Overview
To conduct medical device trials in Colombia, researchers must navigate a structured process that includes regulatory submission to INVIMA, obtaining ethics committee approval, and adhering to Good Clinical Practices throughout the trial. The article outlines essential steps such as initial planning, participant recruitment, trial execution, and post-trial reporting, emphasizing the importance of compliance with regulations to ensure participant safety and the integrity of research outcomes.
Introduction
Navigating the intricate landscape of medical device regulation in Colombia presents both challenges and opportunities for researchers and companies alike. As the demand for innovative medical solutions rises due to a significant burden of chronic and infectious diseases, understanding the regulatory framework established by the National Institute for Food and Drug Surveillance (INVIMA) becomes paramount.
This article delves into the essential components of conducting medical device trials in Colombia, including:
- Classification and registration processes
- Ethical compliance
- Post-market surveillance
By outlining a step-by-step approach to trial execution and emphasizing the importance of skilled personnel and robust data management, this exploration aims to equip stakeholders with the knowledge necessary to thrive in this evolving market. Ultimately, it highlights the potential for growth and innovation within Colombia's medical device sector, underscoring its role as a burgeoning hub for clinical research.
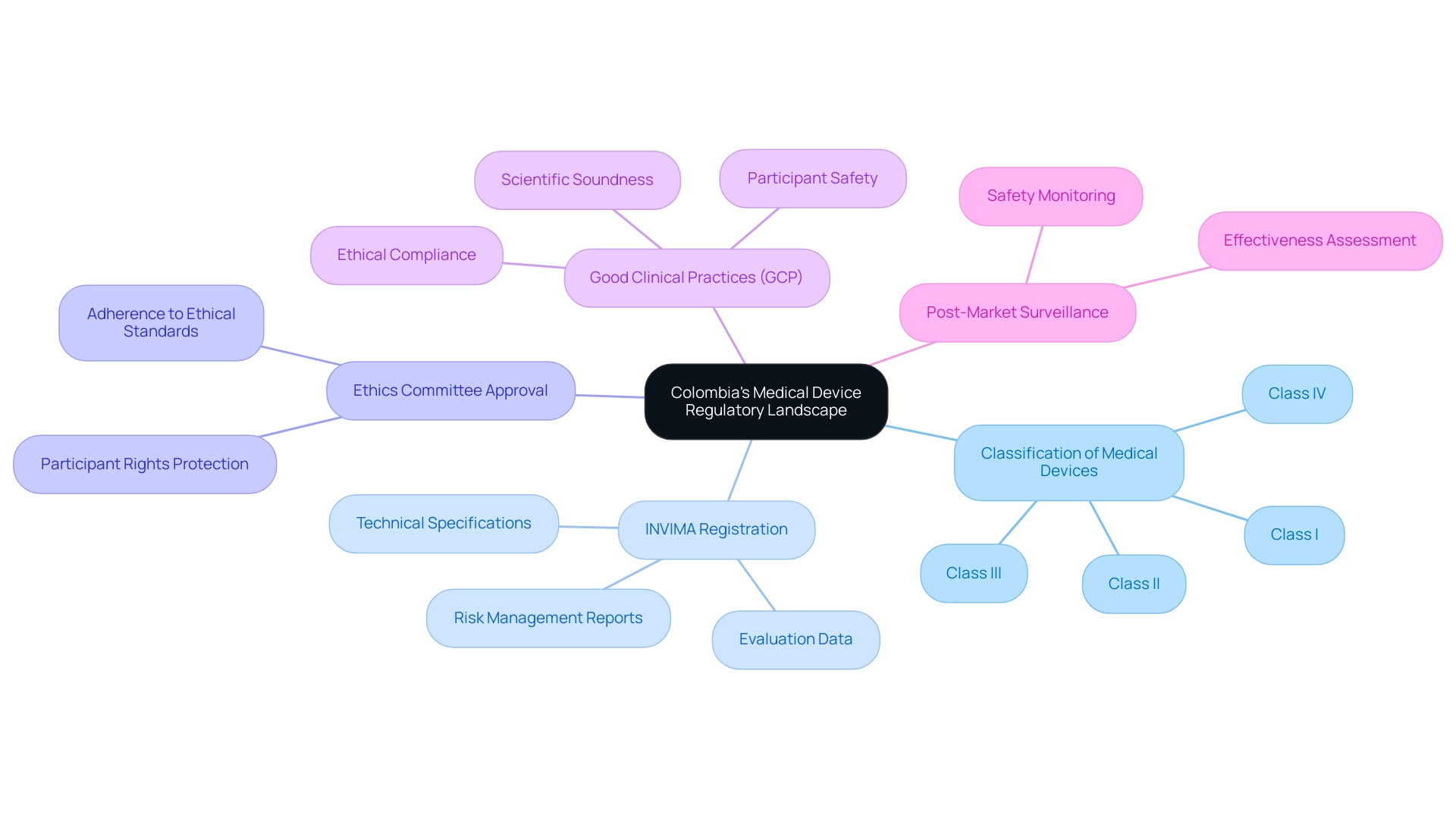
Understanding Colombia's Medical Device Regulatory Landscape
Colombia's medical equipment regulatory landscape is primarily overseen by the National Institute for Food and Drug Surveillance (INVIMA). For investigators in medical studies, a comprehensive grasp of the following key elements is crucial:
- Classification of Medical Devices: Medical instruments in Colombia are categorized into four classifications (I, II, III, IV) based on their associated risk levels, which dictate the regulatory pathway and requirements for each type, significantly influencing the approval process.
