Introduction
Navigating the intricate landscape of medical device development in Latin America presents unique challenges and opportunities, particularly through the lens of early feasibility studies. These studies play a pivotal role in assessing the viability of new technologies, ensuring they meet both regulatory standards and the diverse needs of local populations. In countries like Peru, where regulatory frameworks differ significantly from those in other regions, understanding the nuances of local regulations, such as those established by the Ministry of Health and the National Institute of Health, is essential for success.
By strategically collaborating with local organizations and stakeholders, Medtech companies can not only enhance their research efforts but also leverage the region's potential for innovation. This article delves into the critical aspects of conducting early feasibility studies in Latin America, offering a comprehensive guide to navigating the complexities of this dynamic market.
Understanding Early Feasibility Studies in Latin America
Initial viability assessments are essential in the healthcare product development process, particularly in Latin America, where compliance frameworks and market dynamics can vary greatly from those in other areas. In Peru, these examinations not only assist in evaluating the viability of a medical device by assessing its technical performance, safety, and usability in a real-world setting, but they also reflect a broader trend of Medtech companies, such as PubMed, successfully conducting first-in-human trials in collaboration with local organizations like bioaccess®.
Understanding local regulations, including those set by the Peruvian Ministry of Health (MINSA) and the National Institute of Health (INS), is paramount. Bioaccess® offers essential services such as:
- Regulatory approval
- Clinical research site activation
- Subject recruitment
These services are crucial for navigating these regulatory landscapes. Furthermore, cultural factors—such as the diversity of patient populations and varying levels of healthcare accessibility—must be considered to ensure that research is relevant and ethically conducted.
By recognizing these elements, researchers can customize their feasibility analyses to better align with local needs and expectations, ultimately enhancing the chances of successful product development and market entry while leveraging the potential that Latin America offers for Medtech innovation.
Step-by-Step Guide to Conducting Feasibility Studies in Peru
Conducting early feasibility assessments for medical devices in Peru requires meticulous planning and adherence to local regulations. Follow these essential steps to ensure a successful study:
- Define the Study Objectives: Start by clearly outlining the objectives of the feasibility assessment. Specify the hypotheses to be tested and the data required to support these hypotheses, ensuring that they align with clinical and compliance expectations.
- Engage with Regulatory Authorities: Initiate communication with the Ministry of Health (MINSA) and the National Institute of Health (INS) early in the process. Comprehending legal requirements and securing essential approvals is vital for adherence and seamless project execution. As Julio Martinez-Clark, CEO of Bioaccess, highlights, "Interacting with oversight organizations from the beginning guarantees that your research aligns with local standards and can move forward without avoidable hold-ups."
- Select a Suitable Study Site: Choose healthcare facilities that have the infrastructure and experience to conduct clinical trials effectively. Collaborate with local investigators who are familiar with the patient population and clinical landscape to enhance recruitment and data integrity.
- Develop a Comprehensive Research Protocol: Create a detailed research protocol that outlines the design, methodology, participant recruitment strategies, and data collection methods. Ensure that the protocol adheres to ethical standards and local regulations, which is vital for both participant safety and regulatory compliance.
- Obtain Ethical Approval: Submit the research protocol to an Institutional Review Board (IRB) or Ethics Committee for review and approval. This step is fundamental to ensuring the protection of participants' rights and well-being throughout the research.
- Recruit Participants: Implement effective recruitment strategies tailored to the local population. Utilize culturally appropriate materials and methods to inform potential participants about the research, enhancing engagement and participation rates.
- Conduct the Research: Execute the feasibility assessment in accordance with the approved protocol. Ensure that all data collection processes are conducted ethically and accurately, maintaining the highest standards of scientific integrity. Significantly, the successful first-in-human use of Vascudyne's TRUE vascular graft in end-stage renal disease patients illustrates the potential for innovative health technologies in this region.
- Analyze Data and Report Findings: Upon completion of the research, conduct a thorough analysis of the collected data to evaluate the feasibility of the medical device. Prepare a comprehensive report outlining the findings, challenges faced, and actionable suggestions for future research.
