Introduction
Navigating the intricate regulatory landscape for medical device trials in Chile presents both challenges and opportunities for researchers.
With the guidelines set forth by the Chilean Ministry of Health and the National Agency for Medicines and Food, a thorough understanding of the requirements is essential for successful trial execution.
This article delves into the critical steps necessary for compliance, highlights the advantages of conducting research in Chile, and emphasizes the importance of establishing local collaborations.
By leveraging the expertise of specialized service providers, researchers can not only streamline the trial process but also contribute to the enhancement of healthcare outcomes in the region.
As the demand for innovative medical solutions grows, understanding this landscape becomes increasingly vital for the advancement of clinical research.
Navigating the Regulatory Landscape for Medical Device Trials in Chile
Navigating the regulatory landscape for Medical Device Research Chile requires a comprehensive understanding of the guidelines established by the Chilean Ministry of Health (MINSAL) and the National Agency for Medicines and Food (ANAMED). To facilitate this process effectively, consider the following essential steps:
-
Understand the Legal Framework: Begin by reviewing the Medical Devices Law and accompanying regulations that govern medical research in Chile.
Understanding these legal stipulations is essential for compliance and successful execution.
-
Prepare Required Documentation: Assemble all necessary documentation, including the clinical research protocol, informed consent forms, and any prior approvals from ethics committees. Ensuring these documents are meticulously prepared will streamline the review process.
-
Submit for Approval: Submit your application to MINSAL, which will entail a comprehensive evaluation of your study design, patient safety measures, and data management plans. It's important to note that 80% of the Chilean population currently has access to primary care benefits, yet only 50% utilize these services annually, highlighting the necessity for well-structured studies that can enhance healthcare delivery. The execution of these tests is anticipated to decrease unnecessary emergency room visits and hospitalizations, further highlighting their significance in the healthcare environment.
-
Engage with Ethics Committees: Collaborate with local ethics committees to secure their approval. This engagement is vital to uphold ethical standards and ensure the protection of participant rights throughout the process.
-
Stay Updated: Continuously monitor for any updates regarding regulations or modifications in the approval process. This vigilance is essential for maintaining compliance as regulatory landscapes can evolve rapidly.
