Introduction
In the realm of clinical trials, the safety of participants is paramount, and understanding Serious Adverse Events (SAEs) is essential for ensuring this safety. Defined as any untoward medical occurrence that results in significant consequences, such as death or hospitalization, SAEs pose critical challenges to researchers and regulatory bodies alike.
With the increasing complexity of clinical studies, the accurate identification, reporting, and management of these events have become more crucial than ever. This article delves into the multifaceted nature of SAEs, exploring their:
- Definitions
- Importance of timely reporting
- Challenges faced by researchers
- Regulatory frameworks that govern these incidents
By examining best practices and innovative tools for SAE management, this discussion aims to enhance the integrity of clinical trials and ultimately safeguard participant well-being.
Defining Serious Adverse Events in Clinical Trials
In research trials, a serious adverse event in clinical trial is defined as any untoward medical occurrence that results in death, is life-threatening, necessitates hospitalization, or leads to significant disability or incapacity. These events can occur in the context of any research study, irrespective of the investigational product being tested. Understanding the criteria that define a serious adverse event in clinical trial is crucial for safeguarding participant well-being and maintaining the integrity of clinical research.
For instance, severe allergic reactions, substantial alterations in vital signs, or any serious adverse event in clinical trial that poses a significant risk to a participant's health are all regarded as serious adverse events in clinical trial. Recent analyses have also highlighted the variability in stopping boundaries produced by different safety monitoring methods, revealing notable differences in conservatism and aggressiveness at various stages of trials. This highlights the significance of precisely recognizing and categorizing a serious adverse event in clinical trial to improve the dependability of research results.
Notably, three methods explicitly restrict their analysis to Tier 2 AEs, which is crucial for understanding the context of serious adverse events in clinical trial and their classification. Additionally, various software tools have been implemented to analyze these events, with some sources providing publicly available R packages that enhance accessibility and reproducibility in statistical analyses. As Dr. Eric Leifer mentioned, 'The writers express gratitude to Dr. Nancy Geller and Dr. Eric Leifer for evaluating this work and for significant recommendations that enhanced the manuscript,' highlighting the importance of teamwork and expert advice in comprehending serious adverse events in clinical trials.
The Importance of Reporting Serious Adverse Events
The documentation of a serious adverse event in clinical trial is not only a regulatory necessity but a cornerstone of clinical trial integrity, as emphasized by authorities such as the FDA and EMA. In 2023, hospitals documented rates that were 2.0 points higher than the previous year, indicating a growing acknowledgment of the significance of these records. Significantly, the fall event type represented 2.0% of submissions from other acute care facilities, in contrast to 11.3% from all acute care facilities, emphasizing the differing trends in SAE documentation.
Timely and accurate reporting of serious adverse events in clinical trial is essential for participant safety and the integrity of study results. Non-compliance can lead to significant regulatory penalties and potential harm to participants, thereby compromising the overall trustworthiness of the study. Furthermore, the analysis of serious adverse events in clinical trial furnishes critical data that informs the risk-benefit analysis of investigational therapies, supporting the development of safe and effective treatments.
As part of our comprehensive clinical trial management services, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Import permits
- Nationalization of investigational devices
- Thorough documentation processes
we emphasize the importance of these elements to uphold ethical standards. As mentioned by Rebecca Jones, the director of Data Science & Research at the Patient Safety Authority, the proactive identification and documentation of patient safety events are vital for enhancing research outcomes. In 2023, over half of the acute care facilities in Pennsylvania raised their submission levels, with the five facilities demonstrating the greatest increases representing 37.0% of the total rise, indicating that specific facilities are becoming more proactive in identifying and documenting patient safety events.
Researchers are therefore encouraged to prioritize the documentation of serious adverse events in clinical trials to adhere to ethical standards and ensure regulatory compliance, particularly in light of the latest requirements that emphasize accountability in research involving humans. For further clarification, investigators should consult their IRB of record and the NIAAA SAE Coordinator.
