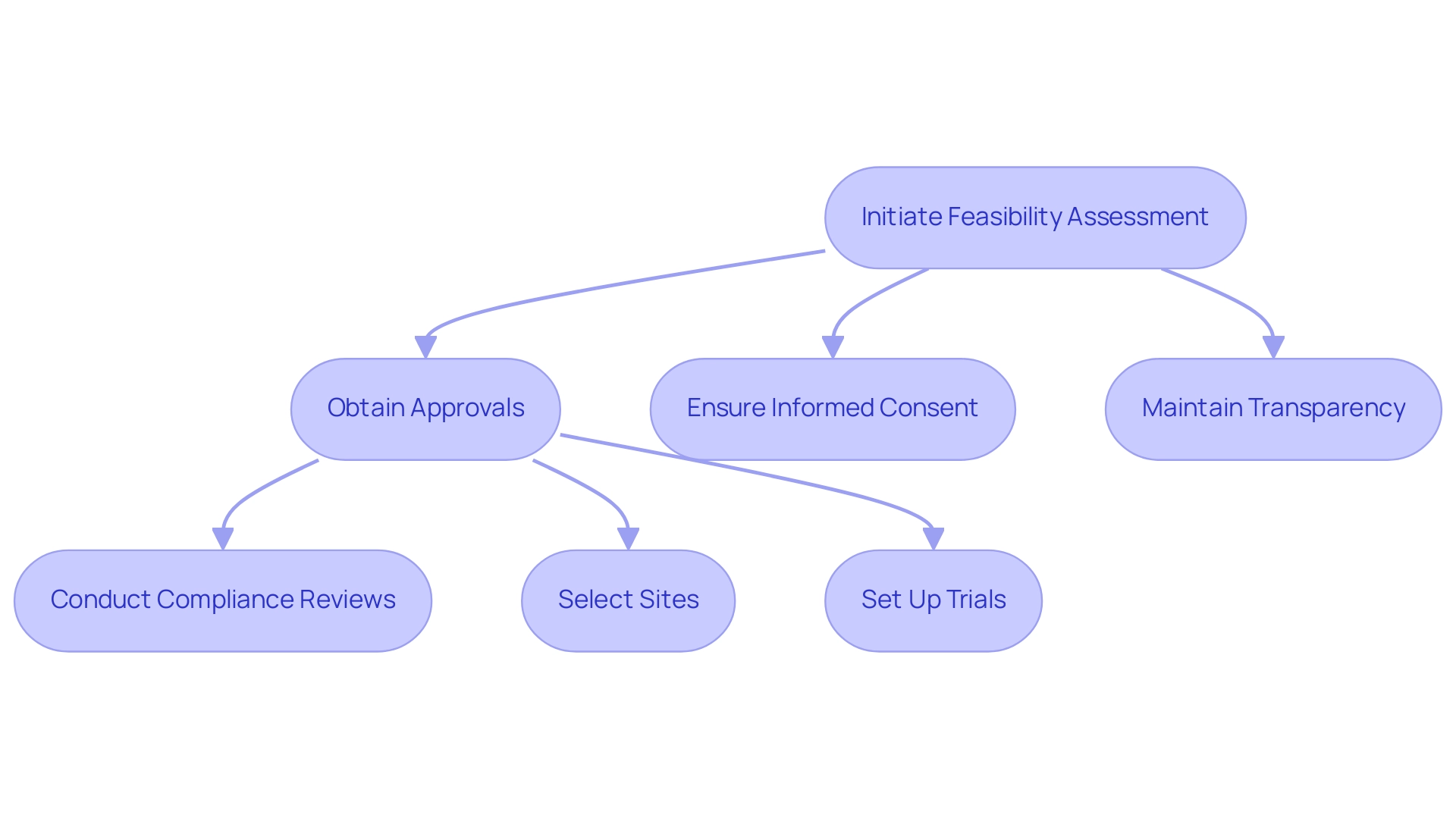
Conducting early feasibility assessments in Paraguay necessitates strict adherence to regulatory frameworks established by health authorities. Familiarity with the guidelines set forth by the National Directorate of Medicines and Health Technologies (DINAMED) is crucial, as these guidelines dictate key processes and ethical standards that must be followed. Many experts believe that DINAMED guidelines provide a solid foundation for conducting feasibility assessments, though some suggest that additional flexibility could enhance their application in diverse contexts. Essential considerations include:
- Obtaining requisite approvals prior to research initiation
- Ensuring that informed consent is thoroughly communicated and understood by participants
- Maintaining transparency throughout the reporting of results
Our comprehensive clinical trial management services tackle these challenges, providing assistance in:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup—including import permits and nationalization of investigational devices
- Project management
- Reporting
Engaging with regulatory bodies at the outset can facilitate a more efficient approval process and significantly enhance the research's credibility. Notably, Katherine Ruiz, our Regulatory Affairs expert, has a proven track record advising medical equipment startups in navigating complex regulations and obtaining market clearance in Colombia. As noted by Dr. Juan Pérez, a regulatory expert in Paraguay, "Adhering to DINAMED guidelines not only ensures compliance but also fosters trust with stakeholders in the medical community." Staying informed about anticipated updates to DINAMED guidelines in 2024 will be vital for the successful execution of medical device research in the region.