- INVIMA Registration: Before starting research studies, all medical devices must be registered with INVIMA. This registration process requires the submission of comprehensive documentation, including technical specifications, evaluation data, and detailed risk management reports. Ensuring meticulous preparation of these documents is crucial for facilitating a smooth registration process.
- Ethics Committee Approval: Researchers must secure approval from an ethics committee (CEI) before starting any study. This step is vital to guarantee that the study adheres to ethical standards and adequately protects the rights of participants, reinforcing the integrity of research.
- Good Clinical Practices (GCP): Adherence to Good Clinical Practices is a mandatory requirement. This includes conducting scientifically sound trials, ensuring ethical compliance, and prioritizing participant safety throughout the research process. GCP guidelines serve as a framework for maintaining the highest standards in clinical research.
- Post-Market Surveillance: After the approval and market introduction of a healthcare product, ongoing post-market monitoring is required to assess its safety and effectiveness in real-world applications. This ongoing assessment is vital for recognizing any possible problems and ensuring long-term patient safety.
The need for new healthcare instruments in Colombia is highlighted by the significant burden of chronic and infectious diseases, with a cancer prevalence of 29,3524 in 2020 and over 9.4 cases of malaria per 100,000 individuals. Knowledge of these elements is crucial for researchers involved in Colombia Medical Device Trials to effectively navigate the regulatory environment and uphold compliance throughout the clinical study process, ultimately aiding in the advancement of efficient healthcare instruments.
Significantly, bioaccess™ has successfully partnered with Welwaze Medical Inc. for the launch of the Celbrea® healthcare product in Colombia, showcasing effective regulatory access and market entry strategies.
This collaboration involved comprehensive services from bioaccess™, including trial setup, obtaining import permits, and managing project reporting on study status and adverse events, which are critical for ensuring compliance with INVIMA regulations. Furthermore, a case study on market segmentation and growth shows that the Colombian healthcare equipment Regulatory Affairs market is divided into in-house and outsourced services, with outsourcing accounting for a revenue share of 65.76% in 2024, highlighting a structured growth trajectory for the market. Moreover, comparable patterns are expected in the healthcare industry, highlighting the potential for growth in healthcare equipment demand.
Step-by-Step Process for Conducting Medical Device Trials in Colombia
Carrying out evaluations of medical equipment in Colombia entails various essential stages that utilize the nation's advantageous research setting and extensive study management services:
-
Initial Planning:
- Clearly define the objectives of the trial and outline an effective study design that aligns with regulatory requirements and the capabilities of services available, including feasibility studies and the selection of a principal investigator (PI).
- Identify the target population and suitable site locations, taking into account Colombia's cost-effective environment that facilitates extensive studies benefiting both patients and the advancement of medical knowledge.
-
Regulatory Submission:
- Prepare and submit the necessary documentation to INVIMA, the Colombia National Food and Drug Surveillance Institute, for device registration, including a detailed technical dossier and a comprehensive clinical evaluation report.
- Submit the study protocol to the relevant ethics committee for approval, ensuring adherence to ethical standards and regulations critical for compliance.
