Overview
The future of virtual clinical trials for medical devices is being shaped by advancements in digital technologies that enhance accessibility, cost-effectiveness, and participant engagement, while also addressing regulatory and data security challenges. The article illustrates this transformation through examples of market growth, innovative partnerships, and the integration of AI and wearable technologies, which collectively promise to improve the efficiency and inclusivity of clinical research.
Introduction
The clinical research landscape is undergoing a profound transformation with the emergence of virtual clinical trials, which are rapidly becoming a crucial alternative to traditional methodologies. This shift is driven by the need for more efficient, patient-centric approaches that leverage digital technologies, enabling participants to engage in studies from their own homes. Such accessibility not only breaks down geographical barriers but also enhances diversity in recruitment, particularly vital for Medtech companies operating in regions like Latin America, where challenges abound.
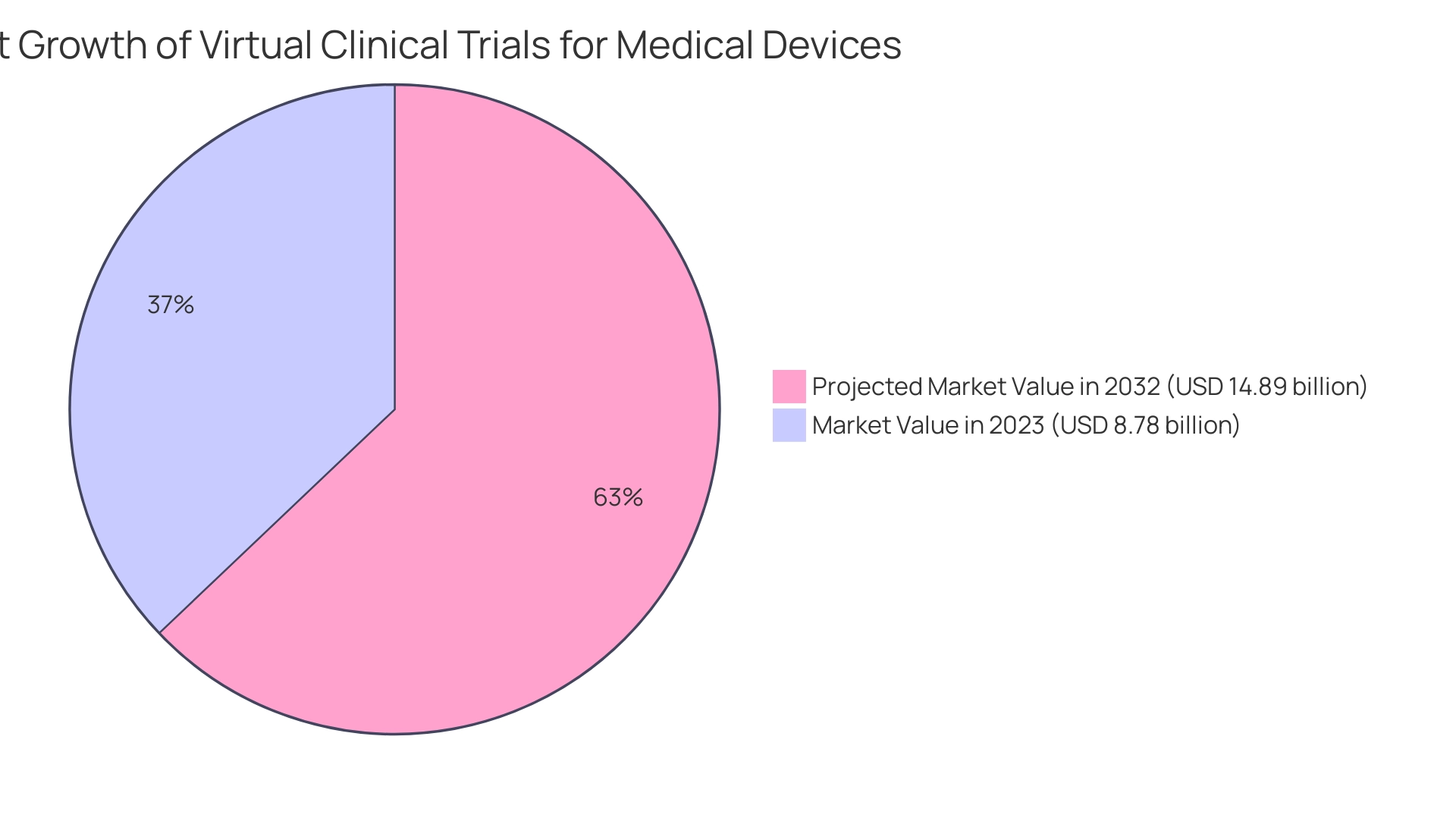
As the market for virtual trials is poised for significant growth, reaching an estimated USD 14.89 billion by 2032, the implications extend beyond research to encompass local economies, job creation, and healthcare improvements.
