Overview
The article emphasizes the critical nature of effective post-market study strategies in Bolivia, which are essential for the success of medical technology (Medtech) companies. It underscores the necessity of tailored clinical research services, regulatory compliance, and stakeholder engagement, while also highlighting the integration of technology to bolster the effectiveness and reliability of evaluations following market releases.
Introduction
In the dynamic landscape of the Medtech industry, navigating the complexities of post-market studies is crucial for driving innovation and ensuring patient safety. With the rise of advanced technologies and regulatory challenges, companies must adopt comprehensive strategies that encompass everything from regulatory compliance to stakeholder engagement.
Bioaccess® emerges as a pivotal partner, offering tailored clinical research services that not only expedite the post-market evaluation process but also enhance the usability and effectiveness of medical devices. By leveraging over 20 years of expertise, Bioaccess® addresses the unique hurdles faced by Medtech startups, particularly in Latin America, where diverse populations and urbanization present both opportunities and challenges.
This article delves into the essential elements of successful post-market studies, highlighting best practices and innovative approaches that empower Medtech companies to thrive in an increasingly competitive environment.
bioaccess®: Accelerate Post-Market Studies with Expert CRO Services
bioaccess® distinguishes itself by delivering tailored clinical research services that expedite after-sales evaluations for medical technology firms. With over 15 years of specialized experience, bioaccess® possesses a keen understanding of the unique challenges faced by medical technology startups, including regulatory hurdles and recruitment issues. Latin America emerges as a compelling region for conducting Medtech early feasibility assessments, attributed to its ethnically diverse population and notable urbanization, making it an ideal location for evaluations post-market release.
Their cost-effective solutions not only ensure compliance with local regulations but also guarantee high-quality outcomes. By leveraging their extensive knowledge of the regulatory landscape and conducting comprehensive evaluations through post-market study strategies in Bolivia following market launch, bioaccess® positions itself as an essential partner for firms aiming to bolster their market presence in Bolivia and beyond. This is exemplified by their collaboration with Avantec Vascular for a first-in-human clinical trial, where bioaccess® facilitated the selection of a principal investigator, managed regulatory submissions, and oversaw trial setup and ethics committee approvals.
The organization’s commitment to patient safety and ethical practices is paramount, as critical measures are enacted to ensure the safety of participants in clinical trials. This dedication enhances the reliability of their research, establishing bioaccess® as a trusted option for medical technology innovators navigating the complexities of post-market study strategies in Bolivia. Furthermore, the integration of comprehensive reporting, as illustrated in a case example involving the use of research documents, demonstrates how bioaccess® contributes to medical understanding through meticulous data collection and analysis. As Adrian Ebner, a pioneer in MedTech clinical research in Paraguay, notes, his involvement in over 70 first-in-human trials underscores the significance of expertise in achieving successful outcomes.
Moreover, the engagement of 179 community hospitals and 279 communities through COUCH Health's initiatives highlights the critical role of community involvement in after-market evaluations, further solidifying bioaccess®'s status as a leader in the medical technology sector.
Ensure Regulatory Compliance: Navigate Bolivia's Medtech Regulations
To effectively implement post-market study strategies in Bolivia, Medtech companies must adeptly navigate the regulatory framework established by the National Service of Sanitary Control (SENASAG) and the Ministry of Health. This process involves securing necessary approvals and ensuring compliance with local laws. Staying informed about regulatory changes is imperative; recent updates in Bolivia's medical device regulations underscore the evolving nature of compliance requirements.
Engaging with local experts, such as Katherine Ruiz, a recognized authority in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can provide invaluable insights into these complexities, offering guidance on specific regulatory pathways and best practices for compliance. Regular training sessions and updates on regulatory requirements are essential for maintaining compliance and preventing costly delays.
Notably, only 11% of companies currently rate their supply chain management as 'excellent,' highlighting the need for robust strategies in regulatory navigation, as effective supply chain management is closely linked to compliance success. As Tiffany Ryder aptly states, "Find your harshest critic, not your biggest fan. That’s where real product improvement happens," emphasizing the importance of addressing skepticism in regulatory compliance discussions.