-
Site Selection and Preparation:
- Select qualified clinical sites based on their experience and capacity to conduct trials effectively, crucial for successful study outcomes. This includes feasibility assessments to ensure the site is suitable for the trial's needs.
- Train site staff thoroughly on the trial protocol and Good Clinical Practice (GCP) requirements, emphasizing meticulous documentation, which is essential for accountability and transparency.
-
Recruitment of Participants:
- Develop robust recruitment strategies to enroll eligible participants, ensuring that informed consent is obtained at every stage.
- Monitor participant recruitment closely to meet enrollment targets, adjusting strategies as necessary to optimize engagement and enhance the study's integrity.
-
Trial Execution:
- Implement the trial according to the approved protocol, ensuring strict adherence to GCP and regulatory requirements, which contributes to the credibility of the research outcomes.
- Systematically collect and manage data to ensure accuracy and integrity, facilitating reliable results that can withstand scrutiny.
-
Monitoring and Reporting:
- Conduct regular monitoring visits to clinical sites to ensure compliance and promptly address any issues that arise during the trial.
- Report any serious and non-serious adverse events to INVIMA and the ethics committee without delay, as this is essential for maintaining participant safety and regulatory compliance.
-
Data Analysis and Reporting:
- Analyze the collected data rigorously to evaluate the safety and efficacy of the medical device, incorporating statistical methods that enhance the robustness of the findings.
- Prepare a comprehensive final report detailing the trial outcomes and submit it to INVIMA for review, showcasing the research's impact and relevance in the healthcare landscape.
-
Post-Trial Activities:
- Ensure proper follow-up with participants to monitor long-term effects of the device, thereby enhancing patient safety and satisfaction.
- Engage in post-market surveillance as mandated by INVIMA to track device performance in the market, aligning with best practices in medical device regulation.
By following these meticulously structured steps and utilizing our comprehensive service capabilities, including feasibility studies, project management, and monitoring, researchers can effectively navigate the complexities of conducting medical device trials in Colombia. This not only ensures compliance with regulatory standards but also enhances the quality of their research outcomes. As Julio G. Martinez-Clark, CEO of Bioaccess, notes, 'Colombia stands out as a noteworthy hub for clinical research, offering many advantages that make it an attractive choice for medtech companies.' This environment not only fosters innovation but also significantly contributes to local economies through job creation and healthcare improvement. Furthermore, the Colombian healthcare equipment market was valued at $1.42 billion in 2022, highlighting the potential for expansion in this sector. The advancements in technology, such as those seen in nanotech innovation for coronary disease treatment, exemplify the significant impact of research in the region.