- Plan for Next Steps: Based on the results of the feasibility analysis, evaluate whether to proceed with further development, implement changes, or carry out additional investigations. Utilize insights gained to inform the broader development strategy for the medical device, ensuring alignment with market needs and regulatory frameworks. As emphasized in the case analysis on early feasibility assessments in Colombia, there is significant untapped market potential in Latin America, making it an appealing region for MedTech innovations.
At bioaccess®, we leverage over 20 years of expertise in managing clinical trials, providing services that include feasibility assessments, First-In-Human Experiments, Post-Market Clinical Follow-Up Research, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Our tailored approach guarantees that we navigate the regulatory landscape effectively, establishing us as your reliable partner for successful research in this dynamic region.
Identifying Key Stakeholders and Collaborators
In Colombia, successful viability assessments for healthcare instruments often depend on the cooperation of various stakeholders, including:
- Regulatory Bodies: Engaging with INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial for ensuring compliance with local regulations and understanding the approval process for healthcare instruments. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its competence in overseeing medical device safety and efficacy, including monitoring pre- and post-market programs.
- Healthcare Institutions: Partnering with networks like the Caribbean Health Group, which brings together leading healthcare institutions in Barranquilla, can provide valuable insights and access to patient populations, enhancing clinical trial recruitment.
- Investigators and Research Teams: Collaborating with experienced investigators familiar with the local context can improve research design and execution, as demonstrated in ReGelTec's Early Feasibility Assessment on HYDRAFIL™ for chronic low back pain, which utilized remote proctoring via Zoom to ensure successful treatment procedures.
- Ethics Committees: Close collaboration with ethics committees ensures that research protocols adhere to ethical standards, safeguarding participants' rights throughout the trial process.
- Industry Partners: Engaging with medical device manufacturers, such as those partnering with GlobalCare Clinical Trials, can provide additional resources and support for conducting studies effectively. This collaboration has resulted in improved patient recruitment times by over 50% and retention rates exceeding 95%. Katherine Ruiz, a compliance affairs specialist with vast experience in maneuvering INVIMA's procedures, highlights the significance of early relationship development with these stakeholders to result in smoother execution and improved outcomes.
Addressing Regulatory Challenges in Peru
Carrying out feasibility assessments in Peru necessitates careful navigation of the compliance environment, supported by extensive clinical trial management services. Here are some common challenges and strategies to address them:
- Understanding Local Regulations: Familiarize yourself with specific regulations set by MINSA and INS, including requirements for clinical trial applications and ethical approvals. Regular communication with these bodies can provide clarity.
- Documentation Requirements: Ensure that all necessary documentation is prepared and submitted accurately. This encompasses research protocols, informed consent forms, and investigator qualifications. Incomplete submissions can result in delays.
- Cultural Sensitivity: Be aware of cultural nuances that may influence compliance interactions. Establishing a connection with officials can enable more efficient processes.
- Changes in Regulations: Stay informed about any modifications in rules that may affect your research. Regularly consult industry resources and legal experts to ensure compliance.
- Timelines for Approval: Anticipate potential delays in the approval process and plan accordingly. Allow sufficient time for compliance reviews and be ready to respond to any feedback swiftly.
Alongside these strategies, it is vital to utilize specific service abilities such as acquiring import permits and the nationalization of investigational tools, as well as thorough reporting on project status, inventory, and serious and non-serious adverse events. With the assistance of compliance specialists such as Ana Criado, Director of Affairs and CEO of Mahu Pharma, who possesses extensive experience in biomedical engineering and health economics, and Katherine Ruiz, an expert in compliance matters for healthcare instruments and in vitro diagnostics, you can navigate these challenges effectively and ensure adherence throughout your research.
