Overview
Latin America is emerging as a key region for clinical research due to its rapid growth in research infrastructure, cost-effectiveness, and diverse patient populations, which collectively enhance the region's appeal for global medical studies. The article supports this by detailing significant investments in research facilities, the establishment of patient-centric trial designs, and the advantages of conducting studies at lower costs, all of which contribute to increased participation and improved outcomes in clinical research initiatives.
Introduction
In recent years, Latin America has emerged as a dynamic player in the global clinical research landscape, showcasing rapid advancements and a commitment to innovation. With substantial investments in research infrastructure and a growing number of clinical research organizations, countries like Brazil, Mexico, and Argentina are increasingly attractive to global sponsors seeking high-quality trials.
This article delves into the multifaceted growth of clinical research in the region, highlighting:
- The shift towards patient-centric approaches
- The rise of decentralized trials
- The evolving regulatory environment that supports these initiatives
By addressing operational challenges and leveraging diverse patient populations, Latin America is not only enhancing its clinical capabilities but also positioning itself as a key contributor to the future of medical research. As the region continues to evolve, it promises to play a pivotal role in shaping the global healthcare landscape.
1. Rapid Growth of Clinical Research Infrastructure in Latin America
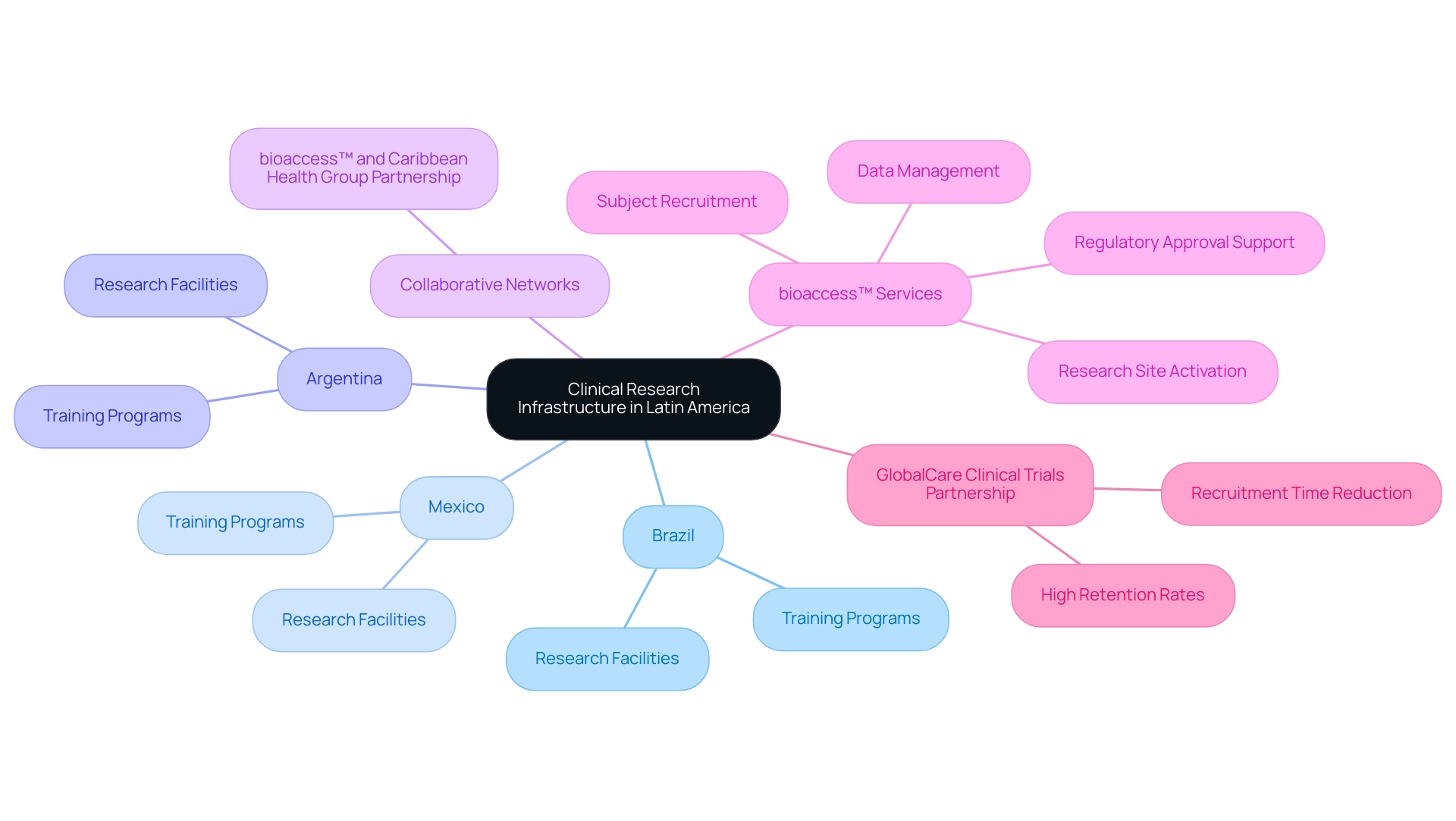
Over the past ten years, Latin America: emerging clinical research region has experienced significant development in its research infrastructure, particularly in Brazil, Mexico, and Argentina. These nations have made substantial investments in state-of-the-art research facilities and comprehensive training programs designed for medical researchers. As of 2024, the count of research organizations (CROs) functioning in Latin America: emerging clinical research region is anticipated to exceed 150, indicating a growing interest from international firms to utilize the region's capacity for high-quality research.
