Introduction
In the realm of clinical research, the meticulous management of data is not merely a procedural necessity but a fundamental pillar that underpins the integrity and success of clinical trials. Clinical Data Management (CDM) plays a pivotal role in ensuring that the data collected during these trials is accurate, consistent, and compliant with regulatory standards.
As organizations navigate the complexities of clinical studies, the importance of implementing robust CDM practices becomes increasingly evident, impacting not only the quality of research outcomes but also the broader economic and healthcare landscape.
This article delves into the multifaceted aspects of CDM, exploring its significance, the various solutions available, key features to consider when selecting a system, and the critical need for compliance and security. Furthermore, it highlights emerging trends and innovations that are shaping the future of clinical data management, offering insights into how organizations can leverage these advancements to enhance their research capabilities and improve patient outcomes.
Understanding Clinical Data Management: Importance and Benefits
Clinical Information Management (CIM) acts as a foundation of the research ecosystem, ensuring the precision, consistency, and regulatory adherence of information gathered during studies. Our extensive research study management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting on adverse events, both serious and non-serious
These capabilities are vital as they directly correlate with enhanced information quality, essential for making informed decisions based on research findings.
For instance, in the old age subgroup, the response rate for the new therapy is 0.7, compared to 0.35 for the control, yielding a relative risk of 4.33. This statistic emphasizes the vital role of clinical data management solutions in ensuring the reliability of research results and highlights the significant impact of Medtech studies on local economies, as we create jobs, promote economic growth, and enhance healthcare through international collaboration. Among the myriad benefits of robust clinical data management solutions are:
- Enhanced patient safety
- Increased efficiency in information collection and analysis
- Streamlined study processes
Furthermore, effective clinical data management solutions fortify the integrity of clinical trials, thereby supporting the advancement of medical knowledge and enhancing patient outcomes. As articulated by Scott R. Evans, Ph.D. from Harvard University, common statistical concerns, including the need for presenting confidence intervals and adherence to the intent-to-treat principle, underscore the necessity of rigorous information management practices. Additionally, it is crucial that the data set be suitable and statistically valid to positively impact the study's outcome.
