Introduction
In the realm of clinical research, the monitoring and management of Serious Adverse Events (SAEs) stand as a cornerstone of patient safety and research integrity. Defined as significant medical occurrences that may lead to serious outcomes, such as death or prolonged hospitalization, SAEs demand rigorous oversight throughout clinical trials.
The implications of these events extend beyond individual patient safety, influencing the ethical framework of research and the trust placed in medical advancements. With recent studies revealing alarming statistics regarding the risks associated with certain treatments, the need for effective SAE reporting and management has never been more critical.
This article delves into the multifaceted nature of SAEs, exploring their:
- Definitions
- Classifications
- Regulatory guidelines
- Essential tools required for comprehensive oversight
By understanding the complexities surrounding SAEs, stakeholders can enhance the safety of clinical trials and uphold the highest standards of medical research.
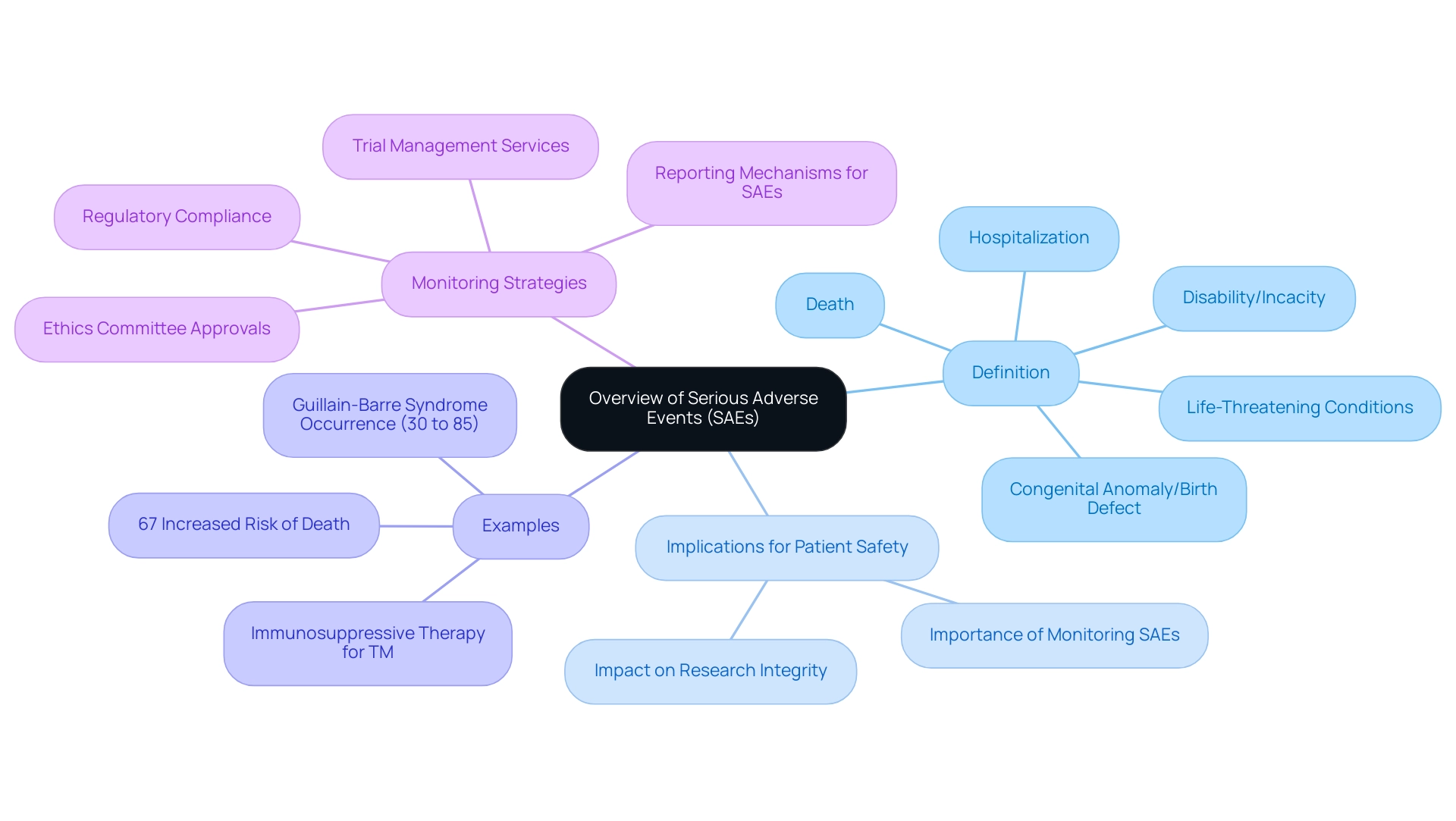
Defining Serious Adverse Events (SAEs): An Overview
Serious Adverse Events are defined by medical authorities as any untoward medical occurrence that leads to either death, is life-threatening, necessitates hospitalization, prolongs an existing hospitalization, results in persistent or significant disability/incapacity, or includes a congenital anomaly or birth defect. These occurrences can emerge during research studies and may be linked to the investigational product, the underlying health condition, or the study protocol itself. The emergence of serious adverse events poses significant implications for patient safety and the overall integrity of clinical research sae definition.