By adhering to these steps, researchers can navigate the regulatory environment effectively, laying a solid foundation for their Medical Device Research Chile. Additionally, bioaccess® provides accelerated study services in Latin America, including feasibility studies, compliance reviews, project management, and reporting, leveraging over 20 years of expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies. This comprehensive support not only aids in regulatory compliance but also contributes to job creation, economic growth, and healthcare improvement in local economies.
As Issam Abousleiman, the World Bank director for Bolivia, Chile, Ecuador, and Peru, stated, 'Primary health care has enabled Chile to take important steps...' This highlights the essential role that medical studies play in advancing healthcare solutions and preparing for emerging health challenges. Additionally, client testimonials emphasize the positive effects of our services on the efficiency and effectiveness of studies in the region.
Benefits of Conducting Medical Device Research in Chile
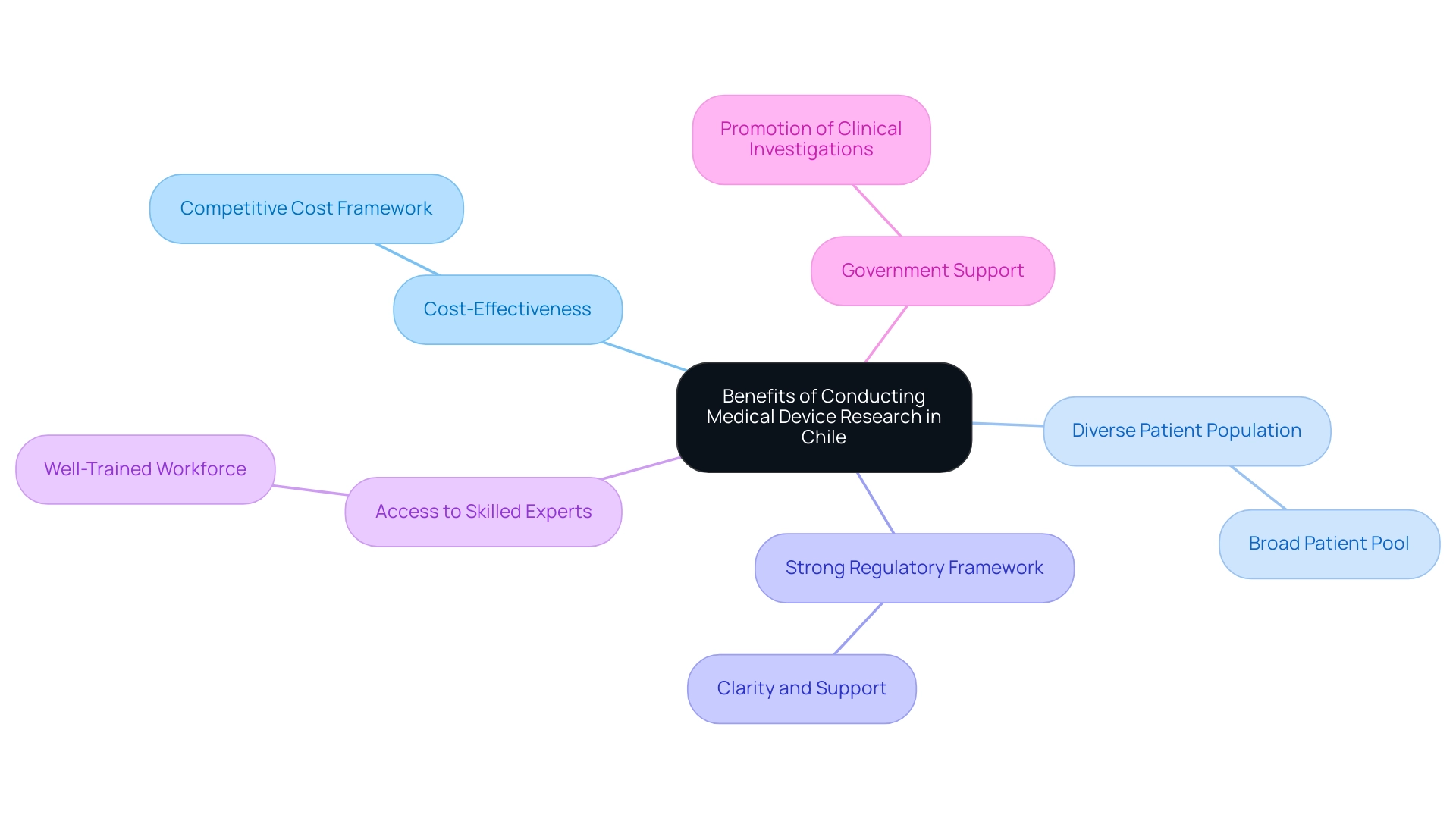
Carrying out Medical Device Research Chile provides various benefits that can improve the success of medical evaluations. By collaborating with bioaccess®, which focuses on expedited medical device research Chile and throughout Latin America, researchers can utilize a comprehensive suite of offerings including Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), feasibility evaluations, site selection, compliance reviews, setup, import permits, project management, and reporting. Consider the following benefits:
- Cost-Effectiveness: Medical Device Research Chile offers a competitive cost framework for studies, making it an appealing choice for funding budgets.
- Diverse Patient Population: With a varied demographic, researchers can access a broad patient pool, which can enhance the generalizability of Medical Device Research Chile results.
- Strong Regulatory Framework: The strong regulatory framework in Chile provides a well-defined environment that offers clarity and support for researchers engaged in Medical Device Research Chile as they navigate the approval process.
- Access to Skilled Experts: Medical Device Research Chile is supported by a well-trained workforce in the medical and experimental fields, ensuring quality in management and execution.
- Government Support: The government actively promotes clinical investigations in the context of Medical Device Research Chile, creating an ecosystem that encourages innovation and collaboration.
By leveraging these benefits and the expertise of bioaccess®, which has a proven background and customized approach to clinical investigations, researchers in Medical Device Research Chile can optimize their projects while contributing to job creation, economic growth, and healthcare improvement in the region. The influence of Medtech research extends beyond the trials themselves, fostering local economies through job creation and improved healthcare outcomes.