The case study regarding subgroup analyses illustrates that while these analyses can evaluate treatment effects in specific patient subsets, they can also be misinterpreted and subject to biases, especially when not pre-specified. This highlights the importance of conducting subgroup analyses selectively and reporting them transparently. With the increasing focus on statistical validity in research studies, the role of clinical data management solutions in ensuring the reliability of these statistics is more crucial than ever, particularly in the context of regulatory oversight by organizations like INVIMA, the Colombian National Food and Drug Surveillance Institute, which plays a key role in maintaining health standards and promoting safety and effectiveness in research.
Furthermore, adherence to national standards during the evaluation and response on research documents is crucial to guarantee that all facets of the study align with local regulations, further reinforcing the beneficial economic effect of our research endeavors.
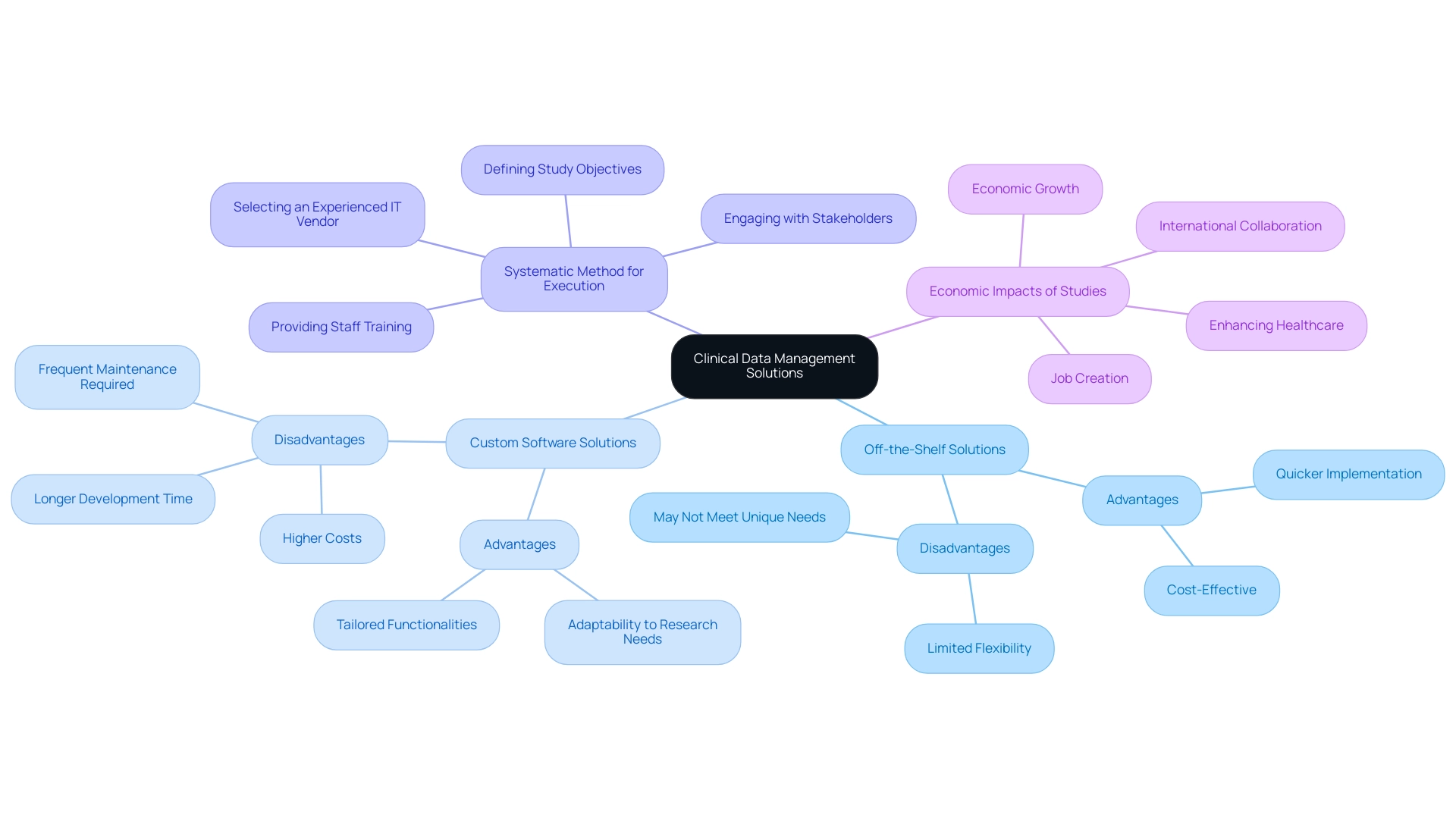
Exploring Different Clinical Data Management Solutions: Off-the-Shelf vs. Custom Software
Selecting the appropriate clinical data management solutions is a vital choice for organizations, often reducing to an option between off-the-shelf software and custom-built systems. Off-the-shelf solutions are typically advantageous due to their quicker implementation timelines and cost-effectiveness, making them ideal for smaller studies or institutions with budget constraints. However, these clinical data management solutions may not provide the flexibility needed to accommodate the unique requirements of specific research trials.
In contrast, custom software solutions offer tailored functionalities that can evolve according to the specific needs of a research project. While these custom systems, which include clinical data management solutions, often come with a higher price tag and extended development time, they provide significant adaptability and can better align with the evolving demands of research. It's essential to consider that custom healthcare software requires frequent maintenance and security updates to ensure optimal functionality and safeguard sensitive information.
Adhering to industry standards is also essential for the integrity of research. A systematic method for execution, as detailed in best practices for clinical data management solutions, involves:
- Defining study objectives
- Engaging with stakeholders
- Selecting an experienced IT vendor
- Providing staff training
Key services such as trial setup, import permits, and project coordination must be carefully integrated into the planning process.
Significantly, when we perform studies, the effect on local economies generates ripples with extensive advantages, including:
- Job creation
- Promoting economic growth
- Enhancing healthcare
- Encouraging international collaboration
Therefore, when evaluating these options, organizations must carefully weigh factors such as project scope, budgetary limitations, compliance with industry standards, long-term research objectives, and the broader implications of their studies on the communities they engage with.