Furthermore, the complexities of product adoption, as explored in the case analysis 'Product Adoption Challenges in Healthcare,' highlight the significance of workflow integration, which is pertinent to the regulatory navigation process. By concentrating on these compliance strategies and leveraging the extensive clinical trial management services provided by bioaccess®, Medtech companies can enhance their operational efficiency and implement effective post-market study strategies in Bolivia to promote successful studies following market release.
Incorporate Human Factors: Enhance Usability in Post-Market Surveillance
Incorporating human factors into post-market study strategies in Bolivia is crucial for enhancing the usability of medical products. This process requires a thorough understanding of user interactions with devices, enabling manufacturers to pinpoint areas for enhancement. Usability assessments, backed by user input, empower manufacturers to implement necessary changes, ultimately improving patient outcomes.
Collaborating with human factors specialists during both the design and assessment stages significantly enhances the effectiveness of post-market study strategies in Bolivia. For instance, bioaccess® boasts over 20 years of medical technology experience, focusing on diverse research areas including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-market study strategies in Bolivia
This ensures that usability remains a primary emphasis. A case study titled 'Choosing the Right Human Factors Partner' underscores the importance of human factors testing in medical product development, highlighting that neglecting this aspect can lead to increased risks for users and patients, as well as potential regulatory challenges.
By partnering with specialists in human-centered design, such as those at bioaccess®, medical technology firms can mitigate risks and enhance product acceptance in the market. Furthermore, comprehending the regulatory landscape, including the role of INVIMA as a Level 4 health authority, is essential for ensuring compliance and successful market entry. By prioritizing human-centered design, medical technology firms can guarantee their products meet user requirements, resulting in safer and more effective healthcare solutions.
Manage Cybersecurity Risks: Safeguard Medical Devices Post-Market
As interconnected medical instruments proliferate, effectively managing cybersecurity risks has emerged as a crucial aspect of post-market surveillance. Medtech firms must prioritize strong cybersecurity practices to protect sensitive patient information and uphold equipment integrity. The substantial rise in cybersecurity threats, resulting from the growth in linked medical equipment, necessitates that organizations implement comprehensive strategies. Key strategies include:
- Conducting regular security assessments to identify vulnerabilities
- Promptly updating software to address potential threats
- Providing comprehensive training for staff on cybersecurity best practices
As noted by the Director of Digital, 'Training on the platform was second to none, with constant feedback from MOBSTR and the technical staff about ongoing usage and how to get the best out of the service.' Collaborating with cybersecurity experts is essential for developing thorough risk management strategies that can effectively mitigate risks. A case study involving a nonprofit healthcare organization illustrates this point; by transitioning to an outsourced managed IT services partner, they improved efficiency and service delivery, successfully safeguarding their operations. By adopting these measures, organizations can enhance the safety and dependability of their medical equipment, ultimately promoting trust and adherence in the evolving landscape of healthcare technology. Bioaccess® offers a unique value proposition for companies in the Medtech industry, ensuring they are well-equipped to navigate these challenges.
Implement Robust Data Collection: Monitor Device Performance Effectively
Implementing robust data collection methods is essential for effectively monitoring medical instrument performance as part of the post-market study strategies in Bolivia. Organizations must establish clear protocols for collecting and examining information related to:
- Equipment usage
- Adverse events
- Patient outcomes
The integration of electronic data capture systems significantly enhances this process, facilitating real-time monitoring and analysis. Regular reviews of the collected data are crucial for identifying trends and potential issues, allowing for timely interventions and ensuring adherence to evolving regulatory requirements. Notably, the recent changes in regulatory frameworks, such as the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), underscore the necessity for thorough data collection and proactive monitoring of performance and safety throughout the lifecycle of medical instruments, which is essential for effective post-market study strategies in Bolivia.
