Overview
Understanding INVIMA clinical trial requirements is crucial for ensuring compliance with Colombia's regulatory standards, which safeguard public health and facilitate medical research. The article highlights the necessity of thorough documentation, adherence to ethical practices, and ongoing reporting obligations, demonstrating how these factors contribute to the integrity and success of clinical trials conducted under INVIMA's oversight.
Introduction
In Colombia, the regulatory landscape for clinical trials is shaped significantly by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), the national authority responsible for overseeing the safety and efficacy of medical research. As the country emerges as a prominent hub for clinical studies in Latin America, understanding INVIMA's role becomes essential for researchers and organizations looking to navigate this intricate process.
From the meticulous evaluation of trial protocols and informed consent documents to the provision of essential tax incentives for research and development, INVIMA's framework not only fosters innovation but also prioritizes public health and ethical standards.
This article delves into the critical components of INVIMA's regulatory oversight, the necessary documentation for clinical trials, and the streamlined approval process that positions Colombia as an attractive destination for medical research.
Overview of INVIMA's Role in Clinical Trials in Colombia
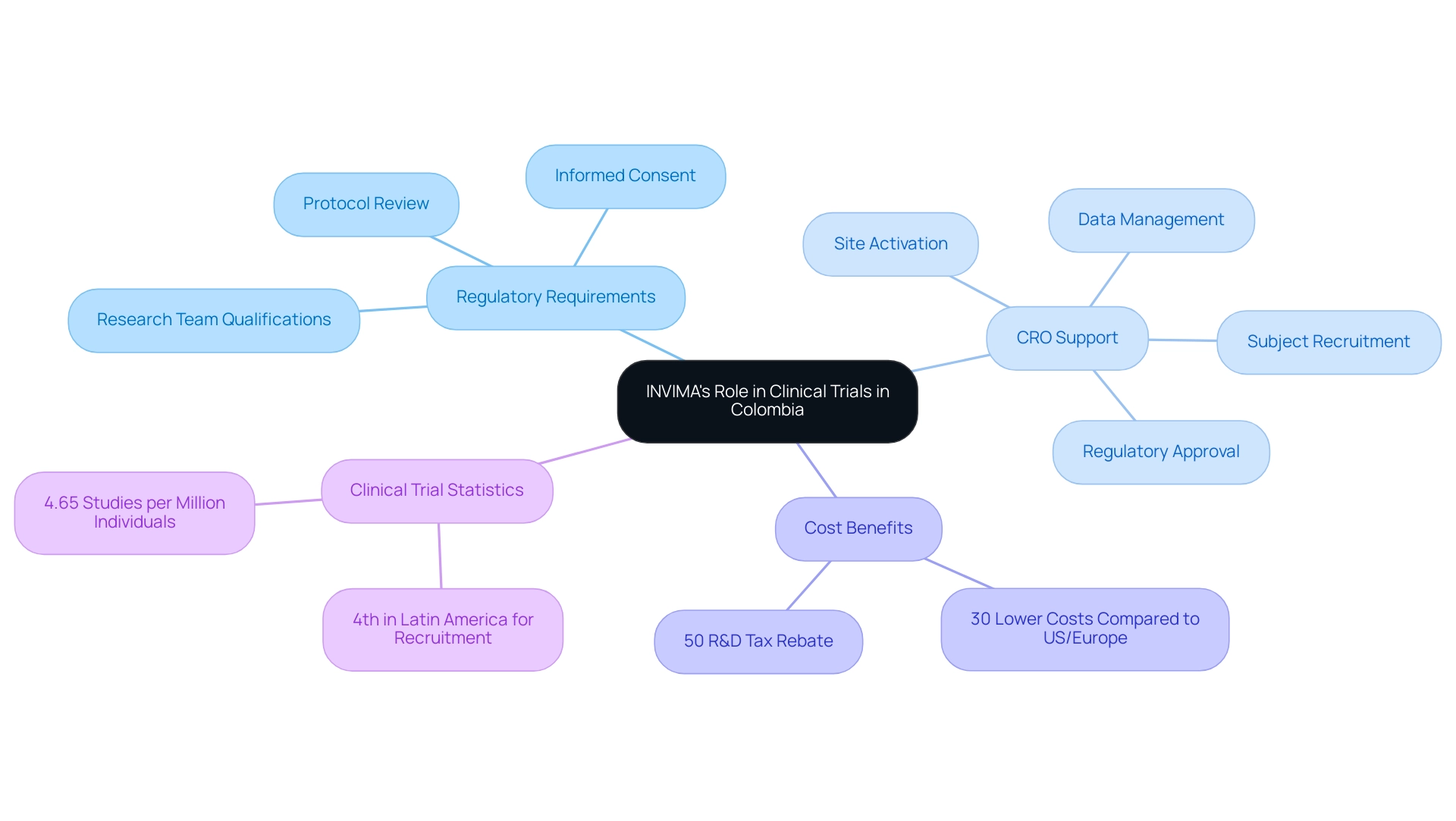
Understanding INVIMA (Colombia) Clinical Trial Requirements is essential as the National Institute of Food and Drug Surveillance plays a crucial role in overseeing research studies in Colombia. Understanding INVIMA (Colombia) Clinical Trial Requirements is essential for the agency as the country's regulatory body, which is responsible for assessing and overseeing research trials to ensure adherence to established ethical and scientific standards, including a thorough review of trial protocols, informed consent documents, and the qualifications of the research team. By rigorously assessing the safety and efficacy of new medical interventions, the agency promotes understanding INVIMA (Colombia) Clinical Trial Requirements while also safeguarding public health.