Key Features to Consider When Choosing a Clinical Data Management System
Choosing efficient clinical data management solutions is essential for enhancing research results, and organizations need to thoughtfully evaluate several important characteristics. Foremost among these is the implementation of rigorous information validation processes that ensure both accuracy and consistency throughout the research lifecycle. This corresponds with our comprehensive clinical trial oversight services, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Review and feedback on study documents
- Project coordination
Complementing information validation are comprehensive audit trails, essential for tracking changes and maintaining integrity, regulatory compliance, and quality control. A user-friendly interface is vital, facilitating ease of use for researchers and enhancing operational efficiency. Furthermore, the ability to integrate seamlessly with other systems, robust support for electronic data capture (EDC), and advanced reporting capabilities significantly contribute to efficient data management.
The impact of our Medtech research studies extends beyond investigation, fostering job creation and economic growth while improving healthcare outcomes through international collaboration. Notably, the clinical data management solutions market is expected to grow at a 13.6% CAGR during the forecast period for 2024-2031, underscoring the increasing importance of these solutions. Recent advancements, such as those observed with Oracle's Clinical Experiment Management System (CTMS) Cloud Service chosen by ONO Pharmaceutical Co., Ltd. in March 2024, emphasize the importance of workflow automation in optimizing research processes.
As mentioned in the case study titled 'Workflow Automation and Task Management in Clinical Research,' CTDMS integrates workflow automation to improve accountability and adherence in research studies. By prioritizing these functionalities, including import permit and nationalization of investigational devices, organizations can enhance their research processes, ensuring transparency and adherence to regulatory standards, ultimately leading to improved outcomes in trial studies.
Ensuring Compliance and Security in Clinical Data Management
Ensuring adherence and safety in research information handling is crucial for safeguarding patient details and maintaining the integrity of medical studies. Organizations must align with regulations such as Good Clinical Practice (GCP) and the Health Insurance Portability and Accountability Act (HIPAA) to safeguard sensitive data effectively. Our comprehensive clinical data management solutions include clinical study oversight services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Project coordination
- Detailed reporting
This ensures that every aspect of your research adheres to necessary regulations.
The project management process involves meticulous planning, execution, and monitoring of trials to ensure they are completed on time and within budget, while our reporting services cover:
- Study status
- Inventory management
- Serious and non-serious adverse events
Furthermore, we assist with import permits and the nationalization of investigational devices, which are critical for compliance in Colombia. The implementation of robust security measures—including encryption, stringent access controls, and routine audits—plays a crucial role in mitigating the risk of breaches.
Alarmingly, 35% of business and tech executives regard third-party breaches as one of the most concerning cyber threats, with 28% expressing a lack of preparedness to address this issue, as highlighted in PwC's 2025 Global Digital Trust Insights. As one executive remarked, "The growing dependence on third-party services exposes us to external threats, and we must prioritize our security measures to safeguard our information." By prioritizing compliance and security, organizations not only foster trust among stakeholders but also enhance the credibility of their research.
A relevant example can be observed in the role of medical coders, who transform descriptions of adverse events and medical histories into standardized codes, facilitating efficient analysis and significantly enhancing overall quality. Moreover, understanding the regulatory landscape is crucial, especially with INVIMA's oversight on medical devices, ensuring compliance with national standards as a Level 4 health authority recognized by PAHO/WHO. Organizations can stay informed about compliance and security through upcoming speaker events, training series, and free webinars available in the Events & Training center.
Thus, as organizations navigate the changing landscape of compliance requirements, implementing effective information oversight strategies will be essential in future-proofing their processes against emerging regulations and technologies.