This proactive strategy not only safeguards user safety but also assists manufacturers in maintaining compliance and enhancing equipment efficacy. As Dr. Ma from Amgen Inc. emphasizes, 'Effective data collection methods are crucial for understanding the long-term effects of medical instruments on patient health.'
Moreover, bioaccess provides extensive clinical trial management services, encompassing:
- Feasibility studies
- Site selection
- Compliance assessments
- Trial organization
- Import permits
- Project oversight
- Reporting
These services are essential for ensuring that medical instruments remain effective and safe for users throughout their lifecycle. Furthermore, the post-marketing data analysis of the diabetes medication metformin illustrates how efficient data gathering can uncover considerable advantages, such as weight management and decreased cardiovascular risks, ultimately enhancing the value of medical instruments in patient care.
Engage Stakeholders: Foster Collaboration in Post-Market Evaluations
Engaging stakeholders is essential for the success of post-market study strategies in Bolivia within the Medtech sector, particularly in Latin America. Actively involving healthcare providers, patients, and regulatory bodies in the evaluation process allows for the collection of diverse perspectives and insights. This collaboration not only enhances the quality of feedback regarding device performance and usability but also informs necessary improvements. A recent investigation funded by the National Institutes of Health demonstrated the validity and cost efficiency of crowdsourcing as a resource for evaluators, underscoring its effectiveness in broadening stakeholder engagement in health-related research. This funding supports the notion that investing in diverse stakeholder involvement can lead to more comprehensive evaluations.
Consistent interaction with stakeholders cultivates trust and guarantees alignment on the objectives and expectations of post-market study strategies in Bolivia. The significance of teamwork is further underscored by healthcare professionals who stress the value of patient feedback in research. One partner remarked, "This research has opened my eyes personally to how crucial my contribution is. I did not know that until I got involved with this study, how important a patient’s voice is in studies." This insight illustrates how understanding the patient's voice can significantly influence outcomes.
By employing strong stakeholder engagement strategies, Medtech firms such as bioaccess can improve their post-market study strategies in Bolivia after market release. With over 20 years of experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), bioaccess® provides the expertise needed to navigate these complex evaluations effectively. To maximize the impact of these strategies, consider establishing regular feedback loops with stakeholders to ensure their insights are continuously integrated into the evaluation process. This approach not only leads to better device performance but also increases patient satisfaction. Moreover, comprehending the regulatory framework, including the function of INVIMA as a Level 4 health authority in Colombia, is essential for ensuring compliance and improving the efficacy of post-market study strategies in Bolivia after market release.
Provide Ongoing Training: Equip Teams for Post-Market Success
Continuous training for teams engaged in after-market evaluations is essential for achieving success in the Medtech industry, particularly concerning the comprehensive clinical trial management services offered by bioaccess®. Implementing regular training sessions ensures that staff remain informed about the latest regulatory changes, best practices, and technological advancements. This commitment to ongoing learning not only enhances team proficiency but also cultivates an environment of innovation and advancement within the organization.
Encouraging team members to participate in workshops and conferences significantly enriches their knowledge and skills, which leads to improved outcomes in post-market study strategies in Bolivia. For example, a case analysis on classifying changes in the medical device lifecycle highlights the critical need to evaluate modifications based on their impact and necessity. By effectively categorizing changes into premarket and postmarket stages, organizations can streamline their regulatory submissions and certifications, thereby mitigating risks associated with cumulative changes.
Moreover, statistics indicate that the effectiveness of training in clinical research teams correlates directly with improved research outcomes. Organizations that implement tailored training programs experience a remarkable 30% increase in compliance with regulatory standards. Training experts advocate for initiatives that ensure medical technology personnel are well-equipped to navigate the complexities of post-market evaluations, particularly through effective post-market study strategies in Bolivia.
As Jan Bogaerts, Director of Methodology Direction at EORTC Headquarters, notes, 'Continuous education is essential for adapting to the evolving landscape of medical equipment regulations.' By prioritizing continuous training, healthcare technology firms can enhance their operational effectiveness and ultimately contribute to the advancement of medical equipment in the market, leveraging the expertise of bioaccess® in managing various research types.