This expansion is additionally supported by collaborative research networks, such as the partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, aimed at positioning Barranquilla as a premier destination for trials in Latin America. Backed by Colombia's Minister of Health, this initiative demonstrates the dedication to improving research capabilities in the region. Notably, bioaccess® has emerged as a specialized CRO facilitating research for Medtech startups, focusing on rapid, cost-effective, high-quality trial data.
Their services encompass:
- Regulatory approval support
- Research site activation
- Subject recruitment
- Data management
GlobalCare Clinical Trials, in association with bioaccess™, achieved over a 50% reduction in recruitment time and 95% retention rates, showcasing the efficacy of these partnerships. Such advancements place Latin America: emerging clinical research region as a more appealing location for worldwide medical research initiatives, coinciding with its ambitions for expansion in the healthcare field.
Furthermore, Colombia's initiatives to advance research studies are anticipated to encourage social and economic development, in line with its aim to transform into a knowledge-based economy by 2031.
2. Emphasis on Patient-Centricity in Clinical Trials
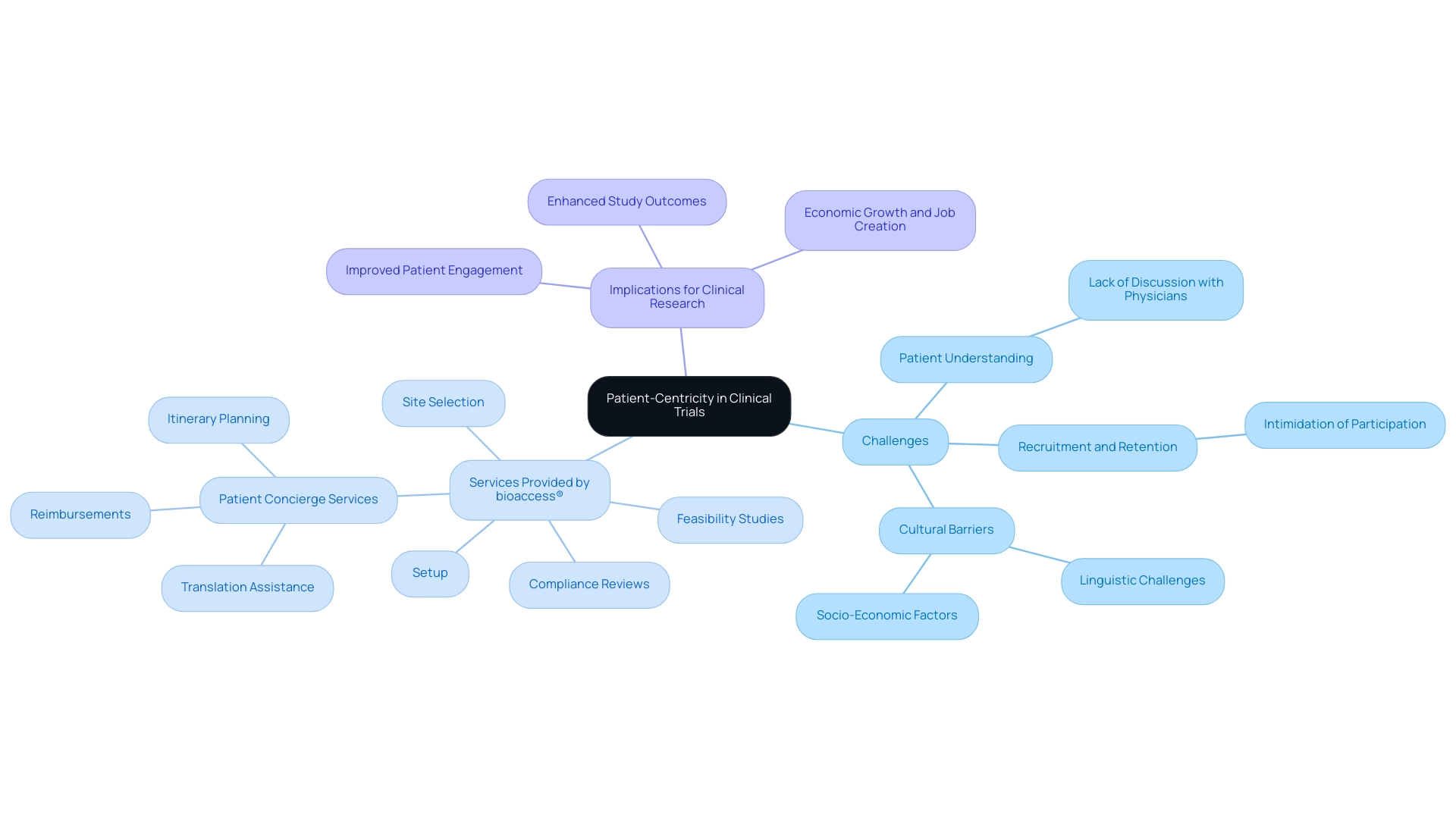
In recent years, Latin America has experienced a notable change towards patient-centricity in research, highlighting the necessity to prioritize the preferences and experiences of participants. This approach encompasses adaptable testing designs, tailored communication strategies, and robust support systems for those involved. Such initiatives are essential, particularly given that only 15% of Healthcare Organizations (HCOs) are presently performing research, signaling a significant chance for enhancement in patient engagement.