Significantly, Colombia ranks as the fourth nation in Latin America for recruiting and not-yet recruiting studies per million individuals, with a rate of 4.65, highlighting the importance of understanding INVIMA (Colombia) clinical trial requirements in facilitating research. Understanding INVIMA (Colombia) Clinical Trial Requirements shows that expenses associated with research studies in Colombia are roughly 30% lower than in areas like the United States or Europe, rendering it a compelling location for research studies. The generous R&D tax incentives for Medtech companies, which allow for a 50% tax rebate on research investments, further enhance this appeal.
Bioaccess®, as a prominent CRO, assists in navigating these regulations by providing services such as:
- Regulatory approval
- Research site activation
- Subject recruitment
- Data management
Their support enables companies to sell innovations locally before obtaining FDA approval, making it particularly beneficial for smaller enterprises. By upholding a high level of regulatory supervision in accordance with Understanding INVIMA (Colombia) Clinical Trial Requirements, the agency improves the integrity of research results and guarantees patient safety in studies carried out within the nation.
This framework was illustrated by eyeFlow, Inc., which recently obtained approval for an 18-month pilot trial on its innovative glaucoma treatment in Barranquilla, showcasing the successful collaboration between Medtech startups and regulatory agencies in Colombia.
Essential Documentation and Requirements for INVIMA Clinical Trials
To initiate a clinical study in Colombia, researchers must meticulously prepare and submit several essential documents, keeping in mind Understanding INVIMA (Colombia) Clinical Trial Requirements, as each document serves a critical role in the approval process. These include:
- Clinical Trial Protocol: A comprehensive plan that delineates the study's design, objectives, methodology, and statistical analysis. This document is foundational to the case's structure and execution.
- Informed Consent Document: A vital document that articulates the study’s purpose, procedures, risks, and benefits to potential participants, ensuring adherence to ethical standards and informed participation.
- Investigator's Brochure: An extensive document that provides crucial information about the investigational product, including detailed preclinical and clinical data to inform both researchers and participants.
- Curriculum Vitae of Investigators: Documentation highlighting the qualifications and experience of the research team members engaged in the project, which supports the credibility and capability of the conductors.