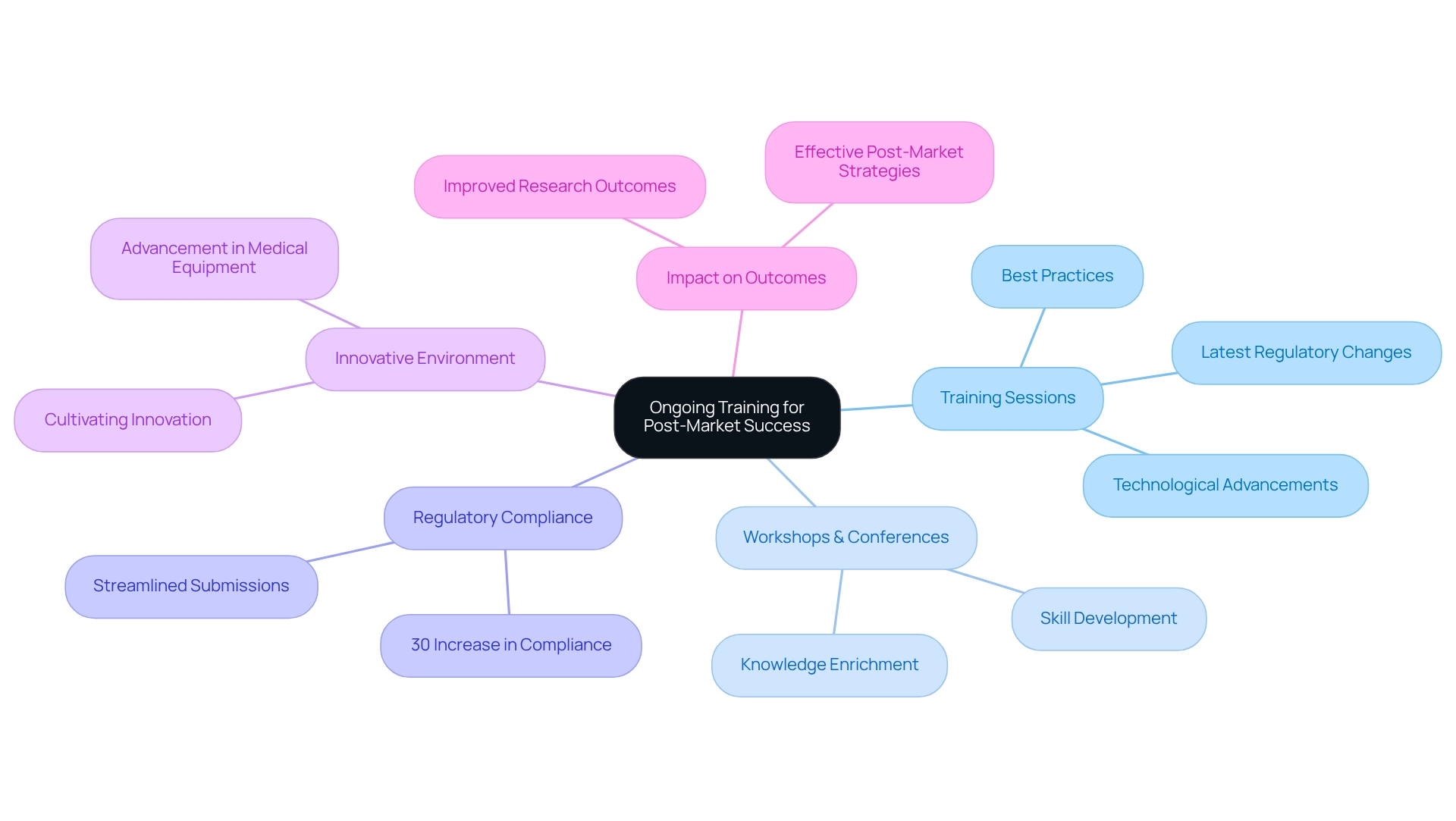
Leverage Technology: Enhance Efficiency in Post-Market Studies
Utilizing technology is essential for enhancing the effectiveness of after-sales research in the medical technology field. Companies must prioritize the adoption of advanced data management systems, electronic data capture tools, and analytics platforms to streamline their operations. With over 20 years of expertise in the medical technology sector, bioaccess® recognizes that these technologies facilitate real-time data gathering, improve data precision, and support efficient evaluation of device performance. By incorporating these technological solutions into their after-sales strategies, Medtech companies can reduce operational expenses and enhance their overall research capabilities, leading to more successful outcomes in clinical trials.
