Overview
The primary focus of this article is to identify the challenges inherent in designing clinical trials in Ecuador and to propose effective solutions to address these issues. It highlights various obstacles, including:
- Regulatory complexities
- Difficulties in participant recruitment
- Financial constraints
To combat these challenges, the article offers tailored strategies such as:
- Early collaboration with local authorities
- Community engagement for recruitment
- Robust training programs for research staff
These approaches are essential to ensure successful trial outcomes.
Introduction
In the dynamic realm of clinical research, Ecuador emerges as a pivotal player, driven by innovative organizations like bioaccess®. With over 15 years of expertise in the Medtech sector, bioaccess® redefines clinical trial design through patient-centric methodologies and collaborative stakeholder engagement. This article explores the multifaceted strategies bioaccess® employs to:
- Navigate regulatory hurdles
- Enhance patient recruitment
- Ensure cultural sensitivity
All while leveraging cutting-edge technology for effective data management. As the demand for diverse and ethical clinical trials escalates, bioaccess® stands at the forefront, dedicated to improving health outcomes and advancing Medtech innovations in the region.
bioaccess®: Streamlining Clinical Trial Design in Ecuador
bioaccess® has established itself as a leader in research design in Ecuador, leveraging over 15 years of experience in the Medtech field. The organization specializes in delivering tailored solutions for a variety of study types, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Trials
- Post-Market Clinical Follow-Up Studies (PMCF)
This customized approach not only aligns with regulatory requirements but also addresses the specific needs of the local population, ensuring that trials are both relevant and effective, particularly considering the challenges in designing trials for Ecuador that highlight the importance of patient-focused methodologies. By concentrating on genuine patient involvement and team collaboration, the system enhances the quality of data gathered, which is essential for the success of Medtech innovations. The successful achievement of Good Practice certification by team members, such as Durgesh Chaudhari, exemplifies the commitment to ethical and scientific standards in research, significantly enhancing their ability to ensure safety and integrity.
Moreover, bioaccess® offers a comprehensive process that includes:
- Feasibility Studies
- Site Selection
- Compliance Reviews
- Trial Setup
- Import Permits
- Project Management
- Reporting
The GCSA certification framework, developed in consultation with industry leaders, has the potential to transform clinical trial capabilities and elevate research sites. The impact of these tailored solutions is evident in the enhanced results of medical studies conducted in the area. By optimizing procedures and fostering collaborative connections among sponsors, CROs, and research sites, the organization accelerates timelines while enhancing the overall quality of the research. As Jacqueline Johnson North, CEO & Co-Founder, states, 'The quality standard emphasizes genuine patient-centricity, workforce involvement and facilitates collaborative efforts between sponsors/CROs and research sites.' This strategic emphasis positions the company as an essential ally for Medtech firms navigating the challenges in designing trials for Ecuador during the complexities of research phases. To explore how bioaccess® can assist you in advancing your studies, consider scheduling a meeting with our team.