This article delves into the multifaceted aspects of virtual clinical trials, exploring their benefits, the role of technology, inherent challenges, and future trends that are reshaping the field of medical research.
The Shift Towards Virtual Clinical Trials: A New Era in Medical Research
The medical investigation environment is experiencing a notable transformation, indicating the future of virtual clinical trials for medical devices as online studies become an essential substitute for conventional approaches. This transformation is fueled by the necessity for more efficient and patient-centric research methodologies, which is essential for The Future of Virtual Clinical Trials for Medical Devices. Utilizing digital technologies, remote studies play a crucial role in shaping The Future of Virtual Clinical Trials for Medical Devices by enabling participants to take part in research from their own locations, thus reducing geographical barriers and improving diversity in recruitment.
This evolution is crucial for Medtech companies in Latin America, where challenges such as regulatory hurdles and fragmented resources persist. As the market for virtual medical studies, valued at USD 8.78 billion in 2023, is expected to expand to USD 14.89 billion by 2032, the future of virtual clinical trials for medical devices will significantly impact local economies, including job creation and healthcare enhancements. Recent collaborations, such as Oracle's partnership with ObvioHealth and Medable's integration of diverse data sets, illustrate the strides being made in this field.
Moreover, extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project oversight
play an essential role in tackling these challenges. The partnership among Medtech firms and regional hospitals is crucial for closing communication gaps and improving clinical study execution. Furthermore, Medable's recent funding of USD 524 million indicates a strong investment environment in digital studies.
This shift not only aligns with regulatory endorsements but also signals a new era in medical research that prioritizes accessibility, efficiency, and collaboration, ultimately transforming lives in Latin America through advanced Medtech solutions and emphasizing the future of virtual clinical trials for medical devices.