- Ethics Committee Approval: Evidence that the study has received the requisite approval from an independent ethics committee, ensuring that ethical considerations are prioritized in the research.
- Insurance Certificates: Proof of insurance coverage for participants, which guarantees compensation in the event of injury associated with the study, thus fostering participant trust and safety.
As Taghogho Apochi, a Clinical Trial Manager, notes, "This article emphasizes the importance of understanding INVIMA (Colombia) Clinical Trial Requirements in drug development and highlights the key components of the regulatory binder, which houses essential documents ensuring the trial's ethical conduct and regulatory compliance."
Furthermore, understanding INVIMA (Colombia) Clinical Trial Requirements is essential for researchers to comply with specific timelines for document submission and actively address any feedback during the review process. Understanding INVIMA (Colombia) Clinical Trial Requirements is critical in this review process to ensure that all documents meet the necessary standards for compliance. Statistics indicate that adherence to documentation standards significantly improves the likelihood of timely approvals, underscoring the importance of thorough preparation.
Furthermore, case analyses, such as those examining innovative payment models for drugs, demonstrate how collaboration among stakeholders can enhance the regulatory landscape and improve drug accessibility. Understanding INVIMA (Colombia) clinical trial requirements is essential, as comprehensive preparation encompassing viability assessments and location selection, along with precision in these documents, ensures adherence and enables the prompt approval of research initiatives in Colombia.

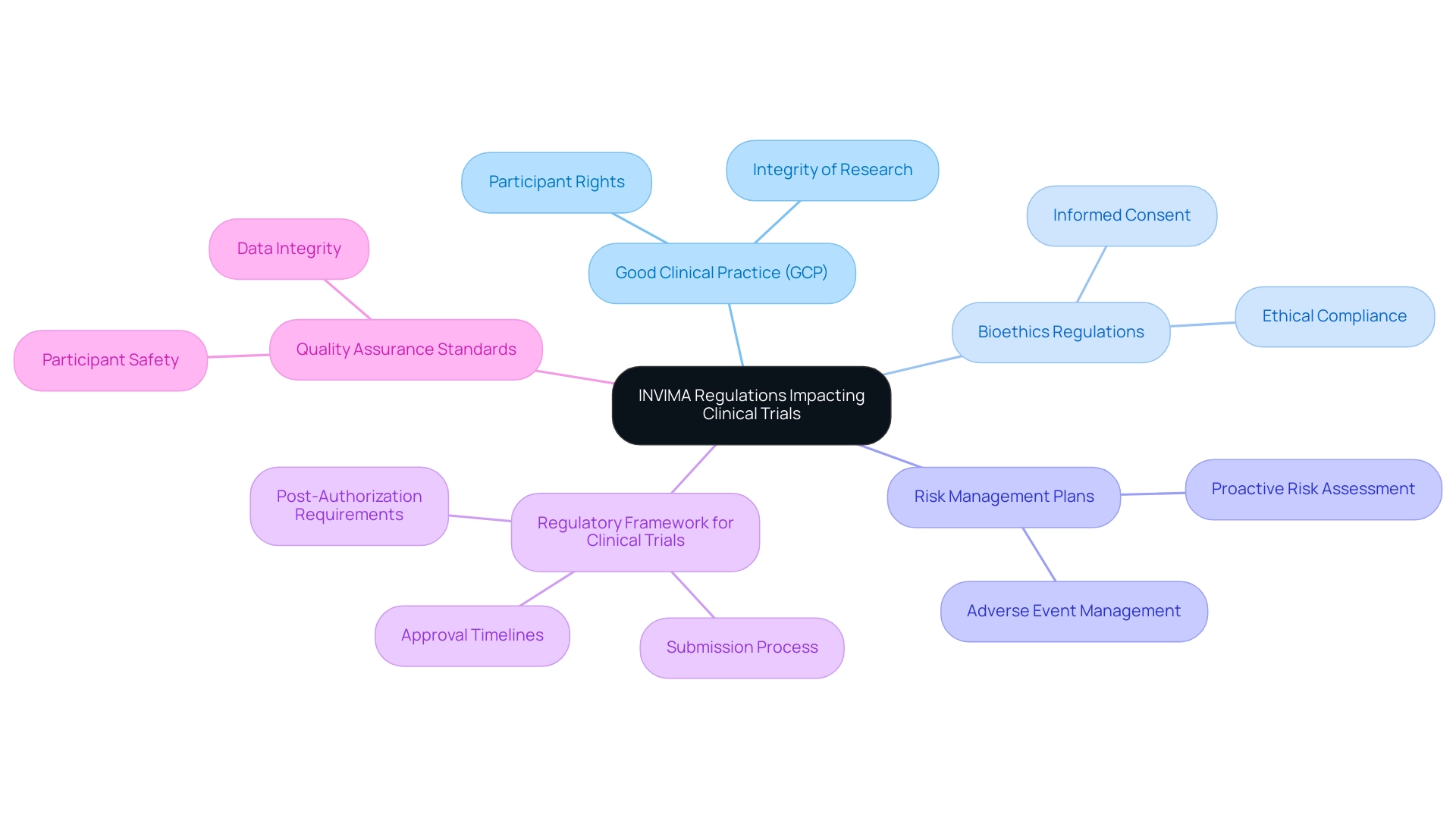
Key INVIMA Regulations Impacting Clinical Trials
Understanding INVIMA (Colombia) Clinical Trial Requirements is essential, as several crucial regulations oversee the conduct of research studies in Colombia under the auspices of INVIMA, shaping the landscape of medical research. Our comprehensive research management services encompass key capabilities such as feasibility studies, site selection, compliance reviews, setup, import permits and nationalization of investigational devices, project management, and reporting (including study status, inventory, and serious and non-serious adverse events), ensuring thorough navigation of these regulations.