Recent studies indicate that the monitoring of serious adverse events is critical, as evidenced by findings that highlight a 67% increased risk of death associated with certain diet and supplement trials. Additionally, classical sensorimotor Guillain-Barre Syndrome (GBS) is reported to occur in 30% to 85% of cases, illustrating the frequency and potential severity of serious adverse events. As Ljubo Znaor notes, 'A.M. and LJ.Z. designed and supervised the study, participated in the interpretation of the results, and critical revision of the manuscript,' underscoring the importance of rigorous research oversight. Furthermore, immunosuppressive therapy may be beneficial for non-responsive Transverse Myelitis (TM) cases, suggesting potential treatment avenues related to serious adverse events.
Comprehending and efficiently overseeing serious adverse events guarantees not only the safety of participants but also the dependability of trial results. In conjunction with our comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, and ongoing project management—we meticulously oversee all aspects of research sae definition. This encompasses acquiring ethics committee approvals, ensuring adherence to INVIMA’s regulatory requirements, and implementing strong reporting mechanisms for serious adverse events.
By aligning our services with regulatory standards, we enhance local economic impact through Medtech research studies, thereby contributing to job creation, economic growth, and healthcare improvement.
The Importance of SAEs in Clinical Research and Patient Safety
Serious negative occurrences play a vital role in the realm of medical research, greatly impacting patient safety and the ethical integrity of studies. Regulatory authorities mandate the prompt reporting of SAEs to swiftly identify and address any potential risks associated with investigational treatments, as outlined in the research SAE definition. Significantly, comprehensive research study management services—including:
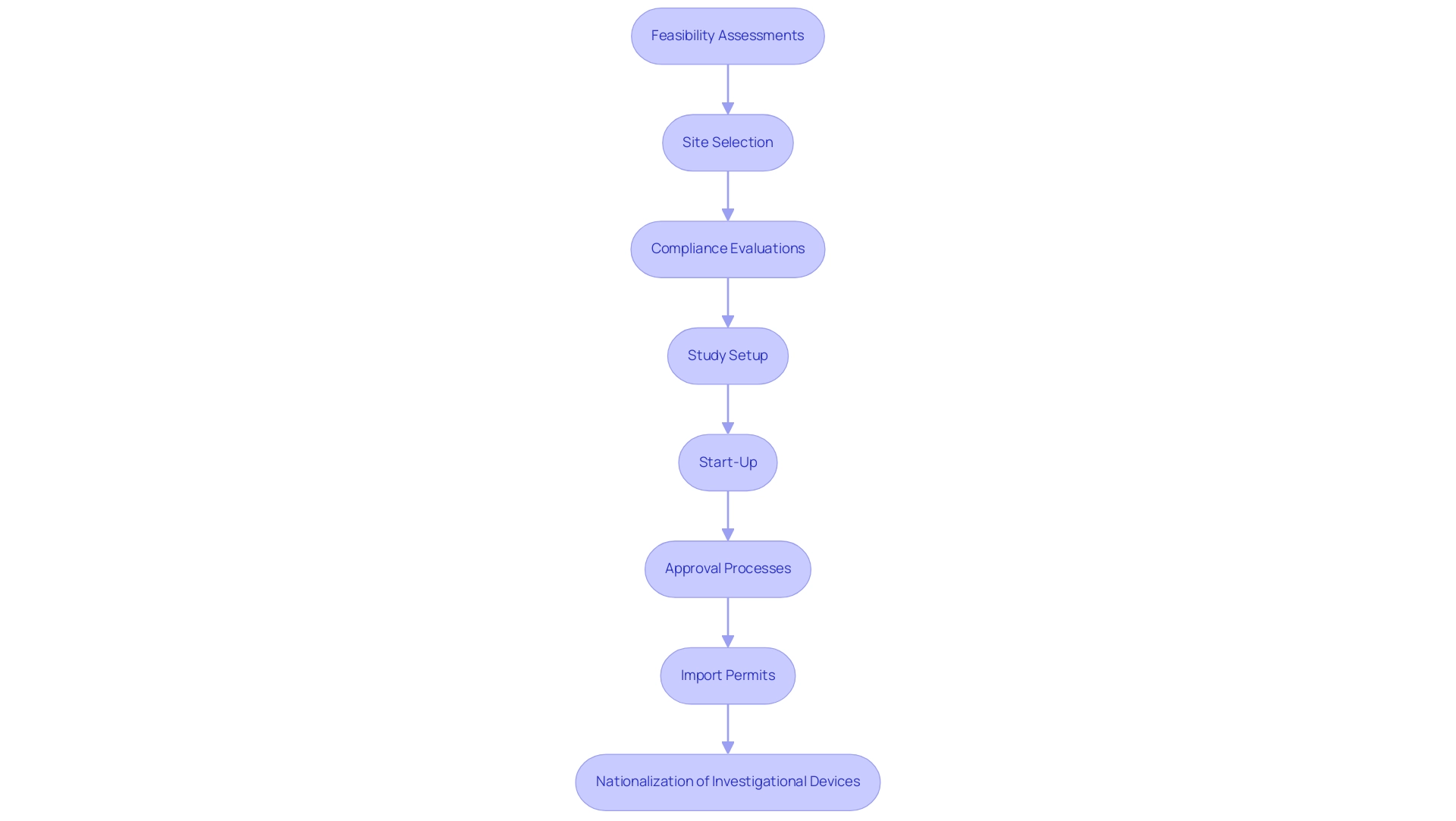
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Start-up
- Approval processes
- Import permits
- Nationalization of investigational devices
play a vital role in this process.
Data reveals that 73.0% of serious adverse events are documented effectively in reports, highlighting the ongoing efforts to improve transparency in this area. As Aron Shapiro highlights, this group is responsible for overseeing participant safety and treatment effectiveness information during the research studies. This vigilance enables researchers to make informed choices about the continuation of studies, prioritizing participant safety above all else.
Moreover, a well-organized SAE reporting system promotes public confidence in the research SAE definition; as John Doe notes,
Ambiguous reporting hinders creating a precise depiction of the safety profile.
In 2024, the research SAE definition will evolve as regulatory requirements for SAE reporting introduce stricter guidelines aimed at enhancing the clarity and comprehensiveness of SAE documentation. These changes underscore the need for researchers to stay informed and adapt their reporting practices accordingly.
Furthermore, the participation of specialists such as Katherine Ruiz in regulatory matters guarantees conformity to compliance benchmarks that are essential for successful research studies in Colombia. The case study titled 'Limitations of the Study' acknowledges potential biases in SAE reporting and highlights good practices that could be adopted to mitigate these issues. Ultimately, the thorough comprehension and documentation of serious adverse events, as outlined in the research SAE definition, not only protect participants but also maintain the ethical standards of medical research, emphasizing the significance of transparent communication and careful oversight throughout the research process.
