Overview
Latin America is emerging as the next frontier for clinical research due to its demographic diversity, supportive regulatory frameworks, and cost-effectiveness, which collectively enhance the feasibility and quality of medical studies. The article highlights how these factors, including a young population, streamlined approval processes, and lower operational costs, create a conducive environment for pharmaceutical companies to conduct diverse and inclusive research, thereby driving significant advancements in the field.
Introduction
As the global landscape of clinical research evolves, Latin America is rapidly emerging as a pivotal player, offering a unique blend of demographic diversity, regulatory advancements, and economic advantages. With a youthful population and a commitment to health equity, the region presents an unparalleled opportunity for pharmaceutical companies seeking to conduct clinical trials that are both cost-effective and scientifically rigorous.
Recent partnerships, such as the collaboration between bioaccess™ and Caribbean Health Group, highlight the strategic positioning of cities like Barranquilla as burgeoning hubs for research. However, while the potential is immense, navigating the complexities of socio-economic barriers and ensuring informed consent remains a critical challenge.
This article delves into the myriad factors that make Latin America an attractive destination for clinical trials, exploring the benefits and obstacles that shape the future of medical research in this dynamic region.
Emerging Opportunities in Latin American Clinical Research
The increasing appeal of Latin America as a destination for medical research illustrates why Latin America is the next frontier for clinical research, driven by a combination of demographic diversity and supportive regulatory frameworks. Recent collaborations, like the alliance between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, in Miami, FL, seek to establish Barranquilla as a prominent center for research in the region, backed by Colombia's Minister of Health. With approximately 30% of the population under the age of 14, the region presents a unique opportunity for pharmaceutical companies to study medical interventions across a broad spectrum of age groups and health conditions.
This diversity is crucial for assessing the efficacy and safety of treatments in varied populations. Additionally, numerous nations in the area have created regulatory frameworks that promote the authorization and execution of research studies, thus minimizing bureaucratic obstacles for sponsors.
Nonetheless, carrying out medical studies in the region is not without its difficulties. Socio-economic obstacles to informed consent can impede participation, but these can be surmounted through effective cooperation between sponsors and trial personnel, ensuring that participants are well-informed and their rights are safeguarded. As Ruiz notes, "Peru’s illiteracy rate drops to 7.1," which highlights the need for tailored approaches to education and consent processes in diverse populations.
The cost-effectiveness of conducting research in the southern continent illustrates why Latin America is the next frontier for clinical research, enabling substantial savings on operational expenses while maintaining high standards of quality. Key market players, including IDx Technologies and GlobalCare Clinical Trials, are already capitalizing on these opportunities, illustrating a vibrant and competitive landscape. For instance, GlobalCare Clinical Trials has achieved over a 50% reduction in recruitment time and a 95% retention rate in their studies.
As the pharmaceutical sector aims to broaden its international presence, the region in the south stands out, illustrating why Latin America is the Next Frontier for Clinical Research, as it not only fulfills operational requirements but also enhances the quality and significance of research results. Media coverage by Clinical Leader further highlights the increasing interest and advancements in trials in South region and Colombia.
Key Drivers of Change: Health Equity and Diversity in Research
Health equity and diversity have emerged as central tenets in medical research, driven by a growing recognition of the necessity to include underrepresented populations in studies. Latin America, characterized by its rich tapestry of ethnic groups, exemplifies why Latin America is the next frontier for clinical research to address these disparities. By leveraging the expertise of bioaccess® in managing trials—specifically Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—researchers can explore why Latin America is the next frontier for clinical research, tapping into the region's diverse genetic backgrounds, lifestyles, and health challenges to gather data that is far more representative of global populations.
This comprehensive approach not only enhances the scientific rigor of medical studies but also aligns with global health equity initiatives aimed at ensuring fair access to medical advancements for all demographics. As Perri Kasen, Senior Manager at Ernst & Young LLP, articulates,
the vision of equitable experience and outcomes for all is paramount.
The emphasis on health equity is further underscored by recent developments in data analytics, which are proving vital for uncovering health equity challenges and tailoring interventions.
Furthermore, artificial intelligence is quickly becoming a crucial tool in this field, especially as bioaccess® employs these technologies to enhance management and improve data quality. By fostering trust and collaboration between researchers and communities, the focus on health equity paves the way for developing more inclusive healthcare solutions. Notably, over half of health equity leaders prioritize enhancing their research strategies and data quality in 2024, underscoring the urgency of addressing challenges such as outdated data infrastructures and limited collaboration.