Notably, many patients in Latin America may accept participation in clinical studies without fully discussing treatment options or the associated risks with their physician, highlighting a critical gap in patient understanding and engagement.
To address these challenges, bioaccess® offers a comprehensive suite of clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
These services ensure adherence to local regulations and promote effective research outcomes. The testing setup process involves detailed planning and coordination with stakeholders to ensure all regulatory requirements are met. Project management encompasses continuous monitoring and communication with all parties involved, ensuring that the study progresses smoothly and efficiently.
Reporting encompasses routine updates on study status, inventory management, and documentation of serious and non-serious adverse events, which are essential for upholding compliance and transparency.
By implementing patient concierge services, sponsors and Contract Research Organizations (CROs) like bioaccess® are effectively tackling recruitment and retention challenges. These services reduce the intimidation often linked to participation by managing logistics such as itinerary planning, reimbursements, and translation assistance, thus leading to a more positive experience for patients and their families. This direct support not only improves the overall experience but also helps reduce the intimidation that can hinder participation.
Moreover, the focus on patient experience not only boosts recruitment and retention rates but also relates to better study outcomes. As noted by expert Eliseth Leão,
Mais do que momentos de contemplação, a beleza percebida no cotidiano também proporciona sensações e experiencias que nos ajudam a manter-nos mais equilibrados e saudáveis.
This sentiment highlights the importance of incorporating patient feedback and preferences into study designs.
As the number of outsourced medical studies continues to rise in countries like Peru, Colombia, and Chile, tackling linguistic, cultural, and socio-economic barriers becomes even more crucial. Ultimately, prioritizing patient-centricity in research studies within Latin America: emerging clinical research region is not just beneficial for participants; it elevates the overall quality and effectiveness of research initiatives across the region, contributing to job creation, economic growth, and healthcare improvement.
In addition, bioaccess® is committed to ensuring the security of participant data throughout the research process. We implement reasonable security measures to prevent the loss, misuse, or unauthorized alteration of information under our control. However, given the inherent risks, we cannot guarantee absolute security, and consequently, we cannot ensure or warrant the security of any information transmitted to us.
For any concerns regarding data processing, participants can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®').
3. Cost-Effectiveness and Access to Diverse Patient Populations
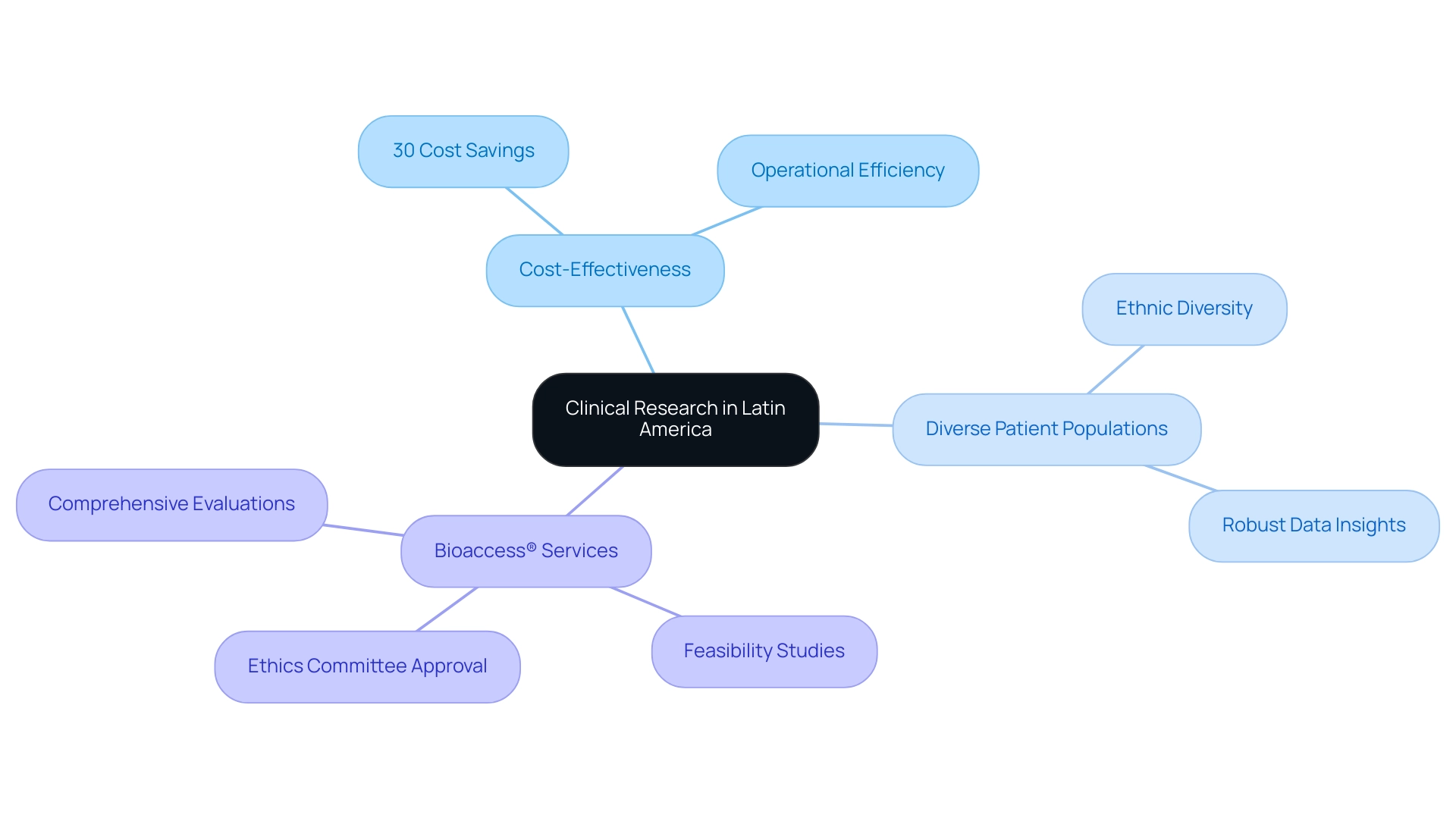
Carrying out medical studies in Latin America: emerging clinical research region offers considerable benefits, especially regarding cost efficiency and extensive study management services. The region is defined by reduced operational costs, such as labor and facility expenses, enabling sponsors to conduct trials affordably—up to 30% less expensive compared to those in North and South regions. This cost advantage is crucial as clinical research budgets face increasing scrutiny and demands for efficiency.