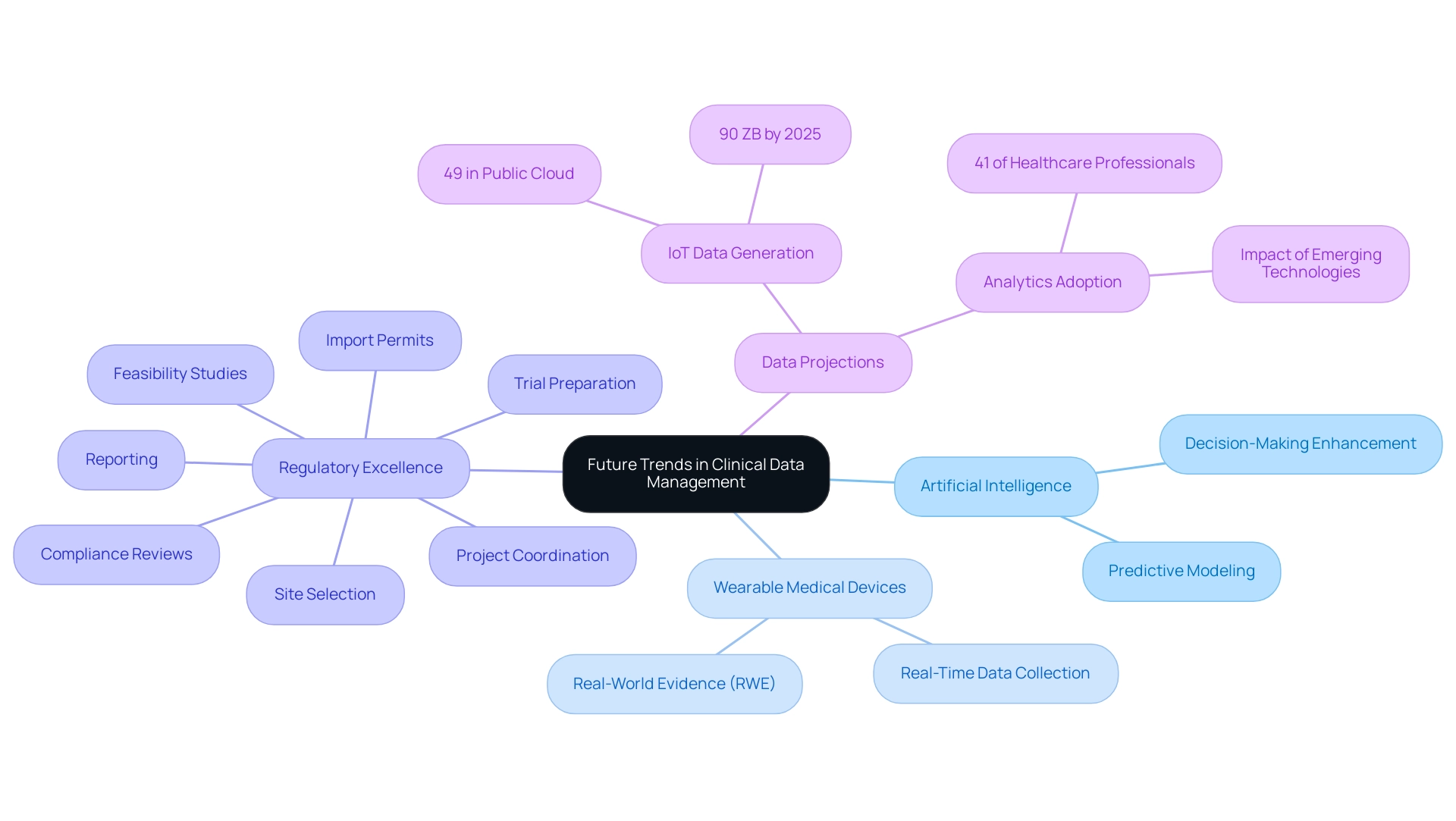
Future Trends in Clinical Data Management: Innovations and Technologies
The area of healthcare information management is undergoing swift change, propelled by technological progress and creative approaches. Dr. Sergio Alvarado, in his role as a Clinical Trial Manager at bioaccess®, embodies this transition by concentrating on cutting-edge medical research and the incorporation of artificial intelligence (AI) for analysis and predictive modeling, improving decision-making processes within research trials throughout Latin America. Dr. Alvarado, who holds an M.D. from Universidad de Los Andes and has extensive experience in medical research, emphasizes the importance of leveraging AI to enhance patient outcomes.
As noted by Warman O'Brien:
- "From the utilization of artificial intelligence and machine learning to enhance the productivity of Clinical Data Management solutions teams to the development of wearable medical devices that enable the collection of real-time data and real-world evidence (RWE), opportunities abound for CDM specialists to modernize the practice of data collection, analysis, and reporting."