Leveraging Technology: The Role of Digital Platforms in Virtual Trials
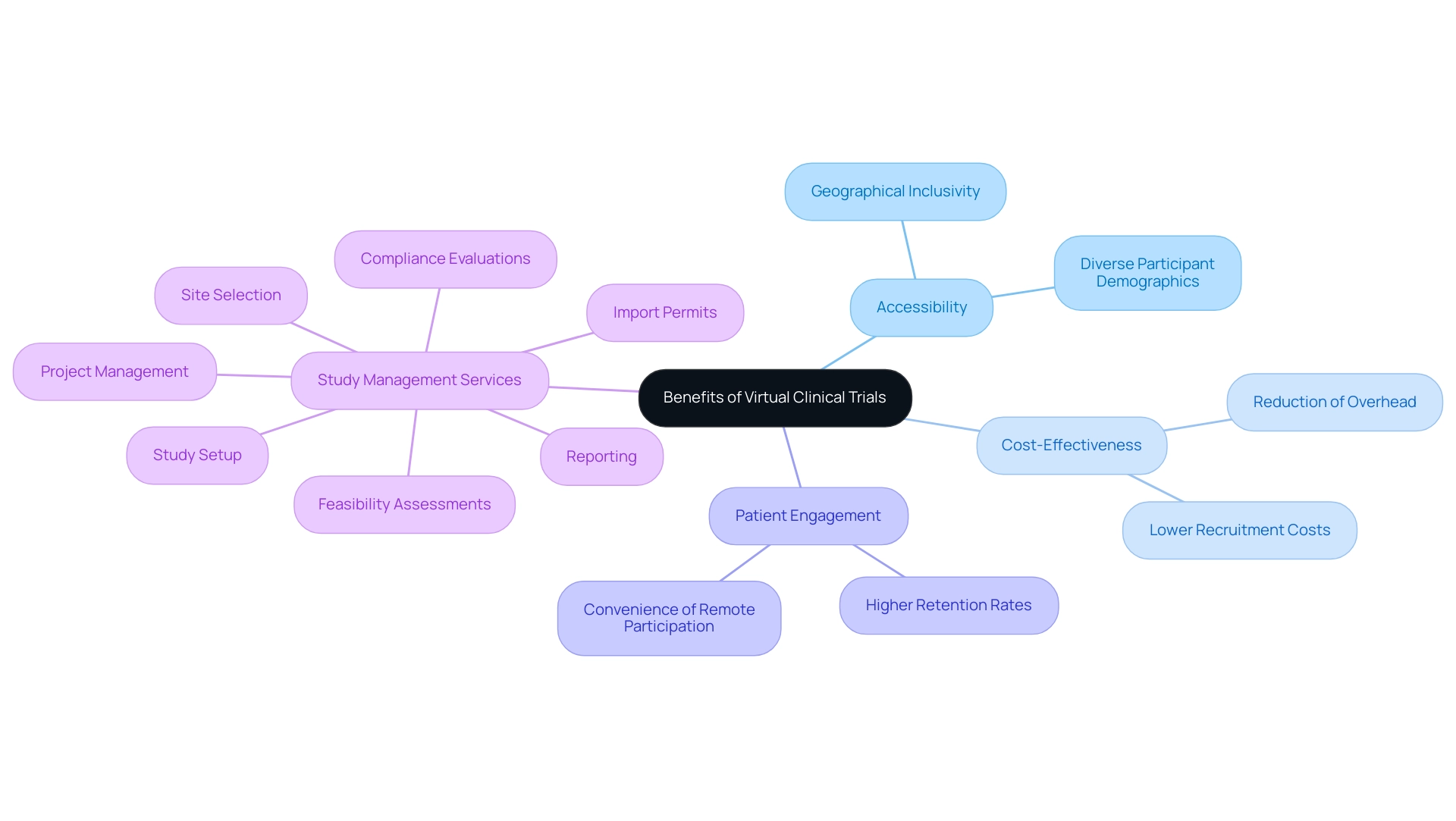
Digital platforms are transforming the environment of virtual health studies, which is vital for shaping the future of virtual clinical trials for medical devices, by serving as essential tools for data gathering, patient observation, and efficient communication. Our extensive research study management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Thorough reporting on serious and non-serious adverse events
This ensures that each study adheres to local and international standards. Technologies like telemedicine, mobile health applications, and remote monitoring devices empower researchers to gather real-time data while ensuring participant engagement from the comfort of their homes.
Notably, in 2023, 49% of smartwatch users in Europe tracked their physical activity daily, illustrating the increasing integration of technology in health management. Apple, acknowledged as the top vendor of wearable devices globally in 2022, illustrates the important role of wearable technology in improving participant engagement. Furthermore, Florence Mowlem, PhD, Vice President of Science for ObvioHealth, emphasizes this shift, stating,
'I hope this can be a turning point for the industry with regard to comparability testing.
We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.'
Additionally, federal government and industry initiatives have launched substantial investments to optimize digitized healthcare delivery, reshaping the healthcare IT landscape and increasing market share. These platforms simplify the data gathering process and significantly improve the participant experience, allowing individuals to take part in studies without the need for frequent in-person visits.
As these technologies keep advancing, their integration into medical studies will become more refined, ultimately enhancing The Future of Virtual Clinical Trials for Medical Devices by improving the overall efficiency of virtual experiments while aiding in job creation, economic development, and global cooperation in the MedTech field.