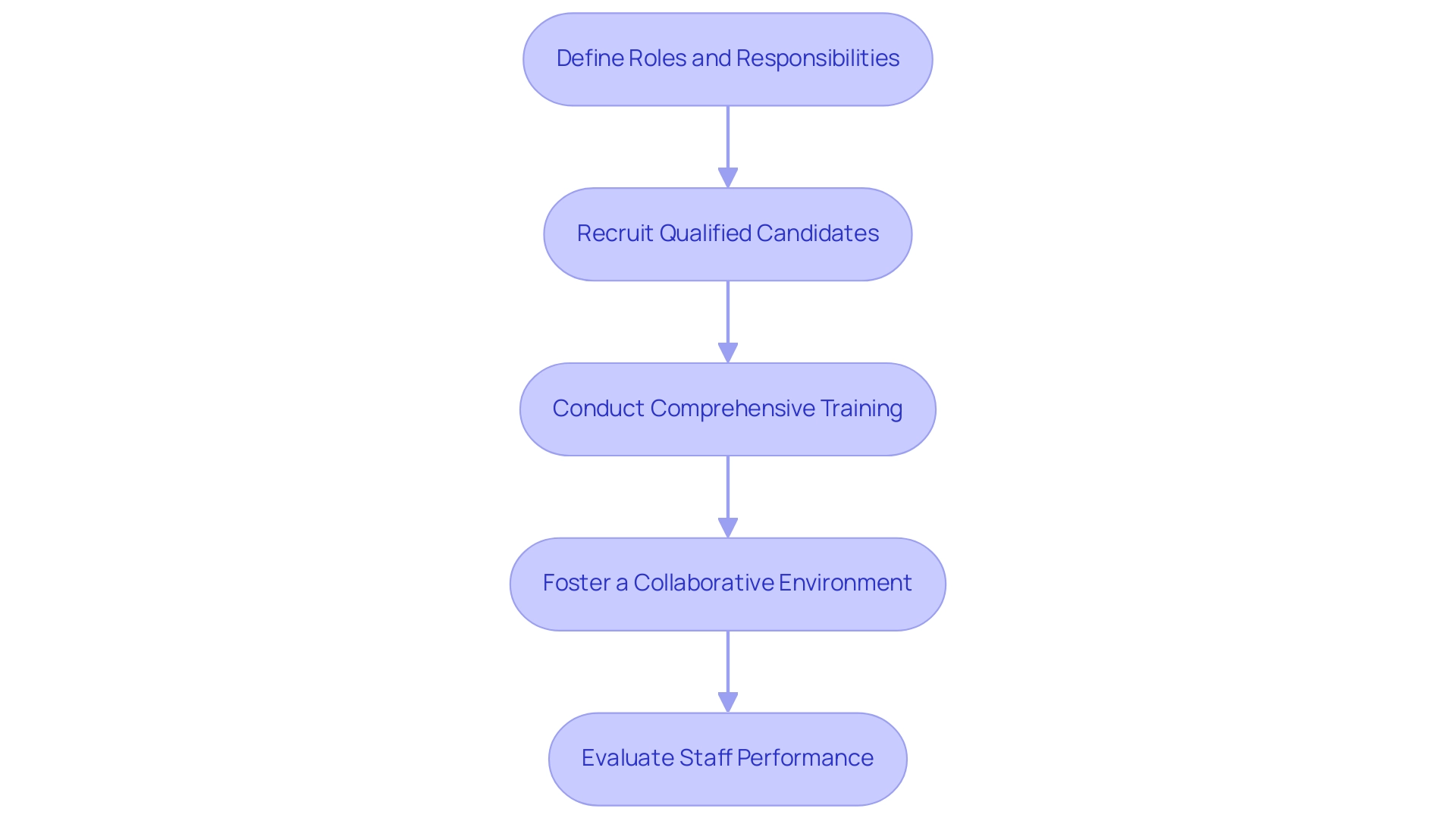
Recruiting and Training Research Staff
Hiring and educating the appropriate staff is essential for the effective implementation of healthcare equipment evaluations. Below are essential steps to follow:
-
Define Roles and Responsibilities:
-
Clearly delineate the necessary roles for the trial, including principal investigators, clinical research coordinators, and data managers.
This clarity ensures that each team member understands their specific contributions to the study.
-
-
Recruit Qualified Candidates:
-
Leverage professional networks, job boards, and specialized recruitment agencies to identify candidates with pertinent experience in clinical research and medical devices.
This targeted approach enhances the quality of applicants.
Evaluate qualifications thoroughly through organized interviews and reference checks to ensure a strong fit for the requirements.
-
-
Conduct Comprehensive Training:
-
Develop a robust training program that encompasses the study protocol, Good Clinical Practice (GCP) guidelines, and regulatory compliance.
Integrating hands-on training sessions will assist in familiarizing staff with data collection methodologies and the software tools used in the project, thereby enhancing efficiency and accuracy.
-
-
Foster a Collaborative Environment:
-
Cultivate an atmosphere of open communication and teamwork among staff to boost collaboration and problem-solving capabilities.
Regular meetings should be scheduled to discuss progress, address challenges, and provide updates related to the experiment, promoting transparency and shared accountability.
-
-
Evaluate Staff Performance:
-
Implement performance evaluation metrics to gauge staff contributions and identify areas for improvement.
Continuous assistance and positive input are essential for nurturing ongoing professional growth within the team.
-
By efficiently hiring and educating research personnel, organizations can greatly improve the overall quality of Colombia Medical Device Trials, ensuring compliance with protocols and regulations. This method is especially significant in areas such as Colombia Medical Device Trials, where the research industry has seen considerable expansion, resulting in over 6% average job increases in the past five years. The expertise of bioaccess® in managing studies—ranging from Early-Feasibility Studies (EFS) to Post-Market Clinical Follow-Up Studies (PMCF)—highlights the critical need for well-trained personnel.
bioaccess® provides extensive services including feasibility studies, site selection, compliance reviews, setup, project management, and monitoring. As highlighted by Olufunmilayo I Olopade from the University of Chicago, investment in training and development is crucial:
Investment in human capital advancement through training of research staff will promote strong research.