Navigating Regulatory Hurdles in Ecuadorian Clinical Trials
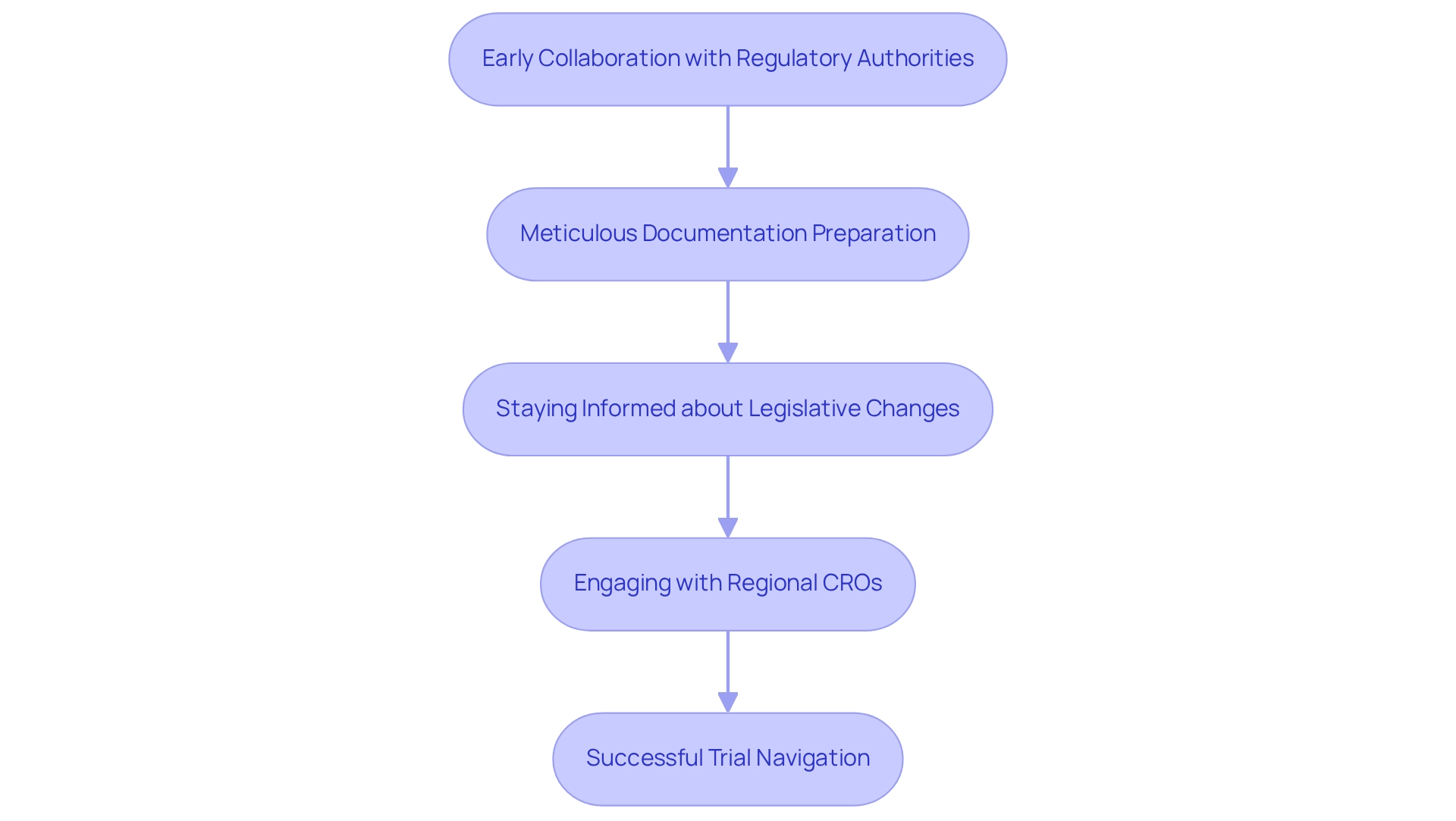
Ecuador's regulatory framework for research studies is undergoing significant evolution, with new guidelines introduced to streamline processes. However, the challenges in designing trials for Ecuador can make navigating these regulations complex. Key strategies for success must address the challenges in designing trials for Ecuador, which involve:
- Early collaboration with local regulatory authorities during the study design phase
- Ensuring that all documentation is meticulously prepared
- Staying informed about legislative changes
Engaging with knowledgeable regional CROs that provide comprehensive research management services—such as feasibility studies, site selection, compliance evaluations, setup, import permits, project management, and reporting—can greatly simplify this process. Furthermore, the organization is committed to ensuring information security and client confidence, addressing any issues through robust complaint and data protection protocols. This dedication guarantees adherence and transparency throughout the research process.