Challenges in Reporting Serious Adverse Events
Despite the critical role of SAE documentation in clinical trials, numerous challenges hinder effective record-keeping. A primary concern is the underreporting of serious adverse events in clinical trials, which can occur when researchers inadvertently overlook or fail to document significant occurrences due to time constraints or lack of familiarity with serious adverse event in clinical trial requirements. This issue is highlighted by recent findings suggesting that unpublished studies frequently include more comprehensive data on adverse events, with 95% of these documents providing such information compared to their published counterparts.
Additionally, complexities in accurately distinguishing between expected and unexpected serious adverse events in clinical trials can create further difficulties. Miscommunication among clinical study team members, compounded by inadequate training on SAE definitions and documentation procedures, further exacerbates these challenges. For instance, a review of 24 comparisons of specific adverse events revealed that in 18 cases, unpublished documentation reported higher instances of serious adverse events in clinical trial, illustrating the potential gaps in published study reports.
To address these issues effectively, it is essential to implement robust training programs, foster clear communication, and cultivate an organizational culture that prioritizes participant safety and compliance. As Rachel Phillips aptly noted, "All authors have read and approved the manuscript," emphasizing the need for thorough oversight in publication practices. Furthermore, randomized controlled studies (RCTs) should concentrate on clinically relevant inquiries and ensure comprehensive documentation of toxicities to alleviate these challenges.
The statistics on dyspnea and peripheral neuropathy, showing a κ of 0.54 and 0.63 respectively, highlight the importance of accurate clinician-patient communication in distinguishing between expected and unexpected serious adverse events in clinical trial.
Regulatory Oversight of Serious Adverse Events
Regulatory agencies, especially the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play an essential role in monitoring Serious Adverse Events within research studies. These organizations create comprehensive guidelines that outline the protocols for reporting serious adverse events, ensuring that researchers adhere to rigorous compliance standards. For example, the FDA mandates that SAEs be reported within specific timeframes determined by the severity and nature of the event, emphasizing the significance of ensuring participant safety and the integrity of clinical studies.
In Colombia, the National Food and Drug Surveillance Institute (INVIMA) serves as a Level 4 health authority by PAHO/WHO, overseeing medical device regulation and compliance. This role is crucial in multinational studies, where understanding local regulations can significantly impact study execution. Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, highlights the importance of adhering to local regulations to ensure successful outcomes.
Moreover, the guidelines set by regulatory agencies for SAE documentation are essential for participant safety and upholding the integrity of research studies. The Pharmacovigilance Risk Assessment Committee (PRAC) has introduced a checklist to prioritize collaborative impact research for identifying safety topics that need further data generation to monitor the effects of regulatory interventions. Recent guidelines from the FDA highlight the significance of using innovative data sources, such as social media and market research, to improve postmarket adverse event documentation.
Initiatives like the WEB-RADR project and the ADVANCE research endeavor aim to refine pharmacovigilance processes and improve vaccine benefit-risk monitoring. By enforcing these regulations and utilizing comprehensive research management services—ranging from feasibility studies to project management—authorities ensure that the data generated during studies is robust, and any potential risks associated with investigational products are thoroughly evaluated. Useful resources such as MedWatch forms for FDA safety documentation further support compliance with these necessary standards.