This corresponds with the FDA's recommendations on statistical principles for research studies, which stresses the significance of precise data reporting in ensuring participant safety and preserving the integrity of medical research.
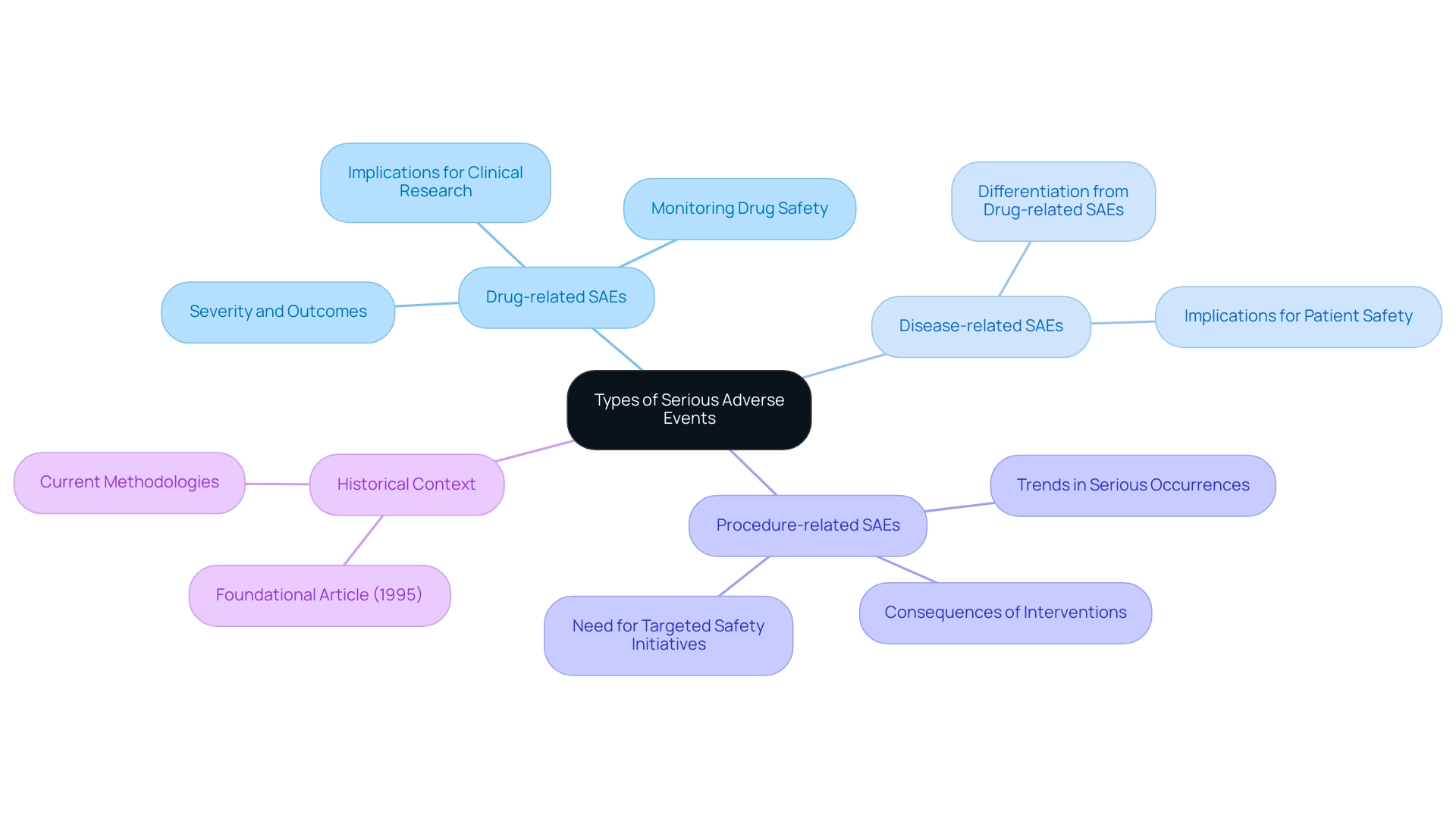
Types and Categories of Serious Adverse Events
Serious adverse events can be categorized into several distinct groups, each with its own implications for clinical research. Drug-related serious adverse events are those that are directly linked to the investigational product, emphasizing the significance of monitoring drug safety throughout the trial. In contrast, disease-related serious adverse events stem from the underlying medical condition of the participants and must be carefully differentiated to avoid misattribution of causality.