- Good Clinical Practice (GCP): This globally acknowledged ethical and scientific quality benchmark is essential for guaranteeing that research studies are carried out with integrity, protecting participant rights during the process.
- Bioethics Regulations: The regulatory body mandates strict adherence to bioethical principles. This includes obtaining informed consent, which is essential for respecting participants' autonomy and ensuring ethical compliance in research.
- Risk Management Plans: The regulatory authority mandates the execution of risk management plans to proactively tackle potential adverse events during clinical studies, thereby improving participant safety and ensuring that any risks are effectively reduced.
- Regulatory Framework for Clinical Trials: The authority offers comprehensive guidance on the submission process, including approval timelines and post-authorization requirements, particularly in the context of adverse event reporting, which is vital for maintaining participant safety.
- Quality Assurance Standards: Highlighting the significance of quality control practices, the organization ensures that data integrity and participant safety are prioritized throughout the process.
A pertinent case analysis titled "Review Process for Clinical Trial Applications" illustrates the organization's thorough review process, which involves a preliminary evaluation of submitted applications, followed by a detailed review by a team of experts who assess scientific, technical, and ethical aspects. This process guarantees that only secure and scientifically valid studies advance to human testing.
To effectively navigate these regulations, research teams must focus on Understanding INVIMA (Colombia) Clinical Trial Requirements, remaining vigilant and well-informed, which enables them to design studies that not only comply with the stringent standards but also uphold the highest ethical principles in medical research. As Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, aptly states,
A comprehensive approach to data management is essential for success in the evolving MedTech landscape.
Moreover, perspectives from regulatory specialists emphasize the significance of ongoing education and compliance with GCP as a fundamental element for successful medical studies.