Moreover, comprehending the regulatory environment, including the role of INVIMA in supervising Colombia Medical Device Trials and assessments of healthcare equipment, is essential for successful execution.
The increasing yearly funding in research studies in Latin America's Andean Region, now surpassing $50 million, along with Cohortias' participation in Colombia Medical Device Trials and studies across Argentina, Brazil, and Mexico, further highlights the changing environment of medical device evaluations.
Managing Data and Compliance
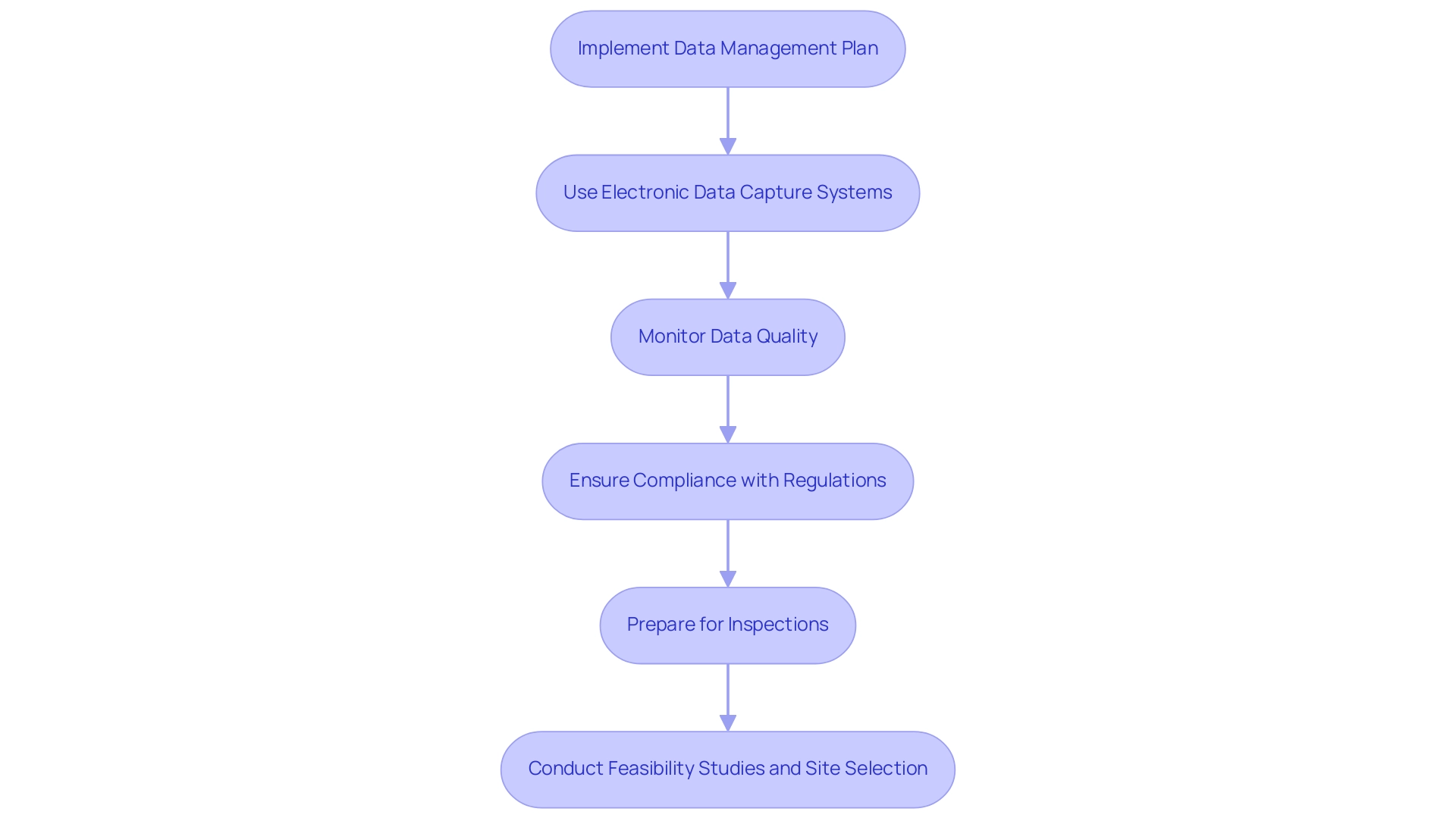
Efficient data handling and adherence are fundamental for upholding research integrity during the study process. To achieve this, consider the following steps:
-
Implement a Data Management Plan:
-
Craft a comprehensive data management plan that delineates data collection methods, storage protocols, and analytical procedures.