Furthermore, 58% of organizations expect their health equity investments to increase in the coming year, highlighting the growing commitment to achieving health equity goals through cross-sector partnerships. Furthermore, the participation of bioaccess® in research studies supports local economic development and global cooperation, generating employment and enhancing healthcare accessibility in the area.
Regulatory Advancements and Supportive Infrastructure
In recent years, the significant progress in Latin America's regulatory environment illustrates why Latin America is the next frontier for clinical research, as nations strive to simplify procedures to attract international research studies. Our extensive research project management services include:
- Feasibility studies
- Site selection
- Compliance assessments
- Project setup, including systematic reviews and feedback on study documents to ensure adherence to country requirements
- Import permits
- Project oversight
- Thorough reporting processes, which are essential for ensuring efficient execution
Regulatory agencies are increasingly implementing best practices drawn from established markets, leading to enhanced transparency and efficiency in approval timelines.
For example, the case analysis named 'Clinical Studies in Mexico (2024)' emphasizes a strong framework that facilitates the prompt initiation and execution of studies, informing stakeholders about the expected environment for research in the area. According to Claudia Yvette Cravioto Guzman, Sr. Director of FSP Clinical Operations and GMBA, 'This approach better positions customers for success as opportunities in the region continue to emerge.' Furthermore, Julio G. Martinez-Clark, CEO of bioaccess, highlights the essential necessity to carry out clinical trials beyond the U.S., which underscores why Latin America is the next frontier for clinical research.
Consequently, sponsors can anticipate faster patient recruitment and improved data reliability, which illustrates why Latin America is the next frontier for clinical research and solidifies the region's status as a favored research hub. Additionally, the observation that dropout rates in South regions are one-third of those in the U.S. and EU reinforces this appeal. With 52 percent of global studies now taking place outside the U.S., and approximately 65% of revenue generated from competitive intelligence and market intelligence teams, the region's regulatory support plays a crucial role in understanding why Latin America is the next frontier for clinical research.
Cost Efficiency and Economic Considerations
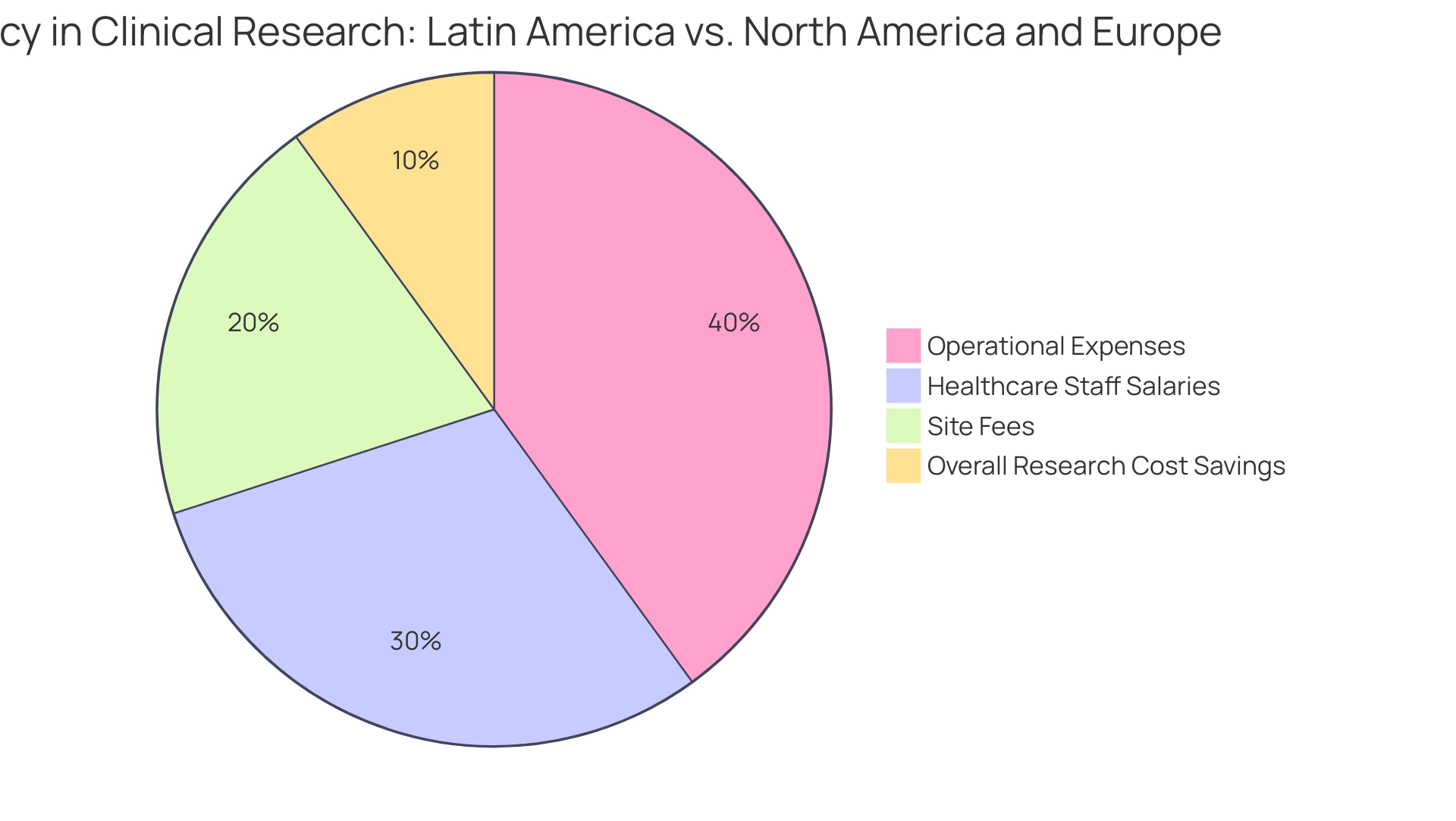
The significant cost savings in comparison to North America and Europe illustrate why Latin America is the next frontier for clinical research. The area features reduced operational expenses, with the research matching software sector producing a revenue of USD 5.8 million in 2023. Additionally, salaries for healthcare staff are considerably lower, allowing for more efficient budget management.