Procedure-related SAEs arise as a consequence of interventions or procedures performed during the study, further emphasizing the complexity of patient safety in clinical settings. Furthermore, these occurrences can be categorized according to severity, duration, and outcomes, which is essential for researchers aiming to identify trends and implement effective safety measures. Recent findings have shown that in 2023, serious occurrences reported by other acute care facilities predominantly involved complications related to procedures and various incident types, accounting for over 90% of serious occurrence reports.
This concentration underscores the necessity for targeted safety initiatives in these specific areas within other acute care settings. As articulated by Tan X., 'A hierarchical testing approach for detecting safety signals in trials' is vital for classifying and understanding these adverse events effectively. The classification of serious adverse events is crucial for safeguarding participant welfare and also contributes to the overall integrity of clinical research SAE definition.
Historical context is also important; a foundational article published in 1995 in the J R Stat Soc Series B Stat Methodology has laid the groundwork for current methodologies in SAE classification.
Reporting Requirements and Regulatory Guidelines for SAEs
Regulatory agencies, including the FDA and EMA, have implemented stringent guidelines that include the research SAE definition for reporting Serious Adverse Events (SAEs). Researchers are mandated to report these occurrences promptly, typically within 24 hours of awareness, in accordance with the research SAE definition to foster a proactive approach to participant safety. Each report must encapsulate comprehensive details about the occurrence, covering aspects such as its nature, severity, and any corrective actions taken, in accordance with the research SAE definition.
The FDA underscores the significance of meaningful information concerning adverse occurrences, stating that 'meaningful AE information' is critical for compliance with reporting requirements, which is not merely a regulatory formality but a vital component of safeguarding participant welfare throughout the research SAE definition. Furthermore, it is essential for multi-site studies to integrate the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) required timeline for reporting adverse events into their internal processes. Alongside these regulatory frameworks, our service capabilities encompass:
- Feasibility studies
- Selection of research sites
- Study set-up
- Compliance reviews
- Monitoring of Sales to ensure adherence to both local and international standards
We also handle import permits and the nationalization of investigational devices, providing comprehensive support throughout the trial process. Notably, WCG IRBs have a custom board of nearly 200 experts to ensure swift determination of protocols and documents, highlighting the importance of efficient processes in the research SAE definition reporting. For instance, the SF VA Medical Center has established distinct timelines and the research SAE definition for reporting certain categories of post-approval occurrences, showcasing the necessity for researchers to navigate varying institutional guidelines effectively.
Additionally, all adverse occurrences must be reported to NIAMS and the monitoring body, regardless of their expectedness, relatedness, or severity, and reported in aggregate as part of routine Data and Safety Monitoring Reports. This adherence not only facilitates regulatory approval but also ensures ongoing monitoring and management of participant safety, aligning with the regulatory oversight provided by INVIMA as a Level 4 health authority by PAHO/WHO.