Enhancing Patient Recruitment Strategies for Ecuadorian Trials
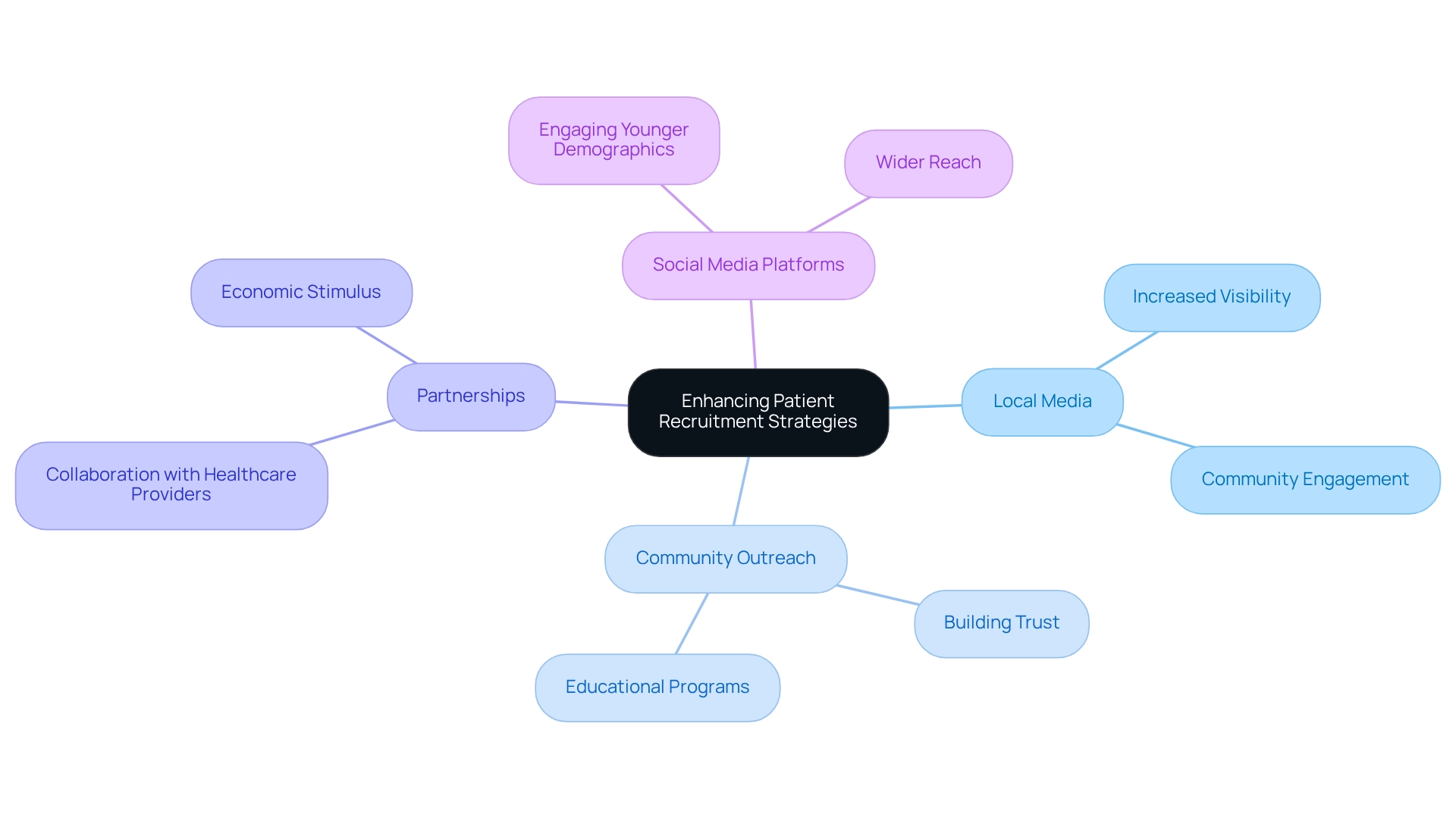
Enlisting participants for research studies in Ecuador involves overcoming the challenges in designing trials for Ecuador with a comprehensive strategy. Employing local media, community outreach programs, and partnerships with healthcare providers significantly enhances visibility and helps to overcome the challenges in designing trials for Ecuador while fostering trust. Additionally, leveraging social media platforms effectively reaches potential participants, particularly among younger demographics.
bioaccess® implements these strategies to ensure diverse and sufficient participant enrollment, which is crucial for the validity of study results. Furthermore, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, with the endorsement of Colombia's Minister of Health. This initiative not only bolsters recruitment efforts but also stimulates the local economy by generating jobs and promoting international collaboration in clinical research.