A well-structured plan is essential for ensuring all team members are aligned on data handling processes.
-
Utilize validated tools for data collection and ensure all staff receive adequate training in these methodologies.
-
-
Use Electronic Data Capture (EDC) Systems:
- Employ EDC systems to enhance the efficiency of data collection while reducing errors commonly associated with manual data entry. These systems are pivotal in streamlining workflows and ensuring accuracy in data handling.
- It is imperative that the chosen EDC system complies with regulatory standards, including data security protocols to protect sensitive information.
-
Monitor Data Quality:
- Establish systematic data monitoring procedures to promptly identify discrepancies and guarantee data accuracy. Regular audits and quality control checks are crucial for verifying adherence to established data management standards.
-
Ensure Compliance with Regulations:
-
Stay abreast of the evolving regulatory landscape pertinent to clinical trials, particularly in Colombia, which is rapidly becoming a prominent hub for clinical research with 240 active studies, making it the fourth country in Latin America for clinical studies per million people.
The role of INVIMA as a Level 4 health authority by PAHO/WHO underscores the importance of regulatory compliance in overseeing medical device trials.
-
Maintain meticulous documentation of all trial activities, including participant consent forms, monitoring reports, and adverse event reports, to ensure transparency and accountability. This encompasses comprehensive documentation on both severe and minor adverse occurrences, which is a vital component of study management.
-
-
Prepare for Inspections:
- Organize documentation systematically to facilitate accessibility during potential inspections by regulatory authorities. This preparedness reflects a commitment to compliance and research integrity.
- Conduct internal audits regularly to assess compliance and proactively address any identified issues, ensuring a culture of continuous improvement.
-
Conduct Feasibility Studies and Site Selection:
- Prior to trial initiation, conduct feasibility studies to assess potential research sites and select qualified principal investigators (PIs). This foundational step is crucial for ensuring the success of the clinical trial.
- Collaborate with selected sites to ensure they are equipped to meet the study's requirements, including necessary approvals from ethics committees and health ministries.
A notable example of effective data management in this landscape is the case study of eyeFlow, which has successfully conducted studies in Colombia, leveraging the favorable conditions for clinical trials.
By applying these approaches, you can maintain the quality and integrity of your research studies, strengthening trust among stakeholders and aiding the progress of healthcare research and local economies. As noted by industry leader Neal D. E. Alexander, 'A comprehensive approach to data management is essential for success in the evolving MedTech landscape.
Post-Trial Reporting and Follow-Up
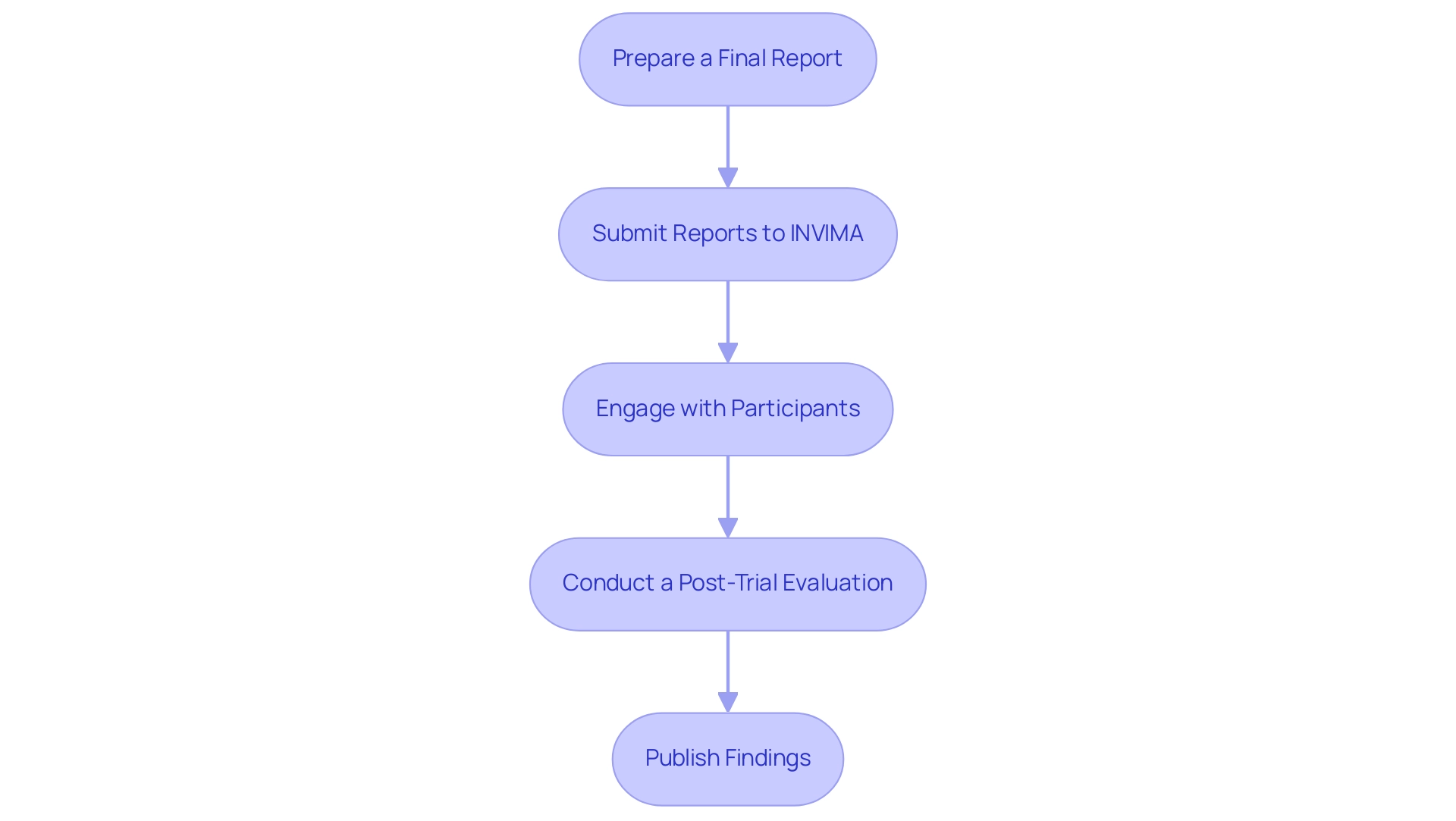