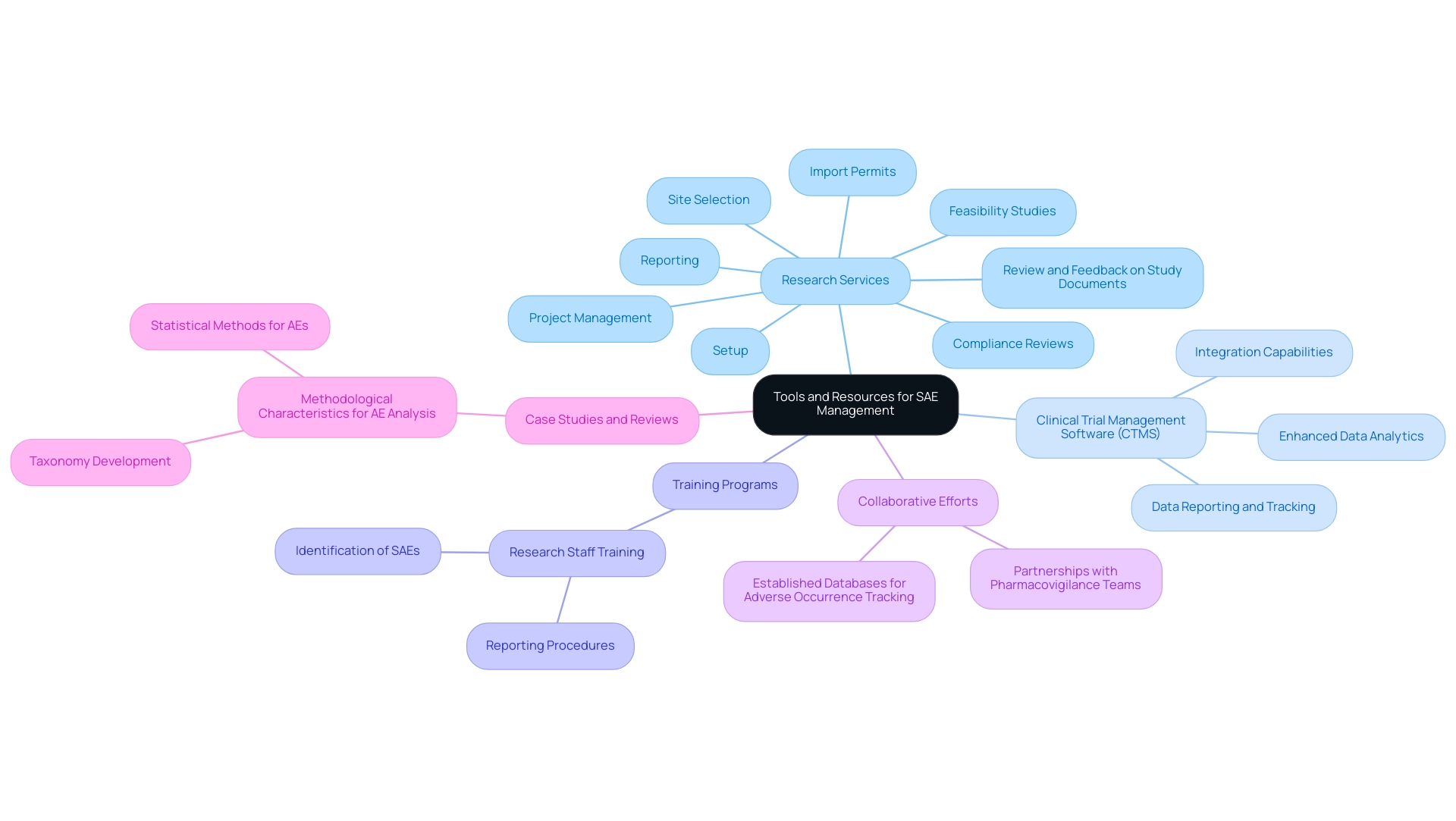
Tools and Resources for Managing Serious Adverse Events
Effectively managing serious adverse events (SAEs) necessitates a robust combination of tools and resources, which underscores the need for a comprehensive research SAE definition and management services. Our capabilities encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Review and feedback on study documents to adhere to country requirements
- Project management
- Reporting
Since September 2006, we have conducted audit site visits for 35 studies across 22 clinical research sites, highlighting the importance of diligent oversight in SAE management.