Establishing Collaborations with Local Institutions
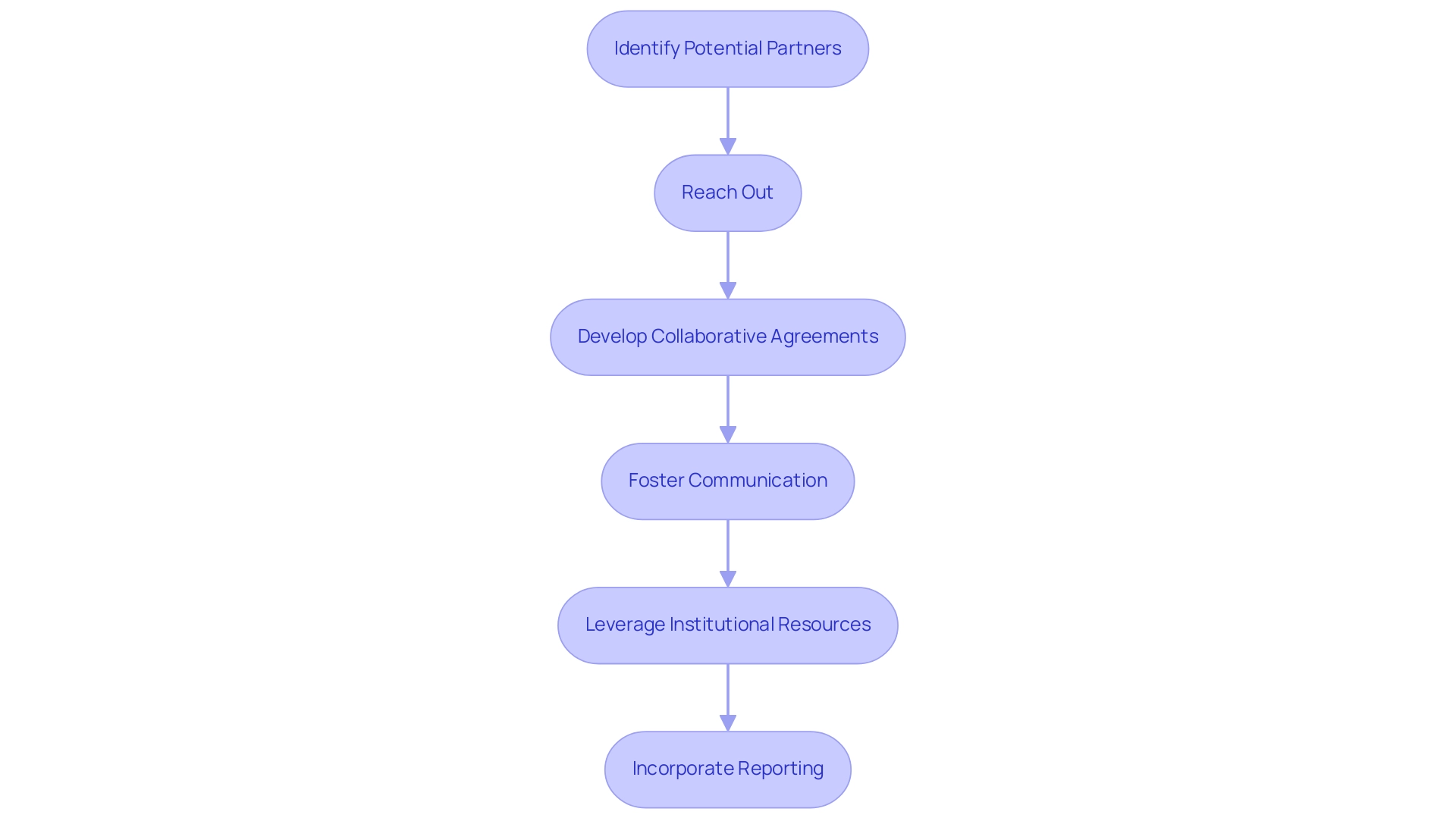
Establishing collaborations with local institutions can greatly enhance your Medical Device Research in Chile. Here are steps to consider:
-
Identify Potential Partners: Research local hospitals, universities, and research organizations that align with your research objectives and have a history of conducting clinical trials.
-
Reach Out: Initiate contact with key stakeholders at these institutions to discuss potential collaboration opportunities and mutual benefits, tapping into their familiarity with regulatory environments and patient recruitment.
-
Develop Collaborative Agreements: Formalize partnerships through agreements that outline responsibilities, resource sharing, and data management protocols, ensuring compliance with local regulations and ethical standards.