Evaluating Results and Preparing for Future Research
After finishing a feasibility analysis, it is crucial to assess the results comprehensively, particularly considering the distinct legal frameworks in Latin America. Here are key steps to consider:
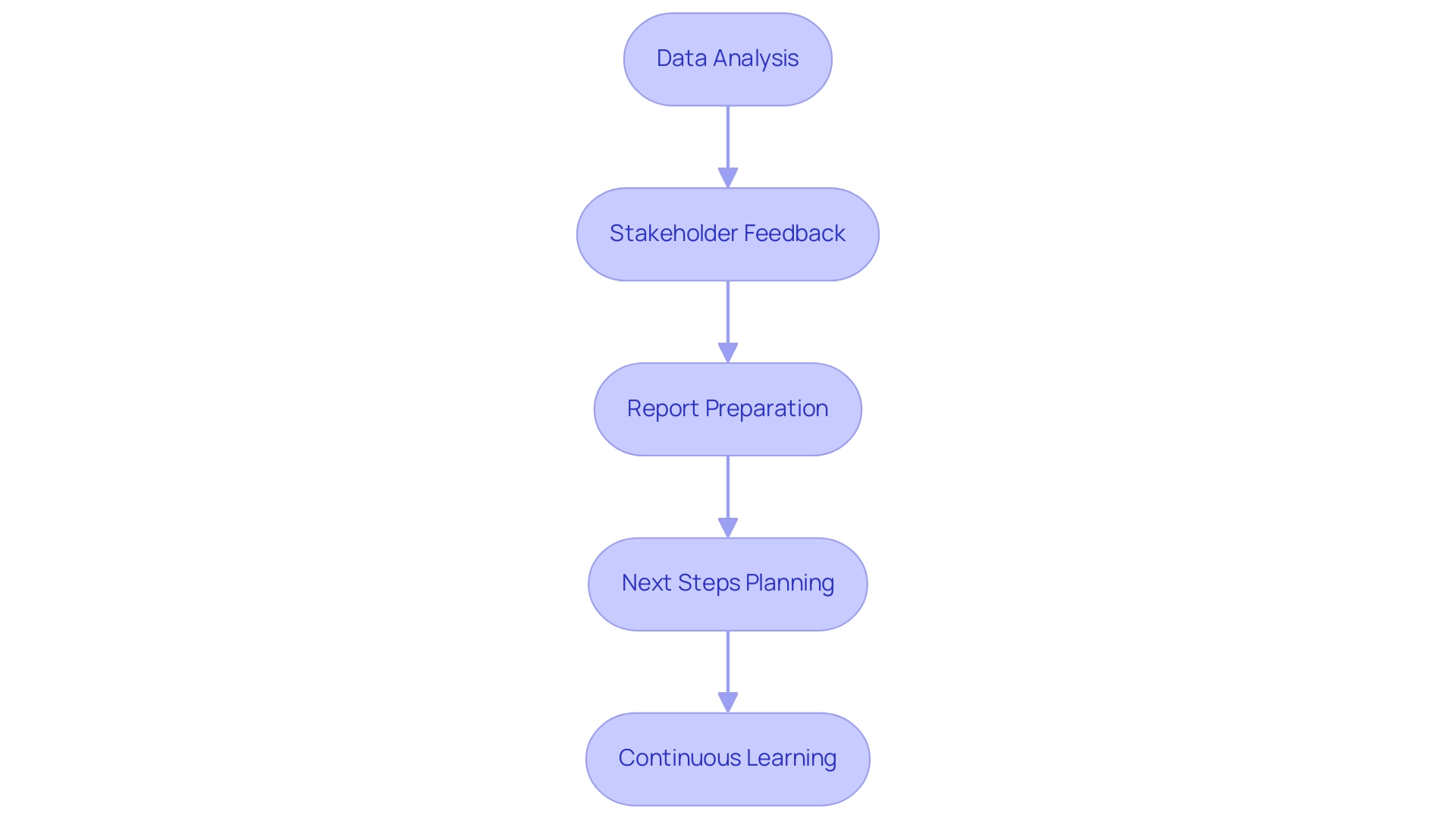
- Data Analysis: Conduct a detailed analysis of the collected data to assess the feasibility of the medical device, such as those seen in ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain or Flow-FX's first-in-human trial of the Flow-Screw device for intraosseous antibiotic delivery. Look for trends, statistical significance, and any unexpected findings, such as improvements in patient outcomes or specific metrics achieved.