Additionally, the region is home to a richly diverse patient population, essential for studies requiring varied demographic representation, enhancing the robustness of collected data. For instance, the inclusion of varied ethnic groups leads to pivotal insights for developing universally applicable medical solutions. Bioaccess® leverages over 20 years of expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring compliance with local regulations and effective project management.
Our services include:
- The feasibility and selection of research locations
- Comprehensive evaluations of study documents to comply with national requirements
- Setup, initiation, and the approval process involving ethics committees and health ministries
As Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, states, 'I am personally and enthusiastically devoted to assisting biotech leaders navigate the complexities of research across various regions, including South America, where diverse patient demographics can significantly impact study results.'
This blend of cost reductions and abundant diversity establishes Latin America: emerging clinical research region as an appealing choice for performing research in 2024 and beyond, aiding in job creation, economic expansion, and global cooperation in healthcare.
The influence of medical research goes beyond study results, supporting local economies through job creation and improved healthcare services, ultimately resulting in a stronger healthcare infrastructure.
4. The Rise of Decentralized Clinical Trials
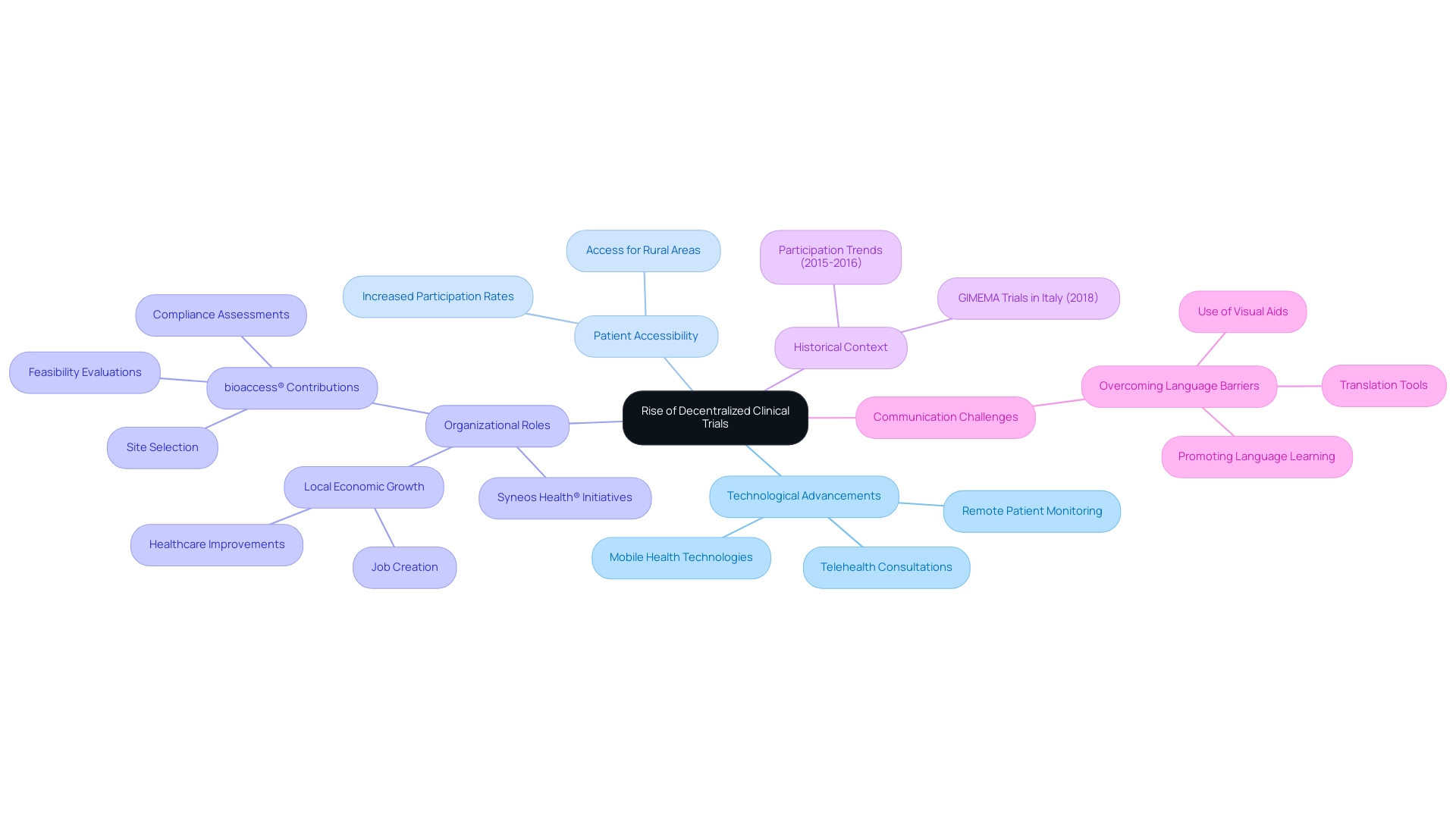
Decentralized research studies are quickly gaining momentum in Latin America: emerging clinical research region, fueled by major progress in technology and a shift towards more adaptable study designs. These innovative experiments facilitate remote patient monitoring, telehealth consultations, and the integration of mobile health technologies, leading to a notable increase in patient participation rates. By reducing the need for patients to visit medical locations, decentralized studies improve accessibility, especially for individuals living in rural or underserved areas.