-
Foster Communication: Maintain open lines of communication with partners to ensure alignment on research objectives and timelines, which can help navigate any regulatory hurdles.
-
Leverage Institutional Resources: Utilize the resources and expertise available at partner institutions to enhance research design, patient recruitment, and data analysis, ultimately addressing financial constraints and improving outcomes.
-
Incorporate Reporting: Ensure that regular reporting on study status, inventory, and adverse events is part of your project management strategy to maintain transparency and compliance.
By effectively establishing collaborations, researchers can access valuable support and resources, ultimately leading to more successful research studies. This collaborative method is further supported by leaders such as Julio Martinez-Clark, who promote Medical Device Research in Chile and highlight the significance of mentorship and strategic alliances. BOOK A MEETING to learn more about how we can assist you in navigating the complexities of clinical studies.
Implementing Effective Patient Recruitment Strategies
Implementing effective patient recruitment strategies is essential for the success of Medical Device Research Chile. At bioaccess®, we understand that a comprehensive approach is key to navigating regulatory environments in Latin America. Consider the following strategies:
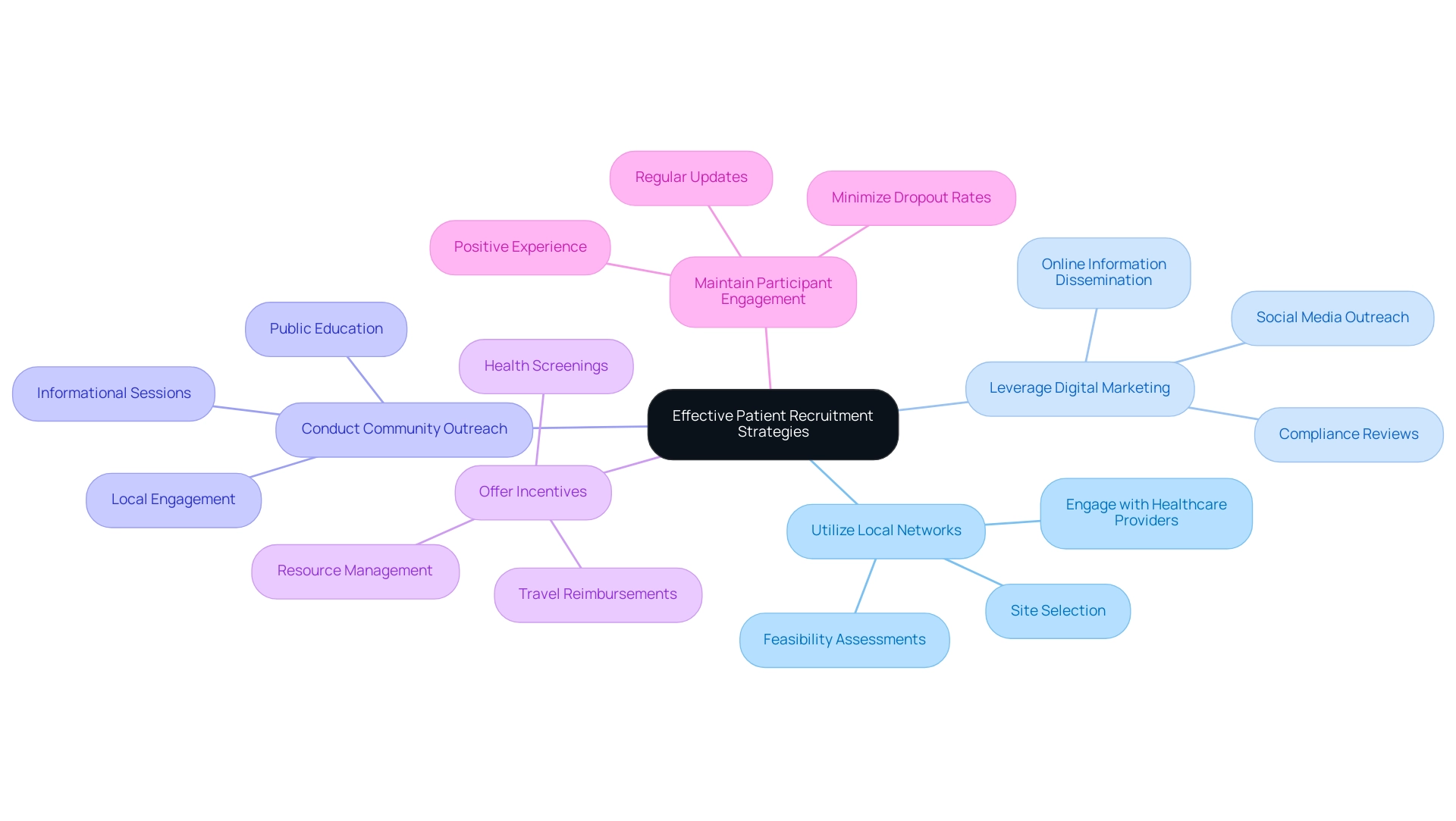
- Utilize Local Networks: Engage with local healthcare providers and institutions to identify potential participants who meet your criteria, leveraging our expertise in site selection and feasibility assessments, which includes thorough trial setup and compliance reviews.