Benefits of Virtual Clinical Trials: Accessibility, Cost-Effectiveness, and Patient Engagement
Virtual medical studies offer a compelling range of advantages that increase their attractiveness for both researchers and participants. One of the primary advantages is improved accessibility, which enables a diverse range of participants to engage in studies without the barriers of travel or geographical limitations. This inclusivity is particularly crucial in expanding participant demographics and enhancing representation in clinical research.
Furthermore, these experiments are often more cost-effective compared to traditional models. Estimates indicate that the cost of bringing a new drug to market can range from $314 million to $2.8 billion, making the reduction of overhead expenses associated with physical site management and recruitment a significant advantage. The convenience of remote participation not only facilitates higher patient engagement but also contributes to improved retention rates, as individuals can partake in studies from the comfort of their own homes.
Enhancing these benefits are our extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
By addressing these critical components, we drive global health improvement through international collaboration and innovation in MedTech. Recent developments highlight this change; for example, the COVID-19 pandemic has expedited the use of remote studies, tackling many challenges encountered by conventional methods.
Based on a geographical assessment of the online healthcare research market, North America presently takes the lead due to progress in digitalization and the extensive acceptance of telemedicine. As mentioned by Laura Wood, Senior Press Manager, 'the shift towards online evaluations is transforming the field of health studies, making it essential for stakeholders to adopt these innovative methods.' Furthermore, the market for online research studies is significantly divided, with key companies such as Dassault Systemes, Medable, and IQVIA representing more than 50% of the market share.
Additionally, hybrid studies, which merge conventional and online components, tackle operational issues like participant retention and logistical limitations, enabling adaptable patient appointments and improving the overall effectiveness of medical investigations. Together, these elements are promoting a growing tendency towards digital approaches in medical research, indicating a notable transformation in how studies are carried out in the digital health and MedTech industries.
Challenges in Virtual Clinical Trials: Navigating Data Security and Regulatory Hurdles
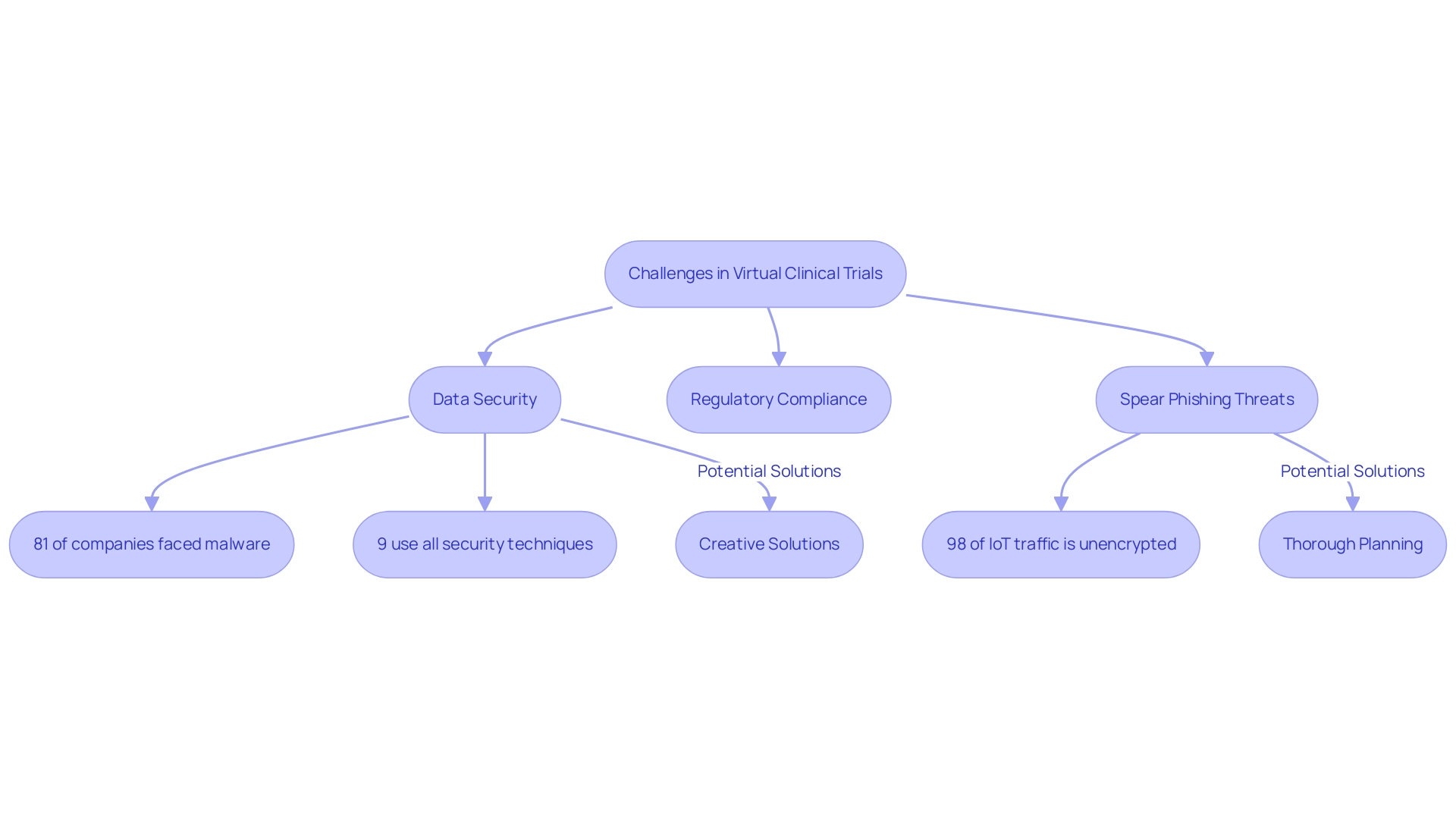