Engaging Stakeholders Effectively
Engaging stakeholders—including healthcare professionals, patients, and regulatory authorities—is fundamental to the success of early feasibility studies, such as ReGelTec's study on HYDRAFIL™ for chronic low back pain in Colombia. This initiative not only showcases the innovative treatment being explored but also highlights how clinical trials can create jobs and foster economic growth in local communities.
A pertinent example is Welwaze Medical Inc.'s collaboration with bioaccess™ for the launch of the Celbrea® medical product, which emphasizes the importance of regulatory access in Colombia. The initial step entails recognizing key stakeholders who have a vested interest in the medical instrument under investigation.
Effective communication strategies are essential; they should aim to keep stakeholders informed throughout the research process. Regular meetings, updates, and feedback sessions can foster collaboration and address stakeholder concerns in a timely manner. According to Lauren Parr, co-founder and Product Director at RepuGen, 'Studies show that good patient-doctor communication has the potential to regulate patients' emotions, and those who feel their doctors communicate clearly are more likely to leave positive reviews online.' This insight underscores the value of transparency and open dialogue in healthcare settings, where environmental factors such as noise and lack of privacy can hinder effective communication.
Research indicates that approximately 30% of patients report feeling anxious in noisy hospital environments, which can significantly impede nurse-patient interactions and negatively influence patient experiences. Furthermore, the analysis by Henderson et al. highlights the importance of cultural competence in community healthcare, which can enhance interactions with diverse stakeholders.
A pertinent illustration is the partnership between bioaccess™ and Caribbean Health Group focused on establishing Barranquilla as a prime location for medical research, backed by Colombia's Minister of Health. This case illustrates how strategic partnerships can facilitate successful regulatory navigation and enhance the overall research landscape.
By establishing trust and keeping open channels of communication, researchers can enhance the overall effectiveness of their work and gain valuable insights that improve the medical device's design and functionality. Overall, the influence of medical studies goes beyond research, aiding in job creation and boosting Colombia's global recognition as a center for innovative healthcare solutions.
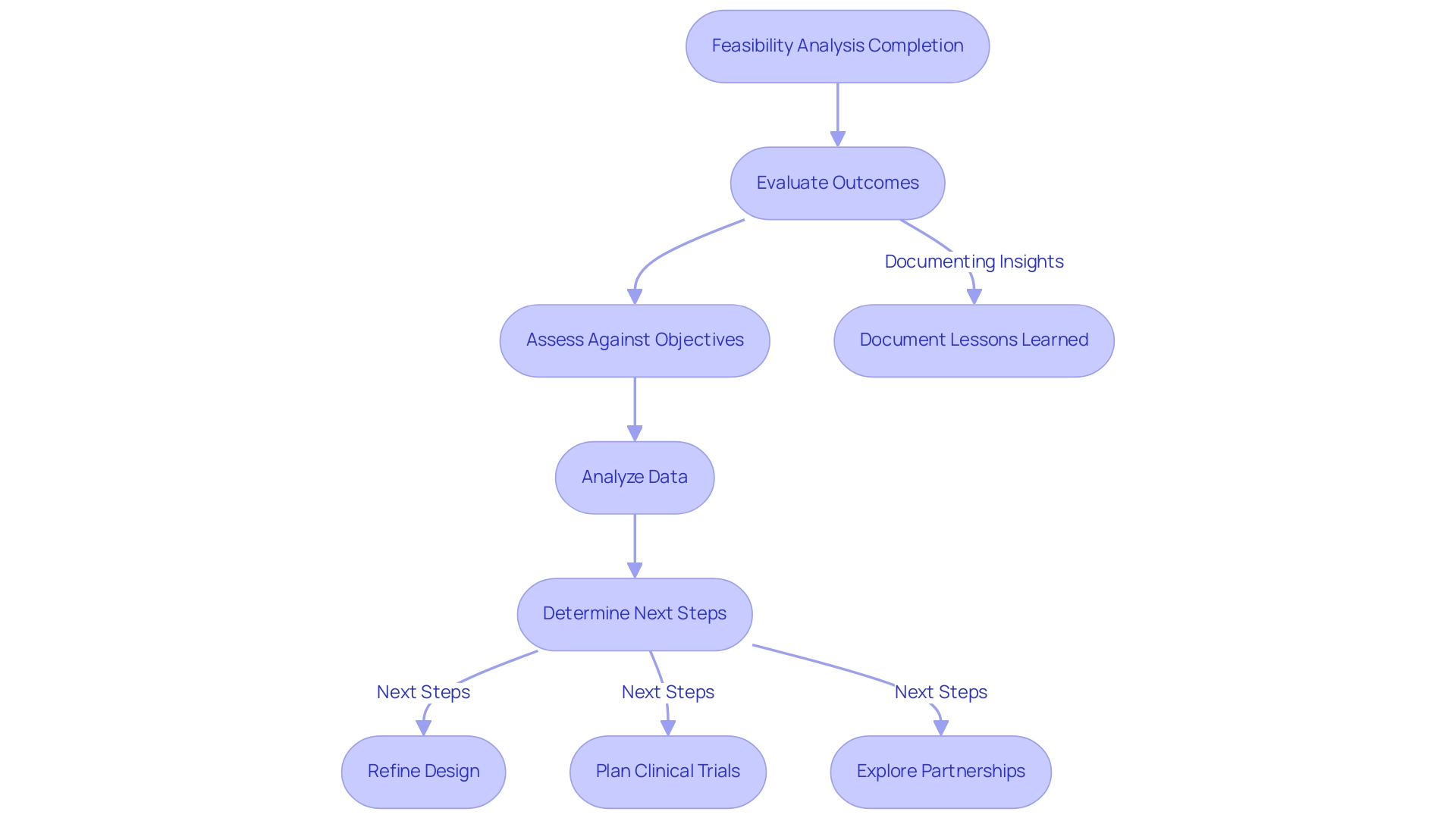
Evaluating Outcomes and Next Steps
After finishing the feasibility analysis, it is crucial to evaluate the outcomes against the initial objectives set at the beginning of the process. With bioaccess®'s knowledge in extensive research management, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
you can analyze the data gathered to determine whether the research achieved its objectives concerning:
- Product viability
- Market potential
- User acceptance
This evaluation will guide the next steps for medical equipment development, which may include:
- Refining the design
- Planning for further clinical trials
- Exploring partnerships for commercialization
Additionally, documenting lessons learned and insights gained during the study, such as specific methodologies applied in project management and monitoring, will not only improve overall project management but also guide future research efforts, aligning with bioaccess®'s commitment to supporting U.S. medical device companies in Latin America.
Conclusion