Incorporating Cultural Sensitivity in Trial Protocols
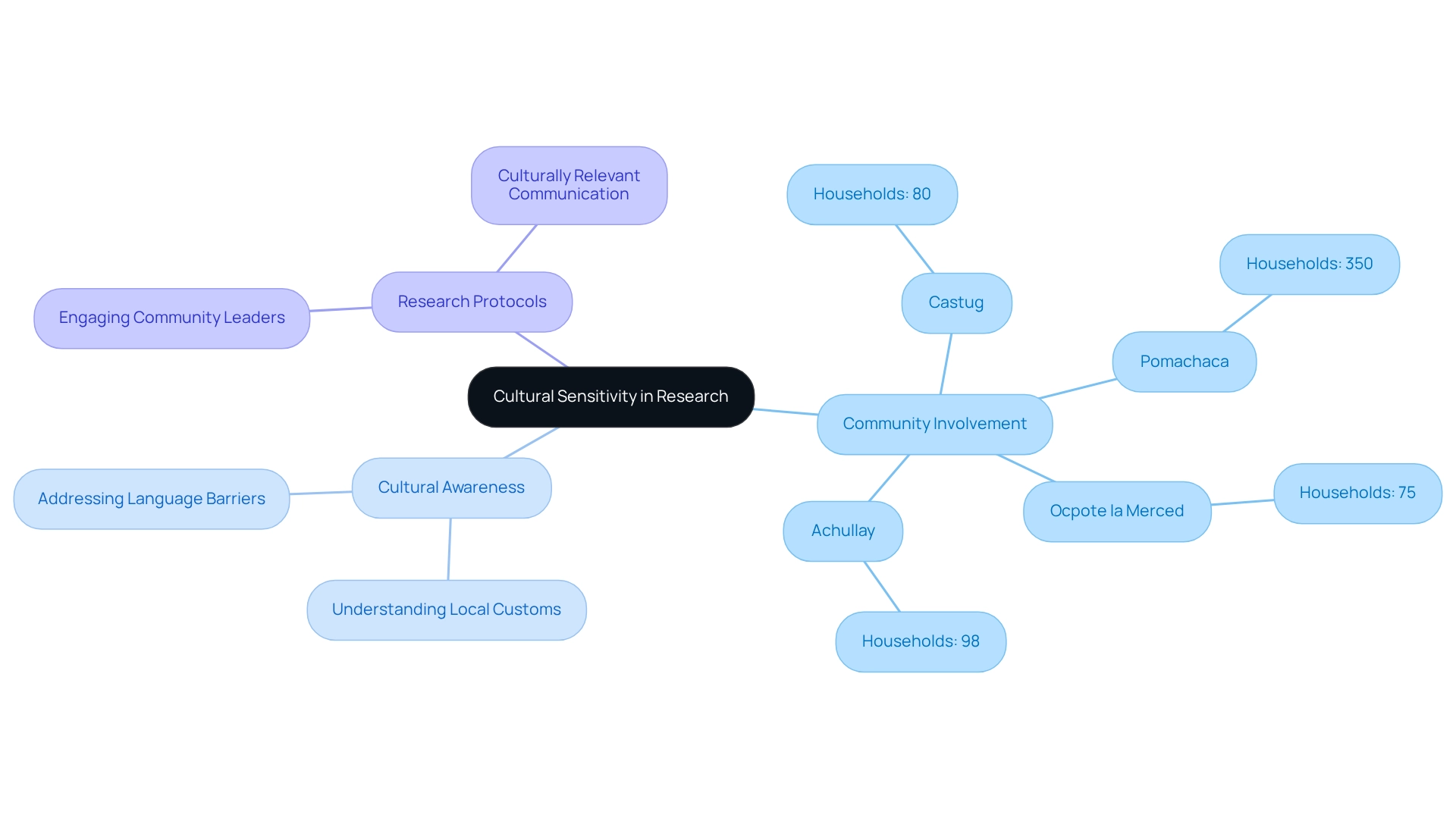
Integrating cultural awareness into research protocols is crucial for establishing trust and promoting collaboration among participants. This integration necessitates a profound understanding of local customs, beliefs, and health practices. Engaging community leaders in the recruitment process significantly boosts credibility and acceptance, as research indicates increased participation when local influencers are involved. For example, community sizes such as:
- Castug with 80 households
- Pomachaca with 350 households
- Ocpote la Merced with 75 households
- Achullay with 98 households
illustrate the impact of community involvement on participation rates.