bioaccess® specializes in managing various research projects, including:
- Early-Feasibility Assessments (EFS)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Assessments (PMCF)
Furthermore, bioaccess® provides comprehensive clinical trial management services, such as:
- Feasibility assessments
- Compliance evaluations
- Project oversight
These services significantly enhance the efficacy of after-market evaluations. This emphasis on technology and expertise positions bioaccess® as a leader in enabling successful after-market research processes.
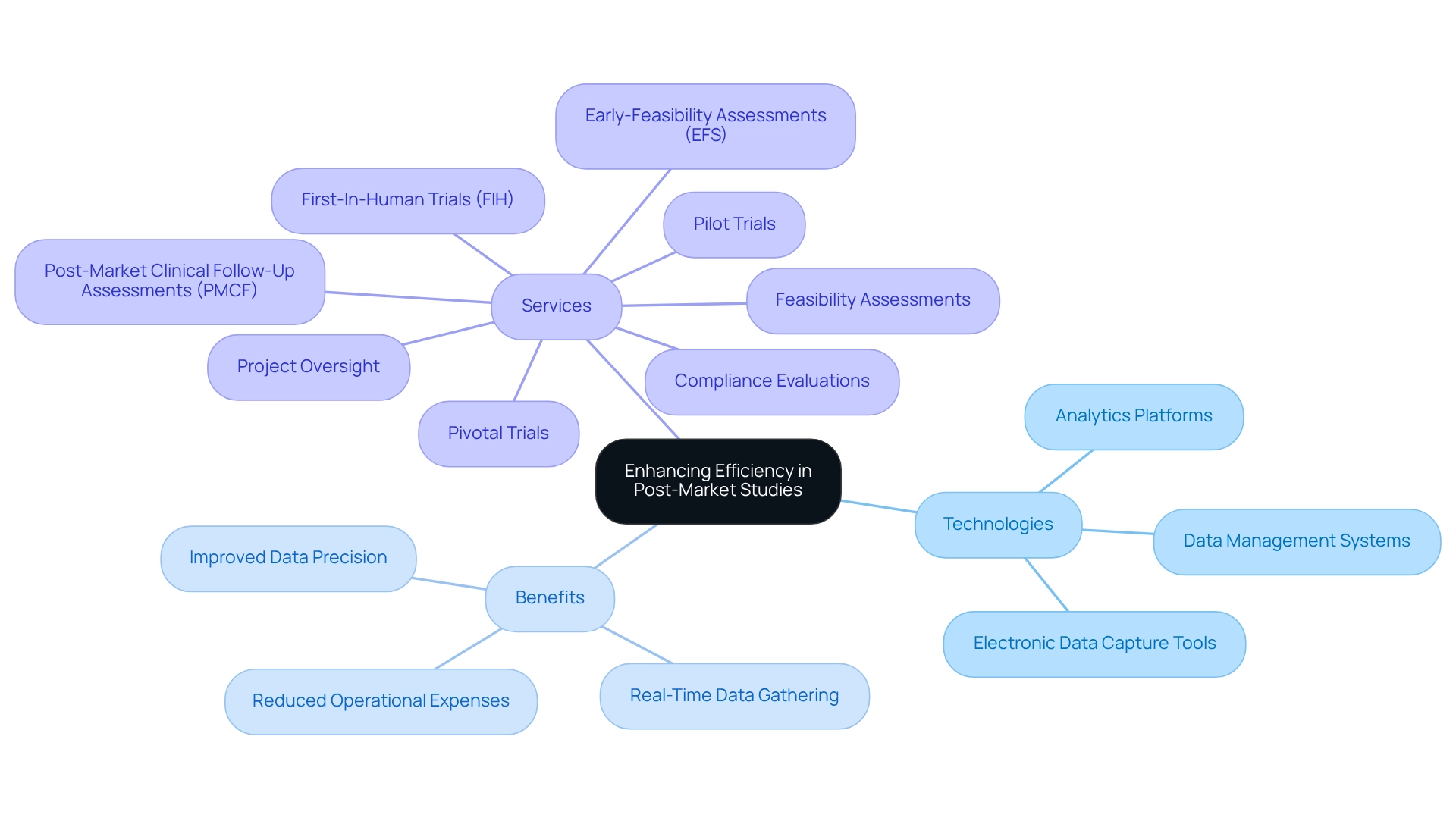
Conduct Regular Audits: Ensure Compliance and Effectiveness
Carrying out regular audits is essential for ensuring compliance and evaluating the effectiveness of after-market research within the medical technology sector. A systematic audit schedule empowers companies to meticulously review their processes, data collection methods, and adherence to regulatory requirements, including:
- Good Clinical Practice (GCP)
- Good Manufacturing Practice (GMP)
- Good Laboratory Practice (GLP)
These audits not only identify potential gaps but also highlight areas for improvement, ensuring alignment with industry standards. Engaging third-party auditors enhances the credibility of the audit process, providing an objective perspective that is invaluable for compliance. In 2025, the emphasis on audit quality is underscored by findings indicating that 53 percent of stakeholders