- Stakeholder Feedback: Collect input from stakeholders, including investigators, regulatory bodies, and participants, to gain insights into the project's strengths and weaknesses. This feedback is crucial for navigating the complexities of conducting research in regions like Colombia.
- Report Preparation: Prepare a comprehensive report detailing the research findings, including data analysis, participant feedback, and recommendations for future inquiries. This report should reflect the compliance and oversight considerations that were addressed during the trial setup, including any compliance reviews and reporting mechanisms utilized by bioaccess®.
- Next Steps Planning: Based on the evaluation, determine whether to proceed with further development of the medical device, make modifications, or conduct additional research to address any identified gaps, ensuring alignment with the regulatory requirements outlined by your CRO, such as bioaccess®. This may include planning for trial setup and obtaining necessary import permits.
- Continuous Learning: Use the insights gained from the feasibility analysis to inform future research efforts. Consider how lessons learned can enhance study design, participant recruitment strategies, and stakeholder engagement in subsequent studies, which is critical for successful trial management in Latin America.
Conclusion
Navigating the landscape of early feasibility studies in Latin America, particularly in Peru, is essential for the successful development of medical devices. The insights gained from these studies not only assess the technical viability and safety of new technologies but also highlight the importance of understanding local regulatory frameworks set by the Ministry of Health and the National Institute of Health. By engaging with local organizations and stakeholders, Medtech companies can enhance their research efforts and tailor their approaches to meet the unique needs of diverse patient populations.
The step-by-step guide outlined emphasizes the necessity of meticulous planning and adherence to local regulations, from defining study objectives to analyzing data and preparing for future research. Collaboration with regulatory authorities, healthcare institutions, and ethics committees is crucial to ensure compliance and ethical conduct throughout the study. Addressing potential regulatory challenges, such as understanding documentation requirements and maintaining cultural sensitivity, further strengthens the feasibility of medical device trials in the region.
Ultimately, the potential for innovation in Latin America is significant, and by leveraging the insights from early feasibility studies, Medtech companies can position themselves for success. As the market continues to evolve, the insights gained from these studies will serve as a foundation for future research, ultimately leading to advancements in healthcare that are both relevant and impactful for local communities. Embracing this opportunity will not only facilitate the development of new technologies but also contribute to the overall improvement of healthcare systems across the region.
Frequently Asked Questions
Why are initial viability assessments important in healthcare product development in Latin America?
Initial viability assessments are crucial in healthcare product development in Latin America due to varying compliance frameworks and market dynamics. They help evaluate the technical performance, safety, and usability of medical devices in real-world settings.
What role does Bioaccess® play in the feasibility assessment process in Peru?
Bioaccess® provides essential services such as regulatory approval, clinical research site activation, and subject recruitment, which are critical for navigating the regulatory landscape in Peru.
What local regulations must be understood when conducting feasibility assessments in Peru?
It is vital to understand regulations set by the Peruvian Ministry of Health (MINSA) and the National Institute of Health (INS) to ensure compliance and successful project execution.
What are the steps involved in conducting early feasibility assessments for medical devices in Peru?
The steps include defining study objectives, engaging with regulatory authorities, selecting suitable study sites, developing a comprehensive research protocol, obtaining ethical approval, recruiting participants, conducting research, analyzing data, and planning next steps.
How can researchers ensure ethical conduct in their feasibility studies?
Researchers must submit their research protocol to an Institutional Review Board (IRB) or Ethics Committee for review and approval, ensuring participant rights and well-being are protected.
What challenges might researchers face when conducting feasibility assessments in Peru?
Challenges include understanding local regulations, meeting documentation requirements, ensuring cultural sensitivity, adapting to changes in regulations, and managing timelines for approval.
How can stakeholder cooperation enhance feasibility assessments in Colombia?
Engaging with regulatory bodies, healthcare institutions, experienced investigators, ethics committees, and industry partners can improve compliance, enhance recruitment, and ensure ethical standards are met.
What should researchers do after completing a feasibility analysis?
Researchers should conduct a detailed data analysis, collect stakeholder feedback, prepare a comprehensive report, plan for next steps based on findings, and apply lessons learned to future research efforts.