Clinical trial management software (CTMS) plays a critical role in streamlining the reporting and tracking of serious adverse events, ensuring that all pertinent data is captured with precision. Training programs aimed at research staff on the identification and reporting of SAEs significantly enhance awareness and prompt responsiveness. Joint efforts with pharmacovigilance teams, along with established databases for adverse occurrence tracking, further enhance compliance and participant safety.
Recent advancements in CTMS, including enhanced data analytics and integration capabilities, have proven instrumental. For example, the review titled 'Methodological Characteristics for AE Analysis' showcases a structured approach to analyzing adverse events through a developed taxonomy that classifies methods based on their requirements for prespecified or emerging events. The authors wish to thank June Tran, Amy Holbert, and Meredith Nahm for their helpful reviews on this manuscript, emphasizing the collaborative nature of this field.
By accessing these tools and resources, we not only facilitate effective SAE management but also contribute to the overall quality and integrity of research SAE definition, positively impacting local economies through job creation and healthcare improvement.
Conclusion
The management of Serious Adverse Events (SAEs) is paramount in ensuring the safety of participants and the integrity of clinical research. From defining SAEs to understanding their classifications, the article highlights that these events can arise from various sources, including investigational products and underlying health conditions. The importance of timely and accurate reporting, as mandated by regulatory guidelines, cannot be overstated. This vigilance not only protects participants but also fosters public trust in the research process.
Moreover, the article emphasizes the critical role that comprehensive clinical trial management services play in monitoring SAEs. By employing advanced tools and resources, researchers can navigate the complexities of SAE management effectively. The integration of clinical trial management software and targeted training programs enhances the ability to identify and respond to SAEs promptly, ensuring a proactive approach to participant safety.
Ultimately, the commitment to rigorous SAE oversight is essential for the ethical conduct of clinical trials. By adhering to regulatory standards and implementing robust reporting mechanisms, stakeholders can uphold the highest standards of medical research. This dedication not only safeguards participant welfare but also contributes to the advancement of medical knowledge and the trustworthiness of clinical outcomes. As the landscape of clinical research evolves, the continuous improvement in SAE management will remain a cornerstone of patient safety and research integrity.
Frequently Asked Questions
What are Serious Adverse Events (SAEs)?
Serious Adverse Events are defined as any untoward medical occurrence that leads to death, is life-threatening, necessitates hospitalization, prolongs an existing hospitalization, results in persistent or significant disability/incapacity, or involves a congenital anomaly or birth defect.
Why is monitoring Serious Adverse Events important in clinical research?
Monitoring Serious Adverse Events is critical for patient safety and the integrity of clinical research. Recent studies indicate a significant risk of death associated with certain trials, highlighting the need for vigilant oversight.
What implications do Serious Adverse Events have for clinical research?
Serious Adverse Events can impact patient safety and the ethical integrity of studies. They require prompt reporting to identify and address potential risks associated with investigational treatments.
What are some examples of Serious Adverse Events?
Examples include life-threatening conditions, hospitalizations, significant disabilities, and congenital anomalies that may arise during research studies.
How are Serious Adverse Events reported and managed?
Serious Adverse Events are documented in reports, and comprehensive study management services oversee their reporting, including feasibility assessments, site selection, compliance evaluations, and adherence to regulatory requirements.
What percentage of Serious Adverse Events are effectively documented?
Data indicates that 73.0% of Serious Adverse Events are documented effectively in reports, reflecting ongoing efforts to improve transparency in this area.
What changes are expected in the reporting of Serious Adverse Events in 2024?
In 2024, regulatory requirements for SAE reporting will introduce stricter guidelines aimed at enhancing the clarity and comprehensiveness of SAE documentation.
Who is responsible for overseeing participant safety during research studies?
A dedicated group is responsible for overseeing participant safety and treatment effectiveness information, enabling informed decisions about the continuation of studies.
How does the participation of specialists impact SAE reporting?
Specialists involved in regulatory matters ensure compliance with benchmarks essential for successful research studies, thereby improving the quality of SAE reporting.
What role does transparent communication play in managing Serious Adverse Events?
Transparent communication and careful oversight throughout the research process protect participants and maintain the ethical standards of medical research.