Despite the numerous benefits of online clinical studies, significant challenges remain, especially in the area of data security. The remote nature of these studies necessitates robust protective measures to safeguard sensitive patient information. Alarmingly, a recent report indicated that 81% of companies have experienced malware infections on their endpoint devices, underscoring the critical need for enhanced security protocols.
Moreover, only 9% of organizations employ all accessible techniques to safeguard against internet-based threats, underscoring the insufficiency of existing security measures in the context of online assessments. As 57% of the workforce now operates remotely for at least two days a week, the implications for data security become even more pronounced. Adhering to regulatory standards introduces an additional layer of complexity, as governing bodies are continuously refining their guidelines for online methodologies.
This evolving landscape means that researchers must adeptly navigate regulatory hurdles while ensuring the integrity of their studies. Additionally, spear phishing is employed by 96% of threat actors, representing a significant threat that must be addressed. The rapid adoption of IoT devices exemplifies these challenges, with 93% of executives acknowledging the benefits of IoT; however, 98% of IoT traffic remains unencrypted, raising substantial concerns about potential breaches and attacks.
The absence of protective measures in IoT devices can result in serious repercussions for data safety in online research. Creative solutions and thorough planning are essential to address these challenges efficiently, paving the way for the successful execution of online assessments, which are crucial to the future of virtual clinical trials for medical devices.
Future Trends in Virtual Clinical Trials: Innovations and Evolving Practices
The terrain of medical research is experiencing considerable change, especially in the area of virtual studies. Innovations in artificial intelligence (AI) and machine learning are poised to transform data analysis and participant recruitment, thereby improving efficiency and precision of the study. For example, Walgreens signed 15 agreements for research recruitment in Q3 of 2023, up from 8 agreements in Q2 of 2023, demonstrating the growing momentum in the sector and the increasing dependence on retail pharmacies for participant recruitment.
These technologies can sift through vast amounts of data to identify suitable candidates and anticipate potential barriers, ultimately contributing to a more targeted recruitment process. Additionally, the integration of wearable technologies enables continuous health monitoring, providing rich datasets that offer deeper insights into patient outcomes and engagement levels. As the FDA's new patient-focused drug development guidelines stress the significance of patient involvement, integrating input from advocacy organizations becomes essential in influencing these studies.