Upon completion of a research trial, meticulous post-trial reporting and follow-up are essential to uphold participant safety and regulatory standards in Colombia:
-
Prepare a Final Report:
- Develop a comprehensive final report encapsulating the study protocol, methodology, results, and conclusive insights. This report should include a thorough analysis of the data collected, emphasizing the safety and efficacy of the medical device under investigation.
-
Submit Reports to INVIMA:
- Ensure the final report and all requisite documentation are submitted to INVIMA, the Colombia National Food and Drug Surveillance Institute, for regulatory compliance and review. Accurate reporting of all findings, including any adverse events, is imperative to maintain transparency and uphold ethical standards in clinical research.
-
Engage with Participants:
- Maintain ongoing communication with trial participants, providing them with updates on the study's findings and implications for their health. This includes offering follow-up assessments or monitoring as necessary, reinforcing the commitment to participant safety.
-
Conduct a Post-Trial Evaluation:
- Undertake a thorough evaluation of the trial process to pinpoint strengths and areas for improvement in future studies. Collecting feedback from both staff and participants is essential for improving study designs and execution, thereby enhancing overall research quality.
-
Publish Findings:
- Aim to publish the trial results in peer-reviewed journals, contributing valuable insights to the broader medical community. Dissemination of findings to stakeholders—including sponsors, regulatory bodies, and the medical community—fosters transparency and collaboration, which are crucial for advancing research and development.
In Colombia, adherence to post-trial reporting requirements is especially pertinent, as recent statistics indicate a concerning trend in compliance. The lack of stringent enforcement has led to numerous overdue results, prompting discussions on the ethical obligation to report. Ethicist Jennifer Miller aptly notes,
Ethics is not minimal compliance with the law.
Moreover, the success rates of medical studies have demonstrated significant improvement, with Phase I success rates increasing to 48% and Phase III rates rising to 66%, emphasizing the crucial role of thorough post-study reporting in ensuring successful outcomes. The lax enforcement of study registration and result reporting, as highlighted in the case study on regulatory challenges, further underscores the need for ethical compliance in research. By diligently following these post-trial steps and leveraging comprehensive trial management services—including feasibility studies, site selection, project management, and monitoring—researchers can effectively communicate trial outcomes while prioritizing participant safety and fostering ethical integrity in trials. Furthermore, addressing the specific challenges faced by Medtech companies in Latin America, such as regulatory hurdles and resource fragmentation, is crucial for enhancing the effectiveness of clinical research in the region.