The Clinical Trial Approval Process in Colombia
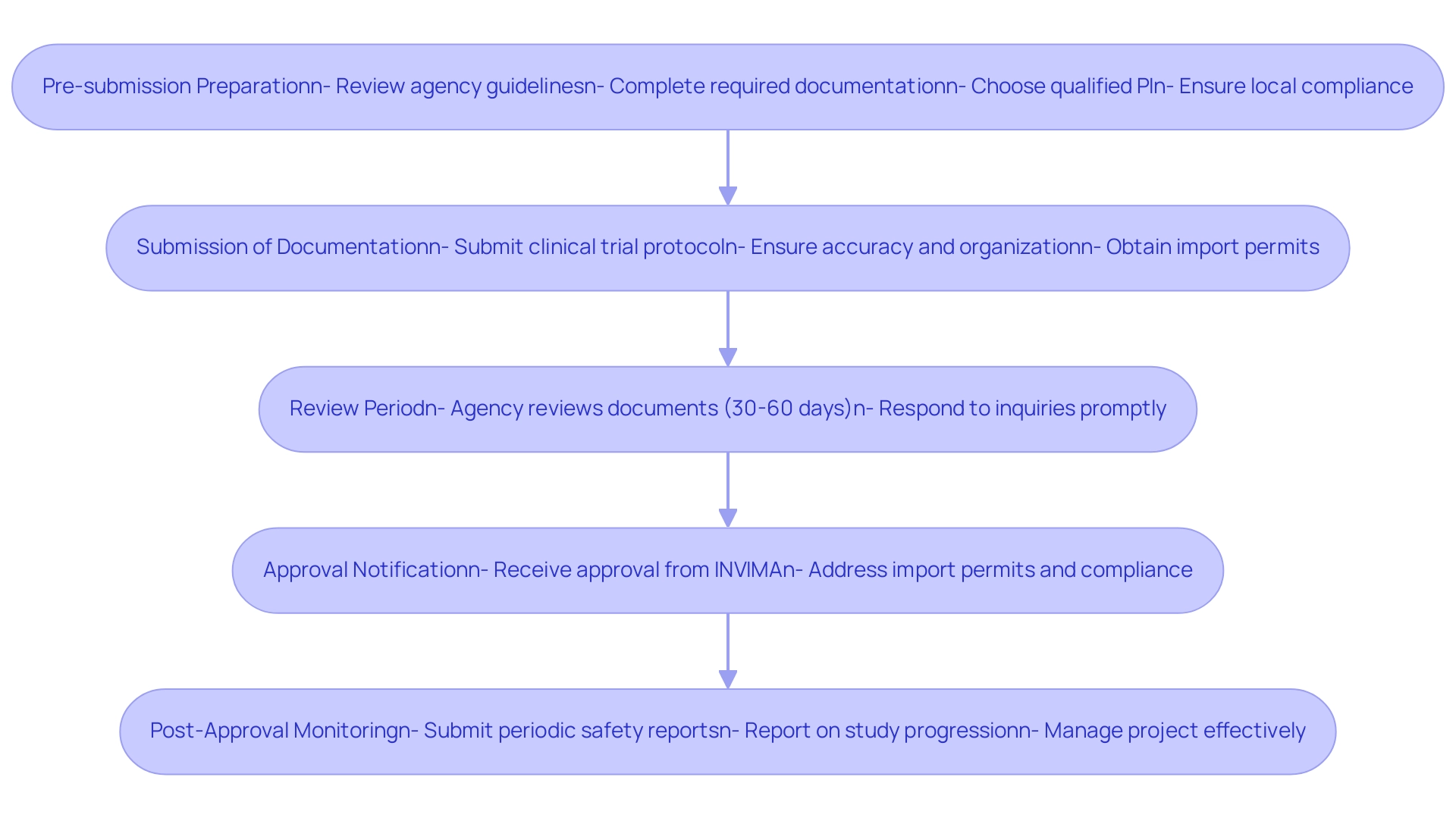
Understanding INVIMA (Colombia) Clinical Trial Requirements is essential for navigating the clinical research approval process in Colombia, as it requires careful adherence to specific steps to ensure successful submissions to the National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO. The process can be outlined as follows:
-
Pre-submission Preparation: Researchers must undertake a comprehensive review of the agency's guidelines to confirm that all required documentation is complete and meets the regulatory standards.