This shift not only encourages greater patient involvement but also enhances the efficiency of execution. As emphasized by Syneos Health®, a leading fully integrated biopharmaceutical solutions firm, the introduction of a decentralized research site network seeks to enhance the adoption of suitable DCTs while delivering high-quality digital health solutions. Moreover, media coverage from Clinical Leader highlights the increasing interest in research in Latin America: emerging clinical research region, particularly in Colombia, demonstrating the region's potential for significant studies.
Particular articles from Clinical Leader describe how decentralized studies are tackling regional healthcare issues and enhancing patient access to research. The continuous expansion of decentralized research studies indicates a crucial point in the advancement of medical investigations, particularly as the Clinical Microbiology Market is expected to attain USD 7.78 billion by 2033, highlighting the growing importance of such innovations in the domain. Furthermore, entities like bioaccess® are establishing themselves as prominent contract research organizations, utilizing approaches such as efficient site selection, feasibility evaluations, and compliance assessments to support medical device research in the southern region of the continent.
Their efforts contribute to local economic growth through job creation and healthcare improvements. To navigate this evolving landscape effectively, it is crucial to address public criticism from team members calmly and constructively, as this can influence the implementation of decentralized studies. Historical information, such as the research trials overseen by GIMEMA in Italy in 2018, and participation figures in the southern continent from 2015 to 2016, offer context for the current growth trends in the region.
Insights from case studies illustrate the importance of overcoming communication barriers, which is particularly relevant in the varied landscape of the regions.
5. Evolving Regulatory Landscape Supporting Clinical Research
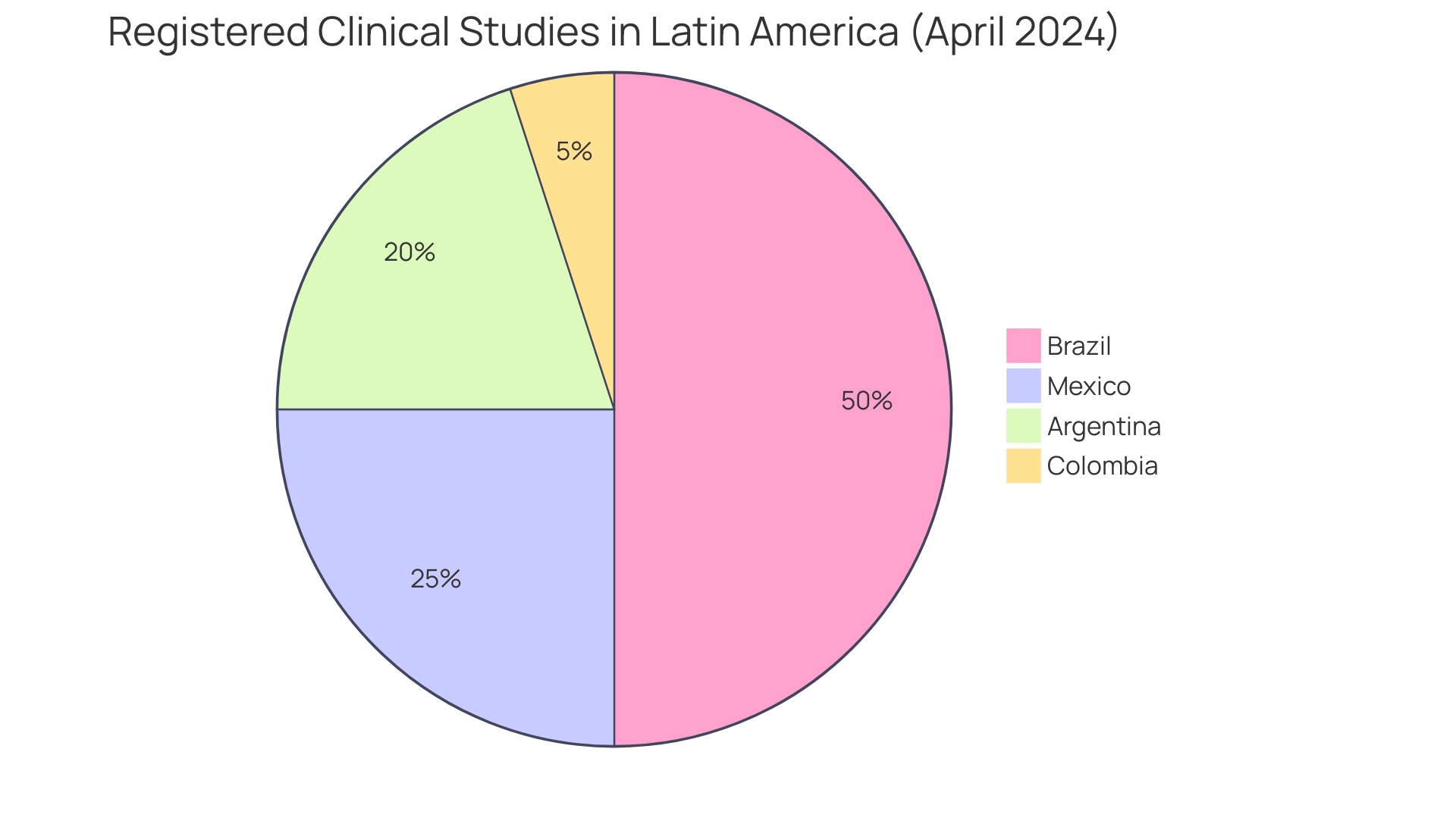
The regulatory environment in Latin America: emerging clinical research region is experiencing considerable change, focused on improving research activities in healthcare. As of April 2024, Brazil boasts approximately 10,000 registered clinical studies, with Mexico at 5,000 and Argentina at 4,000. This increase in activity is closely linked to new initiatives by regulatory agencies in these countries to streamline approval processes and improve transparency.