Conclusion
Navigating the regulatory landscape for medical device trials in Colombia is a multifaceted endeavor that requires careful attention to various critical components. Understanding the classification and registration processes established by INVIMA is fundamental for researchers aiming to ensure compliance and facilitate smoother trial execution. The necessity of obtaining ethics committee approval and adhering to Good Clinical Practices cannot be overstated, as these elements are essential for maintaining participant safety and the integrity of the research.
Moreover, the article emphasizes the importance of skilled personnel and robust data management throughout the clinical trial process. Effective recruitment and training of research staff are pivotal in enhancing the quality of trials, while meticulous data management ensures compliance and supports the credibility of findings. Post-market surveillance and thorough reporting are equally crucial, as they contribute to ongoing monitoring of device safety and efficacy, ultimately fostering trust among stakeholders.
In conclusion, Colombia presents a promising environment for medical device innovation and clinical research, bolstered by its regulatory framework and growing market potential. By embracing the outlined strategies and understanding the intricacies of the regulatory process, stakeholders can not only navigate challenges but also seize opportunities for growth within this expanding sector. As the demand for advanced medical solutions continues to rise, Colombia stands to solidify its position as a key player in the global medical device landscape.
Frequently Asked Questions
What organization oversees the regulation of medical equipment in Colombia?
The regulation of medical equipment in Colombia is primarily overseen by the National Institute for Food and Drug Surveillance (INVIMA).
How are medical devices classified in Colombia?
Medical devices in Colombia are classified into four categories (I, II, III, IV) based on their associated risk levels, which influence the regulatory pathway and requirements for approval.
What is required for the registration of medical devices with INVIMA?
All medical devices must be registered with INVIMA before starting research studies, requiring comprehensive documentation such as technical specifications, evaluation data, and detailed risk management reports.
Is ethics committee approval necessary before conducting medical studies in Colombia?
Yes, researchers must secure approval from an ethics committee (CEI) before starting any study to ensure adherence to ethical standards and protection of participants' rights.
What are Good Clinical Practices (GCP)?
Good Clinical Practices (GCP) are mandatory guidelines that ensure scientifically sound trials, ethical compliance, and participant safety throughout the research process.
What is post-market surveillance in the context of medical devices?
Post-market surveillance is the ongoing monitoring of a healthcare product's safety and effectiveness after market introduction, essential for identifying potential issues and ensuring long-term patient safety.
What are the initial steps involved in conducting medical equipment evaluations in Colombia?
Initial steps include defining trial objectives, outlining study design, identifying target populations, and selecting suitable site locations.
What documentation is needed for regulatory submission to INVIMA?
Necessary documentation includes a detailed technical dossier and a comprehensive clinical evaluation report for device registration and study protocol submission for ethics committee approval.
How should clinical sites be selected for trials in Colombia?
Clinical sites should be selected based on their experience and capacity to conduct trials effectively, with feasibility assessments to ensure they meet trial needs.
What is the importance of participant recruitment in clinical trials?
Robust recruitment strategies are essential to enroll eligible participants, ensuring informed consent is obtained and monitoring recruitment progress to meet enrollment targets.
What is involved in the trial execution phase?
Trial execution involves implementing the study according to the approved protocol, adhering to GCP and regulatory requirements, and systematically collecting and managing data.
What monitoring and reporting activities are required during trials?
Regular monitoring visits to clinical sites are conducted to ensure compliance, and any serious or non-serious adverse events must be reported to INVIMA and the ethics committee promptly.
What steps are taken after data collection in clinical trials?
After data collection, a rigorous analysis is conducted to evaluate the safety and efficacy of the medical device, followed by preparing a comprehensive final report for INVIMA review.
What are post-trial activities that researchers must engage in?
Post-trial activities include follow-ups with participants to monitor long-term effects of the device and engaging in post-market surveillance as required by INVIMA.
How is the Colombian healthcare equipment market projected to grow?
The Colombian healthcare equipment market was valued at $1.42 billion in 2022, with a significant potential for expansion, driven by advancements in technology and a favorable research environment.