Best Practices for Managing Serious Adverse Events
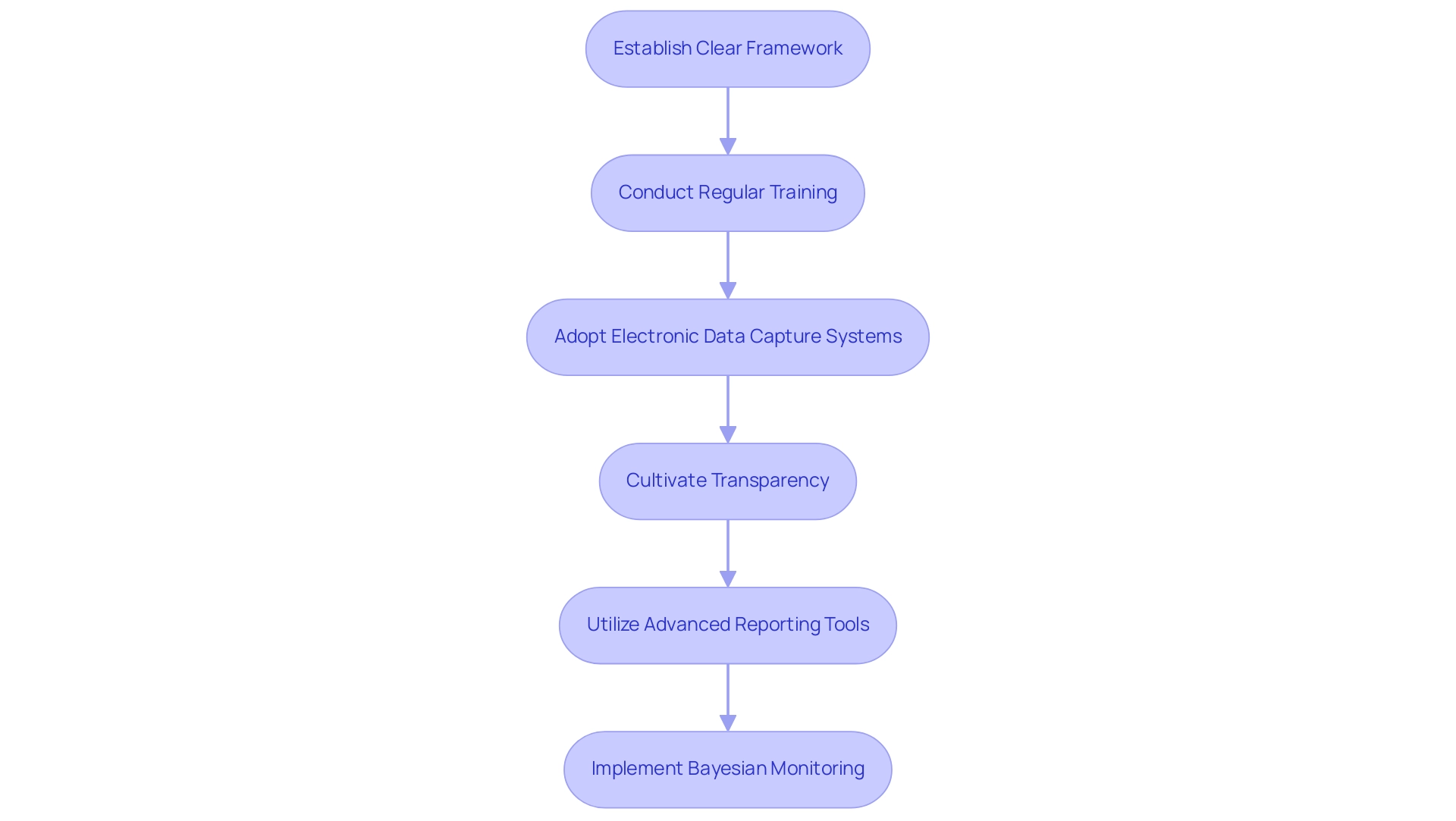
To effectively manage serious adverse events in clinical trials, researchers must implement a series of best practices that enhance both documentation efficiency and participant safety. A foundational step is to establish a clear framework that delineates roles and responsibilities within the research team. This clarity enhances communication and accountability, fostering a structured approach to SAE management.
Furthermore, regular training sessions focused on SAE definitions, reporting processes, and regulatory requirements are crucial. These sessions guarantee that all team members are well-informed and ready to manage serious accidents effectively.
Adopting electronic data capture systems significantly streamlines the documentation process. Such systems reduce the risk of mistakes and enhance data integrity, facilitating easier monitoring and reporting of serious adverse events in clinical trials accurately. Additionally, cultivating a culture of transparency and open communication is essential.
This environment encourages team members to report serious adverse events in clinical trials promptly and accurately, thereby contributing to participant safety and the overall success of research trials.