Colombia stands out in this landscape, providing cost savings of over 30% compared to studies in North and Western Europe, alongside a rapid regulatory review process of 90-120 days for IRB/EC and INVIMA approvals. Gotuzzo from the Universidad Peruana Cayetano Heredia emphasizes this evolution, stating, 'Clinical Research in the region: Constraints and Opportunities.' This quote highlights the dual aspects of growth and the challenges that remain, especially in overcoming linguistic, cultural, and socio-economic barriers in nations such as Peru, Colombia, and Chile, which must be addressed to ensure patient protection and improve study quality.
Moreover, the implementation of unified guidelines throughout the region is promoting enhanced cooperation and uniformity in study execution. Specific examples of streamlined approval processes include:
- Brazil's recent regulatory changes that have reduced review times
- Mexico's implementation of a centralized application system
Both aimed at facilitating quicker initiation. In Colombia, strategic alliances—such as the cooperation between bioaccess™ and Caribbean Health Group—are emerging as essential elements in positioning Barranquilla as a prominent location for medical studies in the region.
Supported by Colombia's Minister of Health, these initiatives not only enhance recruitment efficiency, achieving over a 50% reduction in recruitment time and 95% retention rates, but also improve informed consent through targeted educational programs. Additionally, Colombia offers significant R&D tax incentives, including:
- A 100% tax deduction for investments in science, technology, and innovation projects
- A 25% tax discount
- A 50% future tax credit
- Approximately $10 million in government grants
Collectively, these advancements highlight Latin America: emerging clinical research region as a top research destination.
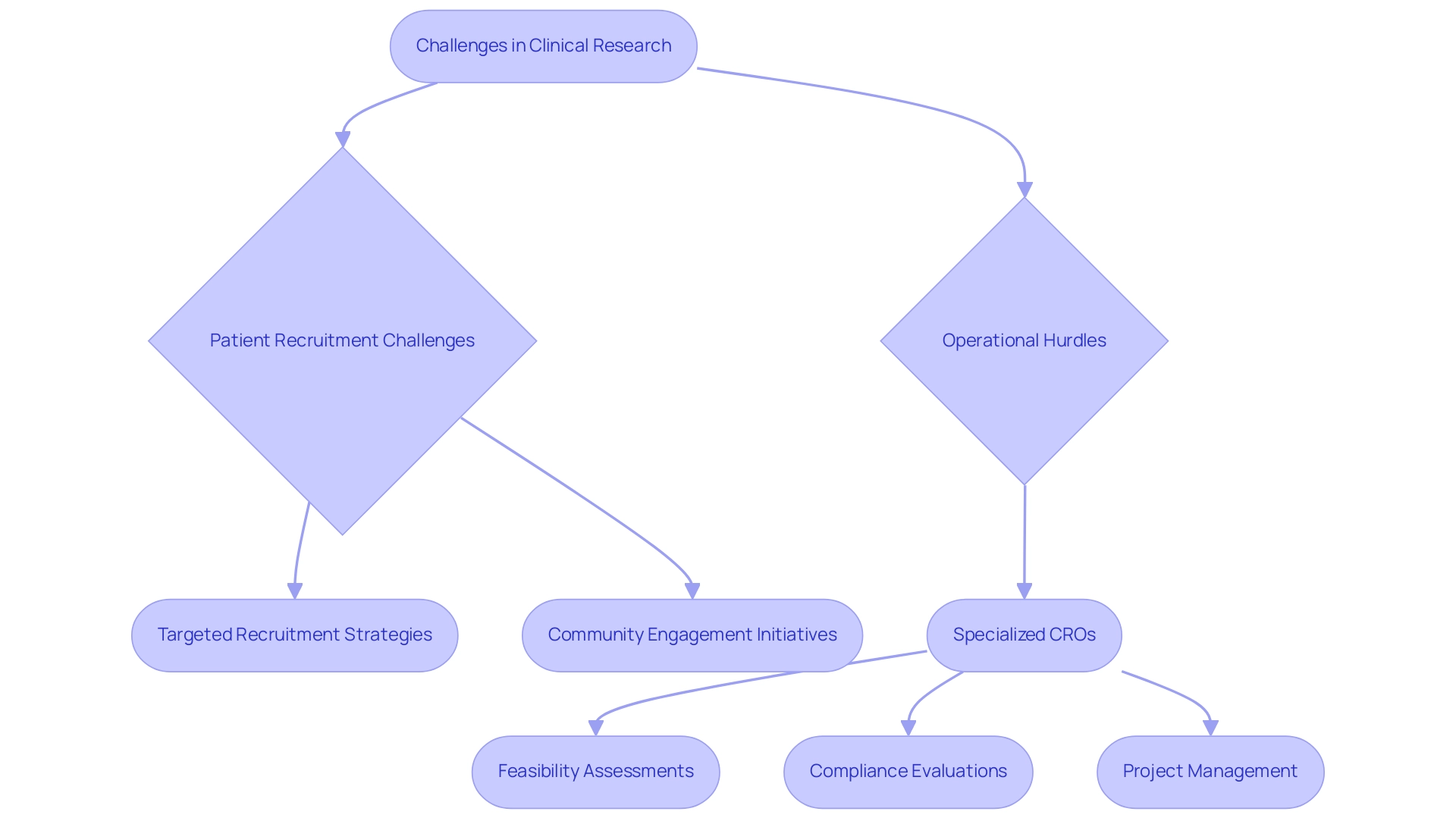
6. Overcoming Recruitment and Operational Challenges
Conducting medical studies in Latin America: emerging clinical research region offers various benefits, such as the seasonal synchronization between the Southern and Northern hemispheres, which allows for year-round examination of seasonal illnesses like respiratory diseases. However, challenges such as patient recruitment and operational hurdles remain significant concerns. The diverse demographics and treatment-naive populations can complicate efforts to reach potential participants and ensure adequate representation.