This encompasses feasibility studies, the choice of a qualified principal investigator (PI), and ensuring that the setup conforms to local requirements.
-
Submission of Documentation: Following preparation, the clinical trial protocol and all relevant documents should be submitted through the online platform. Ensuring that all materials are accurate and well-organized is critical to facilitate review, as compliance reviews are a key aspect of the process.
This also includes obtaining necessary import permits for investigational devices.
-
Review Period: The agency typically conducts a thorough examination of the submitted documents within a timeframe of 30 to 60 days. In comparison, VUCE takes about 5-10 business days to approve an import permit after receiving the necessary documentation, highlighting the importance of timely submissions.
During the review period, researchers should be ready to respond promptly to any inquiries or requests for additional information that may arise.
-
Approval Notification: Upon successful completion of the review, INVIMA will issue an approval notification, allowing the research team to commence their clinical study. Import permits and nationalization of investigational devices should also be addressed at this stage, ensuring that all compliance requirements are met.
-
Post-Approval Monitoring: After obtaining approval, it is essential for researchers to adhere to ongoing monitoring and reporting requirements, including the submission of periodic safety reports and updates on study progression.
This encompasses regular reporting on research status, inventory, and serious and non-serious adverse events. Researchers must also ensure that they have a project management plan in place to oversee the study effectively.
By meticulously following these outlined steps, researchers can improve their understanding of INVIMA (Colombia) clinical trial requirements, streamline the approval process, and thereby enhance the likelihood of receiving timely authorization for their studies. For instance, eyeFlow has successfully navigated this process, leveraging Colombia’s favorable research environment to achieve significant outcomes within the local landscape. Moreover, as pointed out by Katherine Ruiz, a specialist in regulatory matters for medical devices and in vitro diagnostics in Colombia, the changing environment of research studies in Colombia offers distinct possibilities for innovation and collaboration.