This engagement can lead to improved enrollment and retention rates, potentially accelerating product launch timelines by up to 2.5 years. A Senior Manager at Advanced Technology Corporation noted, 'I have reviewed the report and find it very useful and comprehensive,' underscoring the value of these advancements. Moreover, with bioaccess®’s proficiency in overseeing diverse research studies, including Early-Feasibility, First-In-Human, and Post-Market Follow-Up studies, the company guarantees adherence and effectiveness throughout the process.
Their extensive services also encompass:
- Feasibility studies
- Selection of study locations and lead investigators
- Compliance assessments
- Setup
- Import permits
- Project management
These are essential to the success of medical experiments. As regulatory frameworks develop to support these advancements, we can expect a more efficient approval process for online studies. Such developments promise to facilitate rapid advancements in medical device research and clinical outcomes, positioning The Future of Virtual Clinical Trials for Medical Devices as a pivotal element in the evolution of clinical research, while also contributing to local economies through job creation and healthcare improvements.
Conclusion
The emergence of virtual clinical trials marks a significant milestone in the evolution of medical research, driven by the demand for more efficient and patient-centric methodologies. By leveraging digital technologies, these trials not only enhance accessibility for participants but also foster greater diversity in recruitment, particularly in regions facing unique challenges, such as Latin America. The projected growth of the virtual trial market to USD 14.89 billion by 2032 highlights the transformative potential of this approach, with implications extending beyond research to bolster local economies and healthcare systems.
Despite the numerous advantages, including cost-effectiveness and improved patient engagement, challenges such as data security and regulatory compliance remain critical concerns that must be addressed. As the landscape continues to evolve, innovative solutions and robust security measures will be essential to navigate these complexities and ensure the integrity of virtual trials. Moreover, the integration of advanced technologies such as artificial intelligence and wearable devices promises to further enhance the efficiency of participant recruitment and data analysis.
In conclusion, the shift towards virtual clinical trials represents a new era in medical research, emphasizing the importance of accessibility, collaboration, and innovation. As stakeholders embrace these methodologies, the potential for improved clinical outcomes and economic benefits becomes increasingly evident. The future of clinical research is being reshaped by these advancements, which not only prioritize patient needs but also contribute to the overall improvement of healthcare delivery on a global scale.
Frequently Asked Questions
What is transforming the medical investigation environment for clinical trials?
The medical investigation environment is undergoing a transformation with the rise of virtual clinical trials for medical devices, driven by the need for more efficient and patient-centric research methodologies.
How do virtual clinical trials improve participation?
Virtual clinical trials enable participants to engage in research from their own locations, reducing geographical barriers and enhancing diversity in recruitment.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America encounter challenges such as regulatory hurdles and fragmented resources, which impact the execution of clinical studies.
What is the projected market value for virtual medical studies?
The market for virtual medical studies is valued at USD 8.78 billion in 2023 and is expected to grow to USD 14.89 billion by 2032.
How do collaborations contribute to virtual clinical trials?
Collaborations, such as Oracle's partnership with ObvioHealth and Medable's integration of diverse data sets, are crucial for advancing the field of virtual clinical trials.
What services are included in extensive research study management?
The services include feasibility assessments, site selection, compliance evaluations, project oversight, and thorough reporting on adverse events.
What technologies are used in virtual clinical trials?
Technologies such as telemedicine, mobile health applications, and remote monitoring devices are utilized to gather real-time data and enhance participant engagement.
How prevalent is technology use in health management?
In 2023, 49% of smartwatch users in Europe tracked their physical activity daily, highlighting the integration of technology in health management.
What is the significance of wearable technology in clinical trials?
Wearable technology, exemplified by Apple as a leading vendor, plays a crucial role in improving participant engagement in clinical studies.
What impact do federal government and industry initiatives have on healthcare delivery?
These initiatives have launched significant investments to optimize digitized healthcare delivery, reshaping the healthcare IT landscape and increasing market share.
How do digital platforms enhance the participant experience in studies?
Digital platforms simplify the data gathering process and improve the participant experience by allowing involvement in studies without frequent in-person visits.
What is the future outlook for virtual clinical trials?
The ongoing advancement of technologies will refine their integration into medical studies, enhancing efficiency and contributing to job creation and economic development in the MedTech field.