bioaccess® emphasizes cultural competence in its study designs, ensuring that protocols are not only scientifically rigorous but also culturally relevant. This approach enhances participant involvement and retention, aligning with the growing recognition of the necessity for clear and culturally suitable communication in research. By addressing language barriers and misconceptions—such as the common confusion surrounding the term 'research study' in Spanish—bioaccess® strives to create a more inclusive environment for all participants, ultimately leading to more successful study outcomes.
Moreover, the partnership between bioaccess® and Caribbean Health Group to establish Barranquilla as a prominent hub for medical studies in Latin America, supported by Colombia's Minister of Health, illustrates strategic initiatives aimed at improving medical research in the region. This initiative reflects insights shared in the LATAM Medtech Leaders Podcast, where the significance of local engagement and cultural understanding was underscored. Additionally, qualitative interviews conducted in related studies achieved a remarkable 98.4% agreement after coding 10% of transcripts, underscoring the reliability of findings on cultural sensitivity.
To enhance recruitment efforts, medical researchers are encouraged to connect with local leaders or conduct focus groups to gain deeper insights into community perspectives.
Fostering Collaboration Among Stakeholders in Clinical Trials
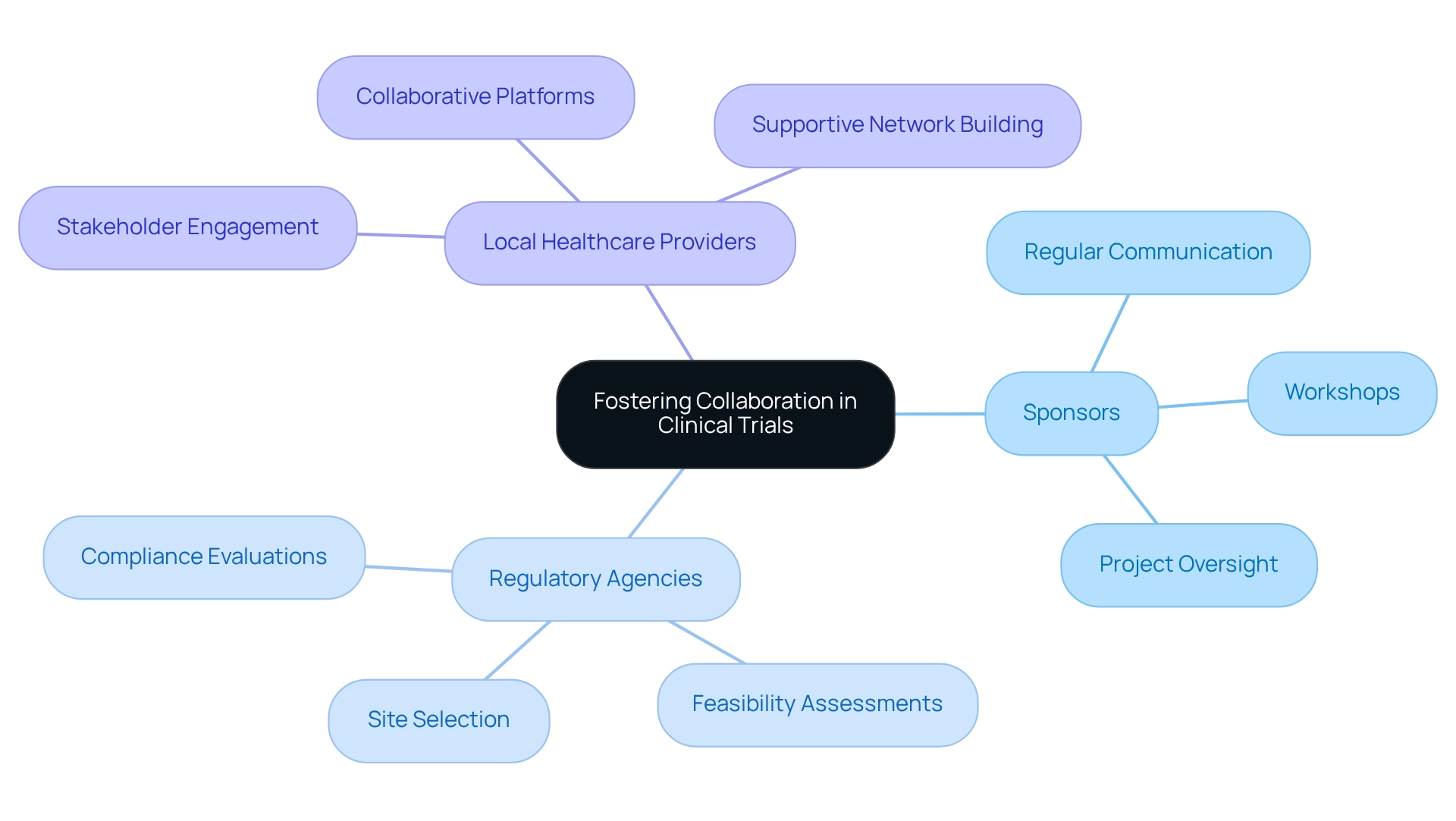
Promoting cooperation among stakeholders—including sponsors, regulatory agencies, and local healthcare providers—is essential for the success of research studies. Regular communication and meetings align goals and expectations effectively.
At bioaccess®, we actively foster stakeholder engagement through workshops and collaborative platforms, ensuring that all parties are informed and invested in the project's success. Our comprehensive research study management services—feasibility assessments, site selection, compliance evaluations, and project oversight—are designed to enhance this collaborative approach.
This not only improves the quality of the research but also builds a supportive network for future studies, ultimately driving economic growth and healthcare improvement in the region. Client testimonials, such as those from Bill Andrews, Ph.D., President & CEO of Sierra Sciences, underscore the positive impact of our services on local economies and highlight the importance of international collaboration in advancing Medtech innovations.
Leveraging Technology for Effective Data Management in Trials