As of 2024, the current landscape also presents new opportunities, including collaborations for foreign manufacturers with local contract manufacturers, further expanding the potential for successful medical endeavors in the Colombian market.
Post-Approval Compliance and Reporting Obligations
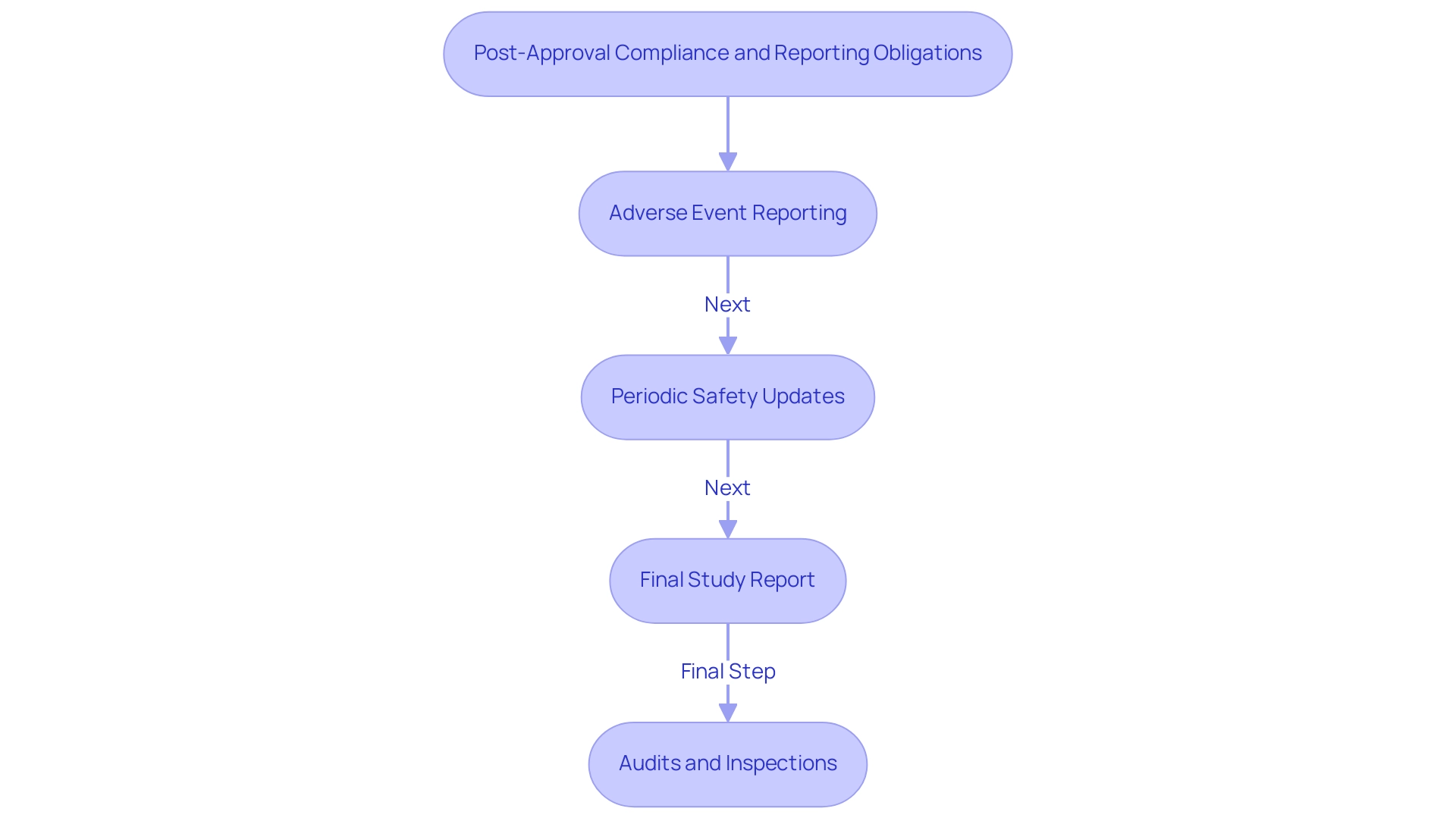
After receiving authorization from the health authority, researchers are required to adhere to various essential reporting and compliance duties that guarantee the integrity and safety of studies. These obligations encompass a range of comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, trial setup, and import permits—all critical to understanding INVIMA (Colombia) clinical trial requirements and navigating the regulatory landscape in Colombia.
- Adverse Event Reporting: It is essential for researchers to promptly report any adverse events or serious adverse events to the relevant authority, adhering to established timelines and formats.
Recent findings indicate that women are particularly susceptible to adverse drug events, with 25.3% experiencing such events in adulthood and 15% in older age, highlighting the necessity for vigilant reporting. The research identified three categories of adverse drug events based on severity—mild, moderate, and severe—each with its related factors, which researchers must consider when reporting.
- Periodic Safety Updates: Researchers must submit regular updates on the safety and efficacy of the investigational product, as specified in the conditions of initial approval.
This ongoing communication is vital for maintaining transparency and ensuring participant safety.
- Final Study Report: After the completion of the study, a comprehensive final report detailing outcomes, methodology, and any deviations from the original protocol must be submitted to the regulatory authority. This report serves as a key document for evaluating the procedure's adherence to its objectives and the regulatory framework.
Understanding INVIMA (Colombia) Clinical Trial Requirements includes the fact that INVIMA reserves the right to conduct audits or inspections to verify compliance with regulatory standards throughout the trial. These audits play a crucial role in ensuring accountability and quality in medical research, as they help identify any lapses in compliance and facilitate corrective actions. Researchers should maintain meticulous records and be prepared for these evaluations.