regard audit quality indicators as crucial. Effective audits become increasingly critical as the MedTech industry navigates profitability challenges and evolving revenue streams, such as direct-to-consumer offerings.
bioaccess® offers extensive clinical trial management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial preparation
- Ethics committee approvals
- Import permits
- Project management
- Reporting
These services are integral to ensuring that audits are thorough and effective. Furthermore, bioaccess® is committed to data protection and transparent grievance procedures, thereby reinforcing client trust and ensuring that any concerns are addressed in compliance with applicable laws.
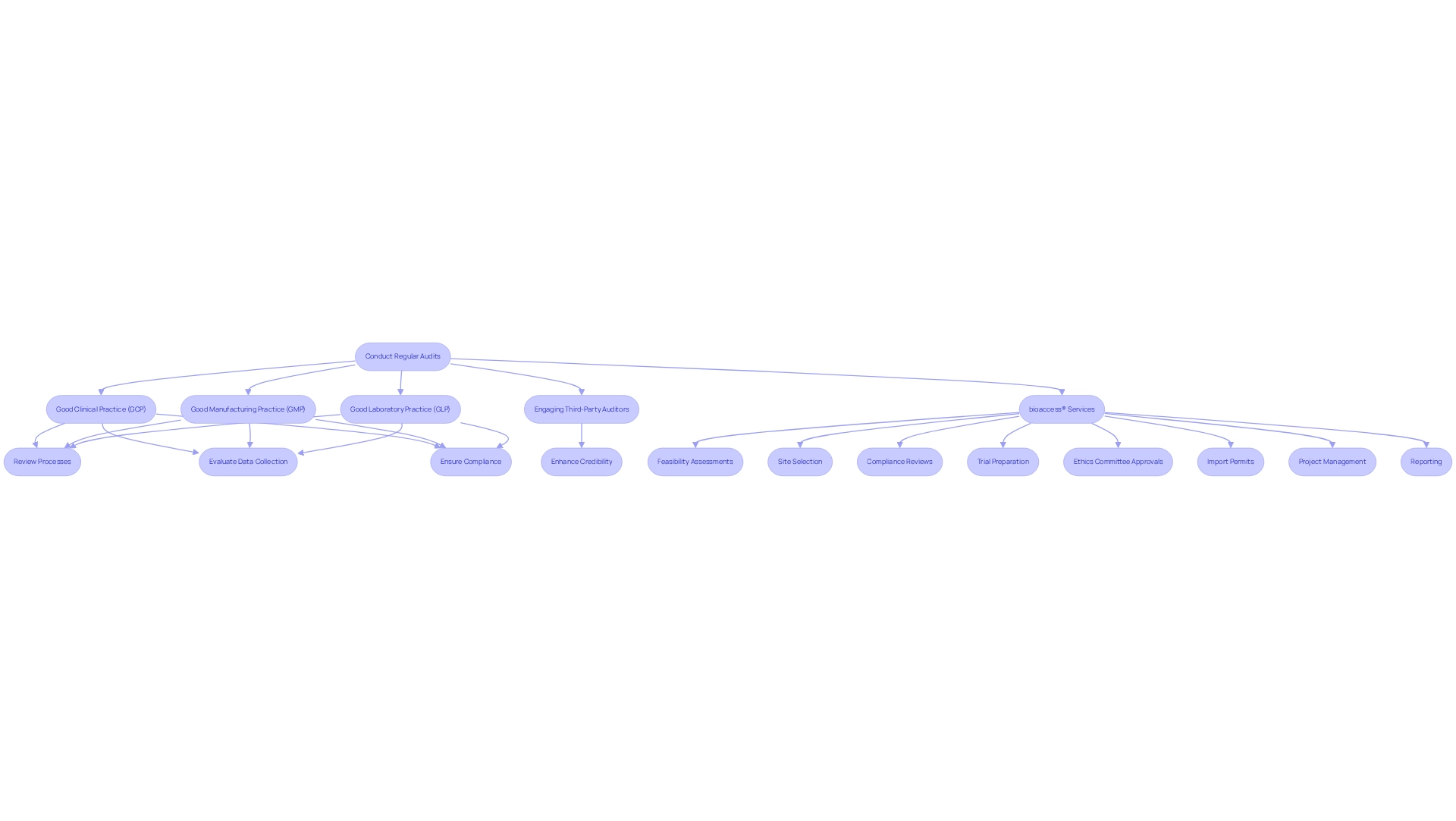
Develop a Communication Strategy: Share Insights from Post-Market Studies
A robust communication plan is essential for effectively conveying insights from after-market research to stakeholders in the Medtech industry. Companies should start by:
- Defining clear objectives for their communication efforts
- Identifying key messages
- Pinpointing target audiences
Utilizing a diverse mix of channels—such as newsletters, webinars, and social media—can significantly enhance the dissemination of findings. For example, Dr. Jorge Hernando Ulloa's presentation of one-year first-in-human VenoValve® data at the Charing Cross International Symposium illustrates how sharing advancements in vascular medicine can engage stakeholders and foster interest in new technologies. Research shows that effective stakeholder communication can reduce the comfort gap regarding new technologies, with notable variations across regions, such as a 24 percentage point gap in the US compared to 10 percentage points in Mexico.
Regular updates on research outcomes, alongside improvements made in response to stakeholder feedback, cultivate trust and involvement. As Jennifer Rymer, MD, emphasizes, "As we explore new targets within our old hypotheses, it's essential to figure out how to implement current therapies and encourage patients to adhere to what we currently have available." This proactive strategy not only strengthens relationships with stakeholders but also lays the groundwork for the success of post-market study strategies in Bolivia.
Furthermore, the impact of medical technology clinical research on local economies—encompassing job creation, economic growth, and healthcare enhancement—highlights the importance of global cooperation in advancing medical technology. By prioritizing effective communication, technology firms such as bioaccess can ensure that their findings are not only disseminated but also valued, ultimately leading to positive research outcomes. Additionally, referencing successful case studies on communication strategies can provide practical examples that further illustrate the significance of effective stakeholder engagement in the Medtech industry.