Recent advancements in SAE reporting frameworks, including the innovative capabilities of tools like JMP Clinical, offer researchers the ability to customize analyses, filter data, and generate regulatory-ready reports. As noted by Marija Lind, a JMP Senior Account Executive, "what a great article Chandramouli R! I have been conversing with numerous scientists from this region and they are primarily fascinated by JMP Clinical that research teams can efficiently analyze and report adverse events, ensuring patient safety and enhancing drug development processes.
And everything is in one tool." The integration of such tools enables research teams to efficiently analyze and report on serious adverse events in clinical trials, ultimately optimizing drug development processes.
Moreover, the implementation of Bayesian approaches for monitoring prespecified harm outcomes related to serious adverse events in clinical trials, as discussed in several case studies, provides valuable insights into effective SAE management practices. These methods allow for continuous monitoring of harm outcomes and guide decisions on study continuation based on predefined thresholds. By embracing these best practices, clinical trials can navigate the complexities of SAE management more effectively.
Conclusion
The multifaceted nature of Serious Adverse Events (SAEs) in clinical trials highlights the critical importance of effective management and reporting processes. From defining what constitutes an SAE to understanding the regulatory frameworks that govern them, it is evident that timely and accurate reporting is essential not only for participant safety but also for maintaining the integrity of clinical research. The increasing awareness of SAE reporting among healthcare facilities demonstrates a positive trend, yet challenges such as underreporting and miscommunication persist.
Addressing these challenges requires a commitment to robust training and clear communication within research teams. By fostering a culture that prioritizes participant safety and compliance, researchers can enhance the accuracy of SAE documentation. Furthermore, the integration of innovative tools and best practices, such as electronic data capture systems and Bayesian monitoring approaches, can significantly streamline the reporting process and improve overall outcomes.
Ultimately, the collaborative efforts of regulatory bodies, researchers, and healthcare facilities are vital in upholding ethical standards and ensuring that clinical trials are conducted with the highest level of integrity. As the landscape of clinical research continues to evolve, prioritizing the management of SAEs will be crucial in safeguarding participant well-being and advancing the development of safe and effective treatments.
Frequently Asked Questions
What is defined as a serious adverse event in a clinical trial?
A serious adverse event in a clinical trial is any untoward medical occurrence that results in death, is life-threatening, necessitates hospitalization, or leads to significant disability or incapacity.
What types of occurrences are considered serious adverse events?
Serious adverse events can include severe allergic reactions, substantial alterations in vital signs, or any event that poses a significant risk to a participant's health.
Why is it important to understand the criteria for serious adverse events in clinical trials?
Understanding these criteria is crucial for safeguarding participant well-being and maintaining the integrity of clinical research.
How do different safety monitoring methods affect the analysis of serious adverse events?
Recent analyses have shown variability in stopping boundaries produced by different safety monitoring methods, revealing notable differences in conservatism and aggressiveness at various stages of trials.
What role do software tools play in analyzing serious adverse events?
Various software tools have been implemented to analyze serious adverse events, with some publicly available R packages enhancing accessibility and reproducibility in statistical analyses.
What is the significance of documenting serious adverse events in clinical trials?
Documentation is a regulatory necessity and a cornerstone of clinical trial integrity, essential for participant safety and the trustworthiness of study results.
What trends have been observed in the documentation of serious adverse events in hospitals?
In 2023, hospitals documented rates that were 2.0 points higher than the previous year, indicating a growing acknowledgment of the significance of these records.
What are the potential consequences of non-compliance in reporting serious adverse events?
Non-compliance can lead to significant regulatory penalties, potential harm to participants, and compromise the overall trustworthiness of the study.
How does the analysis of serious adverse events contribute to clinical research?
It provides critical data that informs the risk-benefit analysis of investigational therapies, supporting the development of safe and effective treatments.
What comprehensive clinical trial management services are emphasized for maintaining ethical standards?
Services include feasibility studies, site selection, compliance reviews, import permits, nationalization of investigational devices, and thorough documentation processes.
How can researchers ensure they are adhering to ethical standards and regulatory compliance regarding serious adverse events?
Researchers are encouraged to prioritize the documentation of serious adverse events and consult their IRB of record and the NIAAA SAE Coordinator for further clarification.