The incorporation of technology in clinical trial information management is essential for enhancing both efficiency and precision. Electronic Data Capture (EDC) systems and Clinical Trial Management Systems (CTMS) are pivotal in streamlining information collection and analysis. For instance, in Ecuador, the implementation of EDC systems has addressed the challenges in designing trials for Ecuador by leading to a significant reduction in entry errors and accelerated information retrieval, facilitating more timely decision-making.
bioaccess® leverages advanced information management technologies that allow for real-time monitoring and reporting. This proactive approach enables the early identification of potential issues, ensuring information integrity throughout the trial process. The advantages of EDC systems extend beyond operational efficiency; they also enhance compliance with regulatory standards, which is critical in the highly regulated Medtech sector. Recent inspections have proceeded smoothly with minimal findings, underscoring the effectiveness of EDC in ensuring compliance and data integrity.
A case study featuring bioaccess® exemplifies the practical application of technology in managing regulatory compliance. Their collaboration with Caribbean Health Group aims to position Barranquilla as a leading research hub in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances the research landscape but also underscores the importance of effective data management practices in achieving regulatory compliance.
Furthermore, a partner's quote underscores their success:
"We helped them by changing and implementing several processes and handled the inspections professionally, which resulted in positive comments by the inspectors and less significant findings."
This statement highlights the critical role of technology in refining legal procedures.
In conclusion, the adoption of EDC and CTMS technologies is not merely a trend but a fundamental necessity for contemporary research, particularly in addressing the challenges in designing trials for Ecuador and South America.
Ensuring Diversity in Clinical Trial Populations
Ensuring diversity in research participant groups is essential for generating valid and applicable results. Actively recruiting participants from a variety of demographic backgrounds—including different ethnicities, ages, and health conditions—enhances the robustness of study findings. The case of Pirtobrutinib in pretreated patients with chronic lymphocytic leukemia (CLL) underscores the necessity for diverse participant inclusion, as targeted therapies have shown improved outcomes but still face limitations due to patient discontinuation from treatment.