- Leverage Digital Marketing: Utilize social media and online platforms to reach potential participants, providing clear information about the research and its benefits, complemented by our compliance reviews to ensure all communications meet regulatory standards.
- Conduct Community Outreach: Organize informational sessions in community centers to educate the public about the research and encourage participation, supported by our extensive project management capabilities and experience in navigating local regulations.
- Offer Incentives: Consider providing incentives for participants, such as travel reimbursements or health screenings, to enhance recruitment efforts, ensuring you have the necessary resources for effective management and reporting.
- Maintain Participant Engagement: Keep participants informed and involved throughout the study to minimize dropout rates and ensure a positive experience, which is essential for the success of Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
By employing these strategies and leveraging the comprehensive management services offered by bioaccess®, including study setup and import permits, researchers can effectively recruit and retain participants for Medical Device Research Chile. Our proven background in successfully implementing these strategies ensures the success of your clinical trials.
Conclusion
Navigating the regulatory landscape for medical device trials in Chile necessitates a thorough understanding of the guidelines established by the Chilean Ministry of Health and the National Agency for Medicines and Food. By following the outlined steps—
- understanding the legal framework,
- preparing documentation,
- submitting for approval,
- engaging with ethics committees,
- staying updated—
researchers can ensure compliance and enhance the likelihood of successful trial execution.
Conducting medical device research in Chile also presents numerous advantages. These include:
- cost-effectiveness,
- access to a diverse patient population,
- a strong regulatory framework,
- skilled professionals,
- a supportive research environment.
Collaborating with local institutions further amplifies these benefits, facilitating access to resources and expertise essential for optimizing study outcomes.
Ultimately, the integration of effective patient recruitment strategies, alongside local collaborations and the support of specialized service providers, positions researchers to significantly impact healthcare outcomes. As the demand for innovative medical solutions continues to grow, understanding and leveraging the opportunities within Chile's regulatory framework is crucial for advancing clinical research and improving healthcare delivery in the region.
Frequently Asked Questions
What is the first step in navigating the regulatory landscape for Medical Device Research in Chile?
The first step is to understand the legal framework by reviewing the Medical Devices Law and accompanying regulations established by the Chilean Ministry of Health (MINSAL) and the National Agency for Medicines and Food (ANAMED).
What documentation is required for Medical Device Research in Chile?
Required documentation includes the clinical research protocol, informed consent forms, and any prior approvals from ethics committees.
How do researchers submit their application for approval in Chile?
Researchers must submit their application to MINSAL, which involves a comprehensive evaluation of the study design, patient safety measures, and data management plans.
Why is it important to engage with ethics committees during the research process?
Engaging with local ethics committees is vital to secure their approval, uphold ethical standards, and ensure the protection of participant rights throughout the study.
What should researchers do to stay compliant with regulations in Chile?
Researchers should continuously monitor for updates regarding regulations or modifications in the approval process to maintain compliance.
What benefits does Medical Device Research in Chile offer?
Benefits include cost-effectiveness, access to a diverse patient population, a strong regulatory framework, access to skilled experts, and government support for clinical investigations.
How can researchers enhance their Medical Device Research through collaborations?
Researchers can identify potential partners such as local hospitals and universities, reach out to discuss collaboration, develop agreements, foster communication, leverage institutional resources, and incorporate regular reporting.
What strategies can be employed for effective patient recruitment in Medical Device Research?
Strategies include utilizing local networks, leveraging digital marketing, conducting community outreach, offering incentives, and maintaining participant engagement throughout the study.