To address these challenges, targeted recruitment strategies are essential. Community engagement initiatives, including partnerships with local healthcare providers, can significantly enhance outreach efforts. As noted by Julio G. Martinez-Clark, CEO of bioaccess, Colombia's ambitious science, technology, and innovation plan aims to establish a knowledge economy, which underscores the importance of integrating local insights into recruitment strategies.
Moreover, specialized CROs like bioaccess® offer comprehensive management services for research studies, including:
- Feasibility assessments
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
These services are essential for navigating the regulatory environment. Statistics indicate that patients in high-income nations tend to use more medications, making them less suitable as subjects for clinical studies. This highlights the potential of Latin America: emerging clinical research region, as an ideal location for research involving treatment-naive populations.
By utilizing technology and offering specialized training, these CROs enhance operational processes, thus increasing management efficiency. By concentrating on these strategies, researchers can improve patient recruitment and operational efficiency, paving the way for successful studies that benefit both local populations and the wider global community.
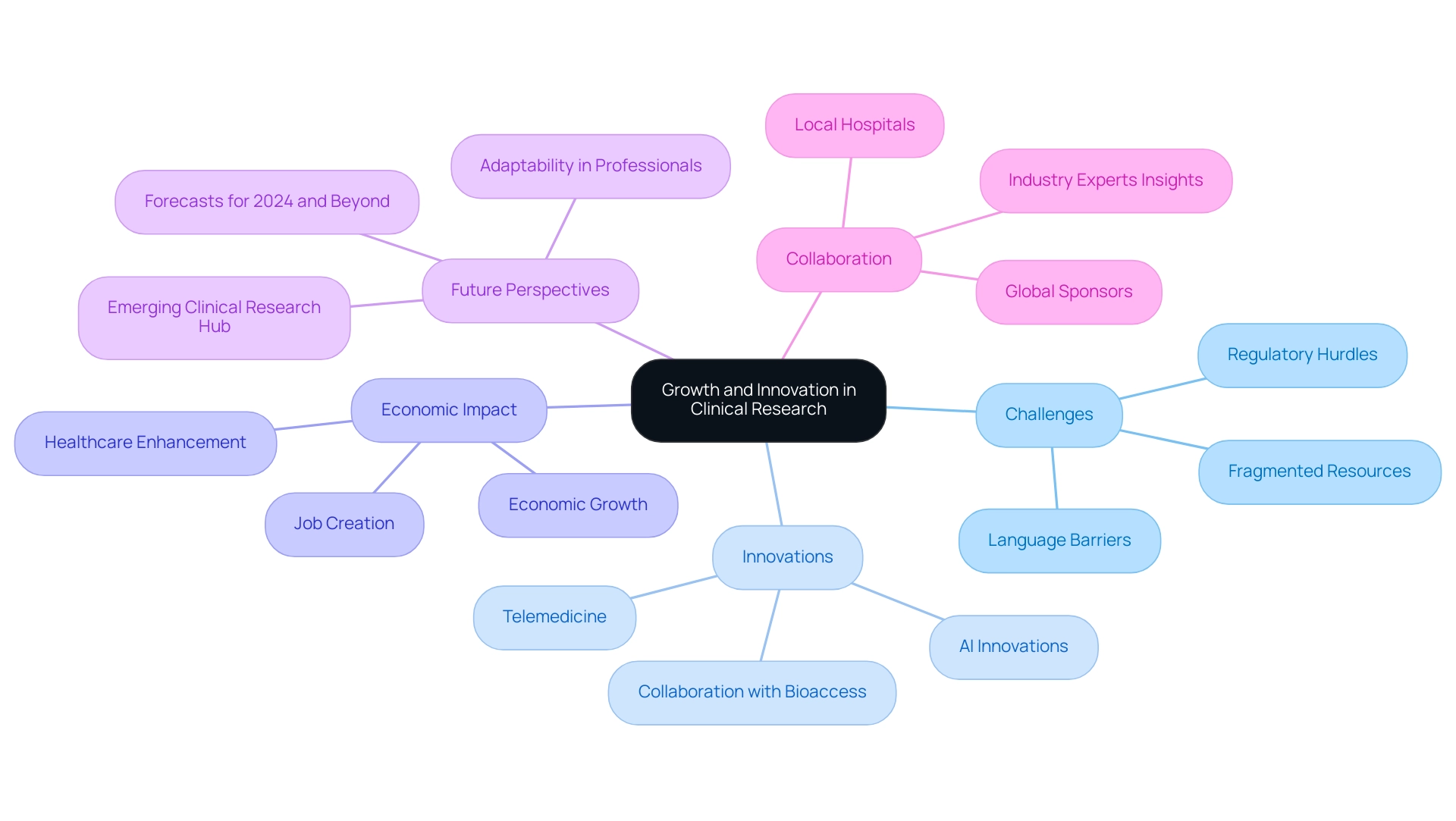
7. Future Perspectives: Growth and Innovation in Clinical Research
The perspective for medical research in the southern continent is becoming more positive, with forecasts suggesting ongoing expansion and creative advancements. For instance, statistics from 2015 to 2016 indicate a notable increase in participation in clinical studies across the region, complemented by studies managed by GIMEMA in Italy, which provides a benchmark for comparison. However, US Medtech firms encounter significant challenges in the region, including regulatory hurdles, language barriers, and fragmented resources, which can hinder effective collaboration with local hospitals.
The region is enhancing its research infrastructure, expected to attract more global sponsors and researchers. As highlighted during a recent Latinos In Clinical Research (LICR) webinar, professionals must remain adaptable to these evolving dynamics. Monica Cuitiva, a seasoned expert in the field, noted that U.S. professionals need to adapt to changing industry dynamics.
Key innovations such as artificial intelligence (AI) are set to revolutionize trial design and data analysis, streamlining processes and fostering greater patient engagement. Additionally, collaboration between Greenlight Guru and bioaccess™ is accelerating Medtech innovations, highlighted by PAVmed's first-in-human study in Colombia. Dr. Sergio Alvarado, a Clinical Trial Manager focused on innovative medical research and AI in the region, emphasizes the importance of adaptability and continuous learning for job seekers amidst hiring freezes, encouraging professionals to build diverse skill sets.