For example, a recent study shows that medical personnel salaries in Latin America are frequently 30-50% lower than those of their European equivalents, which directly affects overall research costs. Furthermore, the affordability of site fees enhances the financial appeal of conducting studies in this region. The diverse populations facilitate faster recruitment processes, significantly reducing delays and associated costs.
As noted by Dr. John B. Simpson, who has conducted Avinger's OCT-guided atherectomy research in Cali, Colombia, collaboration with LATAM CRO experts has proven invaluable, further emphasizing the region's potential for international partnerships. Furthermore, Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shares his experience with bioaccess® during its initial human study in Colombia, emphasizing the company’s commitment to regulatory excellence and innovation in medtech research. The comprehensive services provided encompass:
- Feasibility studies
- Site selection
- Setup
- Ethics committee approvals
- Ongoing project management and reporting processes
All of which are essential for successful execution.
As Peru’s illiteracy rate decreases to 7.1%, it reflects enhancing socio-economic factors that can positively impact research environments. These financial benefits allow sponsors to allocate resources more strategically, enabling them to conduct multiple tests concurrently. Furthermore, access to over 1 million market statistics and 20,000+ reports through subscriptions offers sponsors with vital insights that improve decision-making concerning research studies.
As the demand for innovative therapies rises, the cost-efficient nature of research in the region demonstrates why Latin America is the next frontier for clinical research, positioning it as a more attractive choice for organizations looking to enhance their research investments. In comparison, the Asia Pacific market is expected to achieve USD 25,992.8 million by 2030, highlighting the growth potential of research in the southern continent as a competitive option.
Building Strong Collaborations and Partnerships
The success of medical research is increasingly reliant on strong partnerships among various stakeholders, including academic institutions, healthcare providers, and regulatory bodies. The distinctive environment provided by the region, especially Colombia, illustrates why Latin America is the next frontier for clinical research, as it offers notable competitive benefits for first-in-human clinical studies. With savings of over 30% compared to North and Western Europe, coupled with swift regulatory approval processes—typically taking just 90-120 days—Colombia stands out as a cost-effective and efficient option.
The World Health Organization ranks Colombia's healthcare system as #22 worldwide, and its hospitals are acknowledged among the finest in the region, ensuring high-quality care during tests. Furthermore, with a population exceeding 50 million and about 95% covered by universal healthcare, patient recruitment is facilitated effectively. Colombia also offers enticing R&D tax incentives, including a 100% tax deduction for investments in science and technology, making it an attractive destination for sponsors.