Understanding INVIMA (Colombia) Clinical Trial Requirements allows researchers to meet these obligations, fulfilling legal requirements while significantly contributing to the safety and effectiveness of medical research in Colombia. As noted by Moore N. in > Pharmacovigilance - The next chapter <, the ongoing evolution in monitoring and reporting practices enhances clinical trial outcomes. This evolution is exemplified by the introduction of INVIMA's electronic adverse events reporting tool in August 2015, which allows for easier, more cost-effective reporting and ensures that vital information is captured for analysis.
Conclusion
The regulatory framework established by INVIMA plays a pivotal role in shaping the landscape of clinical trials in Colombia. With its comprehensive oversight, INVIMA ensures that clinical studies adhere to rigorous ethical and scientific standards, thereby protecting public health and enhancing the integrity of research outcomes. The meticulous evaluation of trial protocols, informed consent documents, and the qualifications of research teams illustrates INVIMA's commitment to fostering an environment conducive to innovation while prioritizing participant safety.
The essential documentation and requirements for initiating a clinical trial underscore the importance of thorough preparation. By adhering to INVIMA's guidelines and submitting accurate documentation, researchers can significantly improve the likelihood of timely approvals. The outlined approval process, from pre-submission preparation to post-approval compliance, further highlights the structured approach necessary for successful clinical research in Colombia. This systematic navigation not only facilitates efficient trial initiation but also promotes ongoing monitoring and adherence to safety standards throughout the study.
In conclusion, Colombia's status as a prominent hub for clinical trials in Latin America is largely attributed to the effective regulatory oversight provided by INVIMA. The combination of favorable economic conditions, such as lower trial costs and tax incentives for research and development, further enhances the country's attractiveness for clinical research. As the landscape of clinical trials continues to evolve, understanding and navigating INVIMA's regulatory processes will be essential for researchers and organizations aiming to achieve successful outcomes in their medical studies.
Frequently Asked Questions
What is the role of INVIMA in Colombia regarding clinical trials?
INVIMA, the National Institute of Food and Drug Surveillance, oversees research studies in Colombia by assessing and ensuring adherence to ethical and scientific standards. This includes reviewing trial protocols, informed consent documents, and the qualifications of the research team.
Why is understanding INVIMA clinical trial requirements important?
Understanding INVIMA clinical trial requirements is crucial for ensuring compliance with regulations, which promotes the safety and efficacy of new medical interventions and safeguards public health.
How does Colombia rank in terms of clinical trial recruitment in Latin America?
Colombia ranks as the fourth nation in Latin America for recruiting and not-yet recruiting studies per million individuals, with a rate of 4.65.
What are the cost benefits of conducting clinical trials in Colombia?
Research study expenses in Colombia are approximately 30% lower than in the United States or Europe, making it an attractive location for clinical trials.
What tax incentives are available for Medtech companies conducting research in Colombia?
Medtech companies can benefit from a 50% tax rebate on research investments, enhancing the appeal of conducting clinical trials in Colombia.
What services does Bioaccess® provide to assist with INVIMA regulations?
Bioaccess® offers services such as regulatory approval, research site activation, subject recruitment, and data management to help navigate INVIMA regulations.
What essential documents are required to initiate a clinical study in Colombia?
The essential documents include: 1. Clinical Trial Protocol 2. Informed Consent Document 3. Investigator's Brochure 4. Curriculum Vitae of Investigators 5. Ethics Committee Approval 6. Insurance Certificates.
How does the quality of documentation affect the approval process for clinical trials?
Adherence to documentation standards significantly improves the likelihood of timely approvals, making thorough preparation critical for compliance with INVIMA requirements.
What is the significance of the eyeFlow, Inc. case mentioned in the article?
eyeFlow, Inc. successfully obtained approval for an 18-month pilot trial on an innovative glaucoma treatment in Barranquilla, illustrating effective collaboration between Medtech startups and regulatory agencies in Colombia.
How can collaboration among stakeholders improve the regulatory landscape in Colombia?
Collaboration can enhance the regulatory landscape and improve drug accessibility, as demonstrated by case analyses exploring innovative payment models for drugs.