Moreover, advancements in telemedicine are expected to improve these efforts, while research studies are anticipated to positively affect local economies through job creation, economic growth, and healthcare enhancement. By cultivating a culture of collaboration and innovation, Latin America: emerging clinical research region, particularly in countries like Mexico and Brazil, is well-positioned to become a leading hub for clinical research, with significant developments expected to unfold through 2024 and beyond.
Conclusion
Latin America is emerging as a prominent player in the clinical research sector, driven by substantial investments in infrastructure, a focus on patient-centric practices, and the increasing prevalence of decentralized trials. Countries such as Brazil, Mexico, and Argentina are attracting global sponsors by developing high-quality research facilities and training programs, which enhance local expertise and streamline trial processes.
The commitment to patient-centricity prioritizes participant experiences and preferences, leading to improved recruitment and retention rates, as well as higher-quality research outcomes. With flexible trial designs and comprehensive support systems, clinical trials in the region are becoming more accessible and engaging for participants.
Cost-effectiveness is a significant advantage, as conducting trials in Latin America often results in substantial savings compared to North America and Europe. The region's diverse patient populations also provide invaluable resources for studies requiring varied demographic representation, crucial for developing effective medical solutions. Decentralized trials further enhance accessibility, particularly for underserved communities.
An evolving regulatory landscape is facilitating clinical research growth, with streamlined approval processes and harmonized guidelines promoting collaboration and consistency. Despite some challenges in recruitment and operations, targeted strategies and community engagement can help overcome these hurdles.
As the future unfolds, Latin America is well-positioned to become a leading hub for clinical research. By embracing innovation and a commitment to patient-centric practices, the region is set to play a vital role in advancing global healthcare, ultimately benefiting local economies and improving health outcomes for diverse populations.