By leveraging local knowledge and resources, sponsors can significantly improve both the design and implementation of their trials. Significantly, specialists have noted that dropout rates in South America are roughly one-third of those in the U.S. and the European Union, emphasizing the area's potential for effective patient involvement and retention. This trend highlights the advantages of collaboration, as stronger partnerships can further boost patient trust and involvement in research studies.
Moreover, with 52 percent of worldwide research trials taking place outside the U.S., this region is becoming a more favored site for these studies. As the medical research environment continues to develop, forming robust collaborations will be crucial in demonstrating why Latin America is the next frontier for clinical research. A prime example of this is seen in the field of genomics and personalized medicine, which illustrates why Latin America is the next frontier for clinical research due to its rich genetic diversity and distinct environmental factors driving advancements in global healthcare.
Research initiatives in this region are not only improving diagnostic and treatment protocols but also integrating indigenous medical traditions to create effective and culturally appropriate healthcare interventions. As history has demonstrated, with arguably the most notorious biomedical research study in U.S. history, ethical collaborations are essential for building trust and ensuring the success of research endeavors. Furthermore, extensive research management services in Colombia encompass feasibility studies, site selection, compliance evaluations, study setup, and project oversight, which are essential for navigating the regulatory environment.
The procedures for obtaining trial approval, such as IRB/EC and INVIMA approval, are streamlined, ensuring that trials can commence efficiently. These factors, combined with the economic impact of Medtech clinical studies—such as job creation and healthcare improvement—underscore the importance of collaboration in driving innovation and growth in the regions.
Conclusion
Latin America is emerging as a significant hub for clinical research, driven by its demographic diversity, supportive regulatory frameworks, and cost-effectiveness. The region’s youthful population and focus on health equity create valuable opportunities for pharmaceutical companies to conduct trials across various health conditions and demographics. Partnerships like that of bioaccess™ and Caribbean Health Group position cities such as Barranquilla as key research centers.
Despite challenges such as socio-economic barriers and informed consent, these can be addressed through strategic collaborations and targeted educational programs. The commitment to health equity enhances the scientific rigor of clinical trials and aligns with global initiatives aimed at improving access to medical advancements for diverse populations.
With ongoing regulatory improvements, Latin America offers substantial operational cost savings and quicker patient recruitment, making it an attractive choice for sponsors. Collaborative efforts among stakeholders—including academic institutions and healthcare providers—are crucial for unlocking the region's full potential in clinical research.
In conclusion, Latin America's unique blend of economic advantages, diverse populations, and dedication to health equity establishes it as a strategic option for pharmaceutical companies seeking to expand their clinical research initiatives. Embracing these opportunities will not only drive innovation in medical science but also contribute to a more equitable healthcare future for all.
Frequently Asked Questions
Why is Latin America considered the next frontier for clinical research?
Latin America is seen as the next frontier for clinical research due to its demographic diversity, supportive regulatory frameworks, and a significant portion of the population under the age of 14, which allows for studying medical interventions across various age groups and health conditions.
What recent collaborations have been made to enhance medical research in Latin America?
A notable collaboration is between bioaccess™ and Caribbean Health Group, aimed at establishing Barranquilla as a prominent research center, supported by Colombia's Minister of Health.
What challenges exist in conducting medical studies in Latin America?
Challenges include socio-economic obstacles to informed consent, which can hinder participation. However, these can be addressed through effective cooperation between sponsors and trial personnel to ensure participants are well-informed and their rights are protected.
How does the cost-effectiveness of conducting research in Latin America benefit pharmaceutical companies?
Conducting research in Latin America allows for substantial savings on operational expenses while maintaining high standards of quality, making it an attractive option for pharmaceutical companies.
What are some examples of companies successfully conducting clinical trials in Latin America?
IDx Technologies and GlobalCare Clinical Trials are key market players capitalizing on opportunities in the region. GlobalCare Clinical Trials, for instance, has reported over a 50% reduction in recruitment time and a 95% retention rate in their studies.
How does Latin America contribute to health equity in medical research?
Latin America's diverse ethnic groups provide a rich environment for including underrepresented populations in studies, enhancing the scientific rigor of medical research and aligning with global health equity initiatives.
What role does technology play in improving clinical research in Latin America?
Technologies like artificial intelligence are becoming crucial in managing trials and improving data quality, which helps foster trust and collaboration between researchers and communities.
What is the outlook for health equity investments in the coming year?
Over half of health equity leaders are prioritizing enhancements in research strategies and data quality, and 58% of organizations expect their health equity investments to increase, reflecting a growing commitment to achieving health equity goals through partnerships.