Conclusion
Successful post-market studies are essential for Medtech companies striving to innovate while ensuring patient safety. This article has examined critical strategies for navigating the complexities of post-market evaluations, emphasizing the vital roles of regulatory compliance, stakeholder engagement, and the integration of human factors.
By leveraging expert services like those provided by Bioaccess®, companies can effectively tackle the unique challenges encountered in diverse markets, particularly in Latin America. Engaging stakeholders, including healthcare providers and patients, is crucial for enhancing device usability and performance. Furthermore, incorporating robust data collection methods and conducting regular audits guarantees that medical devices not only comply with regulatory standards but also uphold high efficacy and safety throughout their lifecycle.
As the Medtech industry evolves, the integration of technology and ongoing training will be pivotal in fostering innovation and operational efficiency. By prioritizing these strategies, Medtech companies can meet the demands of regulatory bodies while significantly improving patient outcomes, thereby establishing a solid foundation for future advancements in healthcare technology.
In conclusion, the journey toward successful post-market studies is multifaceted, necessitating a commitment to collaboration, continuous improvement, and adherence to best practices. Embracing these principles will empower Medtech innovators to excel in an increasingly competitive landscape, ultimately enhancing the quality of care delivered to patients worldwide.
Frequently Asked Questions
What services does bioaccess® provide for medical technology firms?
bioaccess® offers tailored clinical research services that expedite after-sales evaluations for medical technology firms, helping them navigate regulatory hurdles and recruitment issues.
Why is Latin America considered a good region for early feasibility assessments in Medtech?
Latin America is appealing for Medtech early feasibility assessments due to its ethnically diverse population and significant urbanization, making it an ideal location for post-market evaluations.
How does bioaccess® ensure compliance with local regulations?
bioaccess® provides cost-effective solutions that ensure compliance with local regulations while delivering high-quality outcomes through its extensive knowledge of the regulatory landscape.
Can you provide an example of bioaccess®'s collaboration in clinical trials?
bioaccess® collaborated with Avantec Vascular for a first-in-human clinical trial, where it facilitated the selection of a principal investigator, managed regulatory submissions, and oversaw trial setup and ethics committee approvals.
What is bioaccess®'s commitment to patient safety?
bioaccess® prioritizes patient safety and ethical practices by implementing critical measures to ensure the safety of participants in clinical trials, which enhances the reliability of their research.
How does bioaccess® contribute to medical understanding?
bioaccess® contributes to medical understanding through meticulous data collection and analysis, as demonstrated in their comprehensive reporting and case examples.
What role does community involvement play in bioaccess®'s evaluations?
Community involvement is critical in after-market evaluations, as evidenced by the engagement of 179 community hospitals and 279 communities through COUCH Health's initiatives.
What are the key regulatory considerations for Medtech companies in Bolivia?
Medtech companies must navigate the regulatory framework established by the National Service of Sanitary Control (SENASAG) and the Ministry of Health, securing necessary approvals and ensuring compliance with local laws.
Why is staying informed about regulatory changes important for Medtech companies?
Staying informed is crucial due to the evolving nature of compliance requirements, as recent updates in Bolivia's medical device regulations highlight the need for ongoing awareness and adaptation.
How can companies enhance their operational efficiency in post-market studies?
By concentrating on compliance strategies and leveraging clinical trial management services, Medtech companies can enhance their operational efficiency and implement effective post-market study strategies.
Why is incorporating human factors into post-market study strategies important?
Understanding user interactions with devices enhances usability, allowing manufacturers to implement necessary changes that ultimately improve patient outcomes.
What areas of research does bioaccess® focus on in medical technology?
bioaccess® focuses on Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and post-market study strategies in Bolivia.
What is the significance of human factors testing in medical product development?
Neglecting human factors can lead to increased risks for users and patients, as well as potential regulatory challenges, making it essential for successful product development.
How does bioaccess® help mitigate risks in medical technology?
By partnering with specialists in human-centered design, bioaccess® helps medical technology firms mitigate risks and enhance product acceptance in the market.