Conducting early feasibility studies for medical devices in Paraguay is a critical step in navigating the complex regulatory landscape and ensuring that innovative solutions can effectively meet patient needs. These studies provide essential insights into technical specifications, market potential, and user acceptance, ultimately guiding design modifications and strategic partnerships that are vital for success.
The collaboration between organizations like Greenlight Guru and bioaccess™ exemplifies how Medtech companies can overcome unique challenges in Latin America, such as language barriers and resource fragmentation. By leveraging comprehensive clinical trial management services, stakeholders can streamline their processes, enhance compliance, and accelerate the pathway for new medical devices to reach the market.
As the medical technology sector continues to evolve, staying informed about regulatory updates and engaging effectively with diverse stakeholders will be paramount. This proactive approach not only fosters trust within the medical community but also contributes to job creation and economic growth, reinforcing the broader impact of clinical trials on healthcare innovation. Ultimately, the commitment to rigorous early feasibility studies sets the foundation for successful medical device development and a healthier future for patients in Paraguay and beyond.
Frequently Asked Questions
What are initial feasibility assessments for medical products?
Initial feasibility assessments are preliminary evaluations that help identify the potential success of a medical product in the market. They analyze technical features, comprehend patient requirements, and evaluate medical practices.
Why are feasibility assessments important in Paraguay?
Feasibility assessments are crucial in Paraguay due to the unique regulatory environment and the need for compliance with local laws and international standards.
What insights can early feasibility research provide?
Early feasibility research can offer essential insights that guide design adjustments and assist in obtaining funding or collaborations for further development.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America face challenges such as language barriers and fragmented resources that hinder effective communication and collaboration.
What services does the partnership between Greenlight Guru and bioaccess™ provide?
The partnership provides extensive trial management services, including feasibility assessments, site selection, compliance reviews, trial setup, import permits, and project management.
What is the significance of engaging stakeholders in feasibility studies?
Engaging stakeholders, including healthcare professionals, patients, and regulatory authorities, is fundamental to the success of early feasibility studies, as it fosters collaboration and addresses concerns.
What steps should be taken in conducting a feasibility assessment?
Steps include defining objectives, conducting literature reviews, selecting research designs, recruiting participants, gathering information, analyzing results, reporting findings, and planning next steps.
How does adherence to DINAMED guidelines impact feasibility assessments?
Adhering to DINAMED guidelines ensures compliance with regulatory frameworks, improves the credibility of the research, and fosters trust with stakeholders in the medical community.
What are the potential outcomes evaluated after a feasibility analysis?
After a feasibility analysis, the outcomes evaluated include product viability, market potential, and user acceptance.
What actions may follow the evaluation of feasibility assessment outcomes?
Following the evaluation, actions may include refining the design, planning further clinical trials, and exploring partnerships for commercialization.