Moreover, bioaccess® is dedicated to regulatory excellence, supervising extensive trial oversight services that include:
- feasibility studies
- site selection
- compliance reviews
- trial preparation
- import permits
- project coordination
- reporting
This robust approach not only supports Medtech associates in navigating the complexities of clinical research but also contributes to local economies by creating jobs, promoting economic growth, and improving healthcare access. The integration of blockchain technology further enhances information integrity and traceability, addressing longstanding challenges in information handling. As organizations adjust to these changing trends, the agility in incorporating new solutions that simplify processes and improve information quality becomes crucial.
Notably, projections indicate that by 2025, IoT devices will generate an astounding 90 ZB of data—49% of which is expected to be stored in public cloud environments. This statistic highlights the extent of information handling challenges and opportunities that lie ahead. Moreover, Accenture states that 41% of healthcare experts have adopted extensive analytics, improving the quality and precision of healthcare decisions.
This real-world example emphasizes the modernization of trials and the critical role of data management in achieving better patient outcomes. In this context, Mahalo Health's integration of traditional healing methods with technology serves as a compelling illustration of innovation in healthcare, demonstrating how personalized healthcare solutions can be enhanced through telemedicine and patient-centric tools. Staying informed about these innovations is crucial for maintaining a competitive edge in clinical research.
Conclusion
Clinical Data Management (CDM) is essential in clinical research, ensuring the accuracy, consistency, and regulatory compliance of collected data. This meticulous management supports the integrity of research outcomes, drives economic growth, and enhances patient safety through efficient data collection.
Choosing the right CDM solution is crucial, with organizations weighing the benefits of off-the-shelf systems against custom software options. Off-the-shelf solutions offer quick implementation and cost savings, while custom systems provide tailored functionalities that better meet specific trial needs. Key features such as:
- Data validation
- User-friendly interfaces
- Seamless integration
are vital for optimizing research and ensuring compliance.
Compliance and security are paramount in protecting sensitive patient information. Adhering to regulations and implementing robust security measures not only safeguard data but also enhance the credibility of research efforts.
As the field of clinical data management evolves, innovations like artificial intelligence and blockchain technology present new opportunities and challenges. Staying informed about these advancements is essential for organizations looking to improve their research capabilities and patient outcomes.
In conclusion, prioritizing effective CDM practices is critical for conducting reliable clinical trials and positively impacting the healthcare landscape. By embracing rigorous data management, organizations can navigate the complexities of clinical research and contribute to a healthier future.
Frequently Asked Questions
What is Clinical Information Management (CIM)?
Clinical Information Management (CIM) serves as the foundation of the research ecosystem, ensuring the precision, consistency, and regulatory adherence of information gathered during studies.
What services are included in the research study management offerings?
The services include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting on both serious and non-serious adverse events.
How does CIM impact the quality of research information?
CIM enhances information quality, which is essential for making informed decisions based on research findings, ultimately improving patient safety and the efficiency of information collection and analysis.
What are the benefits of robust clinical data management solutions?
Benefits include enhanced patient safety, increased efficiency in data collection and analysis, streamlined study processes, and fortification of the integrity of clinical trials.
Why is statistical validity important in clinical research?
Statistical validity ensures that the data set is suitable and statistically valid, positively impacting the study's outcomes and supporting adherence to regulatory standards.
What are the considerations when selecting clinical data management solutions?
Organizations must consider factors such as project scope, budgetary constraints, compliance with industry standards, long-term research objectives, and the economic implications of their studies.
What are the differences between off-the-shelf and custom-built clinical data management solutions?
Off-the-shelf solutions are cost-effective and quicker to implement but may lack flexibility, while custom-built solutions offer tailored functionalities but come with higher costs and longer development times.
What is the role of compliance in clinical data management?
Compliance with regulations such as Good Clinical Practice (GCP) and HIPAA is essential for safeguarding sensitive data and ensuring the integrity of medical studies.
How do clinical data management solutions support local economies?
These solutions contribute to job creation, promote economic growth, and enhance healthcare through international collaboration.
What emerging technologies are influencing clinical data management?
Technologies such as artificial intelligence (AI) and blockchain are being integrated into clinical data management to enhance analysis, predictive modeling, and information integrity.