At bioaccess™, we employ targeted recruitment strategies to engage underrepresented groups, thereby fostering a more representative sample that reflects the population likely to use the medical products being tested. This method aligns with the FDA's focus on monitoring data and ensuring clarity in participant demographics, while also addressing the wider demand for equity in research studies, as emphasized by specialists in the area. As Marcella Nunez-Smith states, 'Clinical study diversity is an issue of fairness,' reinforcing the ethical imperative to include diverse populations.
Furthermore, our partnership with Caribbean Health Group aims to establish Barranquilla as a prominent location for medical research in Latin America, with support from Colombia's Minister of Health. This initiative is bolstered by our collaboration with GlobalCare Clinical Studies, which has effectively improved our ambulatory services in Colombia, achieving over a 50% decrease in recruitment time and 95% retention rates. The recent e-book released in August 2023 addresses the significance of diversity in medical studies, presenting various research methods to confront this issue. By prioritizing diversity, research studies can attain greater validity and relevance, ultimately resulting in improved healthcare solutions for everyone.
Managing Financial Constraints in Clinical Trial Design
Effectively managing financial limitations in clinical study design necessitates meticulous planning and strategic resource distribution. Essential strategies include:
- Creating a comprehensive budget that encompasses all potential expenses
- Exploring diverse funding sources
- Refining experimental designs to minimize costs while maintaining quality
The case study titled 'Impact of Clinical Study Costs on Drug Development' illustrates how escalating expenses can deter pharmaceutical companies from pursuing studies, underscoring the urgent need for cost-effective solutions within the industry. For instance, the typical costs associated with clinical studies in Ecuador can be significantly influenced by these strategies, which illustrates the challenges in designing trials for Ecuador, as financial constraints often necessitate a cautious approach in the sector. Indeed, total per-study expenses for respiratory system studies in Phase 4 can soar to $72.9 million, highlighting the critical importance of effective financial management.
As James Lendall Basford aptly remarked, "The man who never has money enough to pay his debts has too much of something else," a reminder that prudent financial strategies are vital for advancing research. Bioaccess® plays a pivotal role in helping clients navigate these financial challenges by offering tailored strategies that align with the unique needs of each study, ensuring that financial limitations do not impede research progress.
Furthermore, bioaccess's extensive management services for research studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—are designed to enhance the efficiency and effectiveness of these studies. This approach not only aids in overcoming financial constraints but also fosters job creation and economic growth in local communities, promoting international collaboration in the Medtech sector.
Training Clinical Research Staff for Compliance and Quality
Educating research personnel is essential for ensuring adherence to regulatory standards and maintaining the accuracy of study information. Regular training sessions that focus on Good Clinical Practice (GCP), ethical considerations, and data management protocols are imperative. In Ecuador, advancements in education have significantly improved imaging diagnosis and patient care, underscoring the necessity for well-trained staff in clinical studies.
The organization places a high priority on comprehensive educational programs that equip team members with the skills and knowledge crucial for effective and ethical research conduct. With over 20 years of experience in Medtech, bioaccess® leverages its expertise in managing a range of studies—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies—to enhance the quality of evaluations. This commitment not only cultivates a culture of continuous improvement within the organization but also ensures that staff are adept at navigating the complexities of research studies.
Current trends indicate a rising emphasis on GCP training, as numerous organizations acknowledge its significance in maintaining compliance. Expert insights highlight that effective training initiatives are vital for ensuring research personnel are sufficiently prepared to manage the intricacies of medical studies. As G. Elizabeth Zamora remarked, "Without the support from RSNA none of this would be possible in our small country with such limited resources," which highlights the challenges in designing trials for Ecuador and the crucial role of training in addressing them. Furthermore, Lillian Sung noted the benefits of collaborative activities, stating, "The advantage of walking over other activities is facilitation of conversation between CRTI attendees," emphasizing the importance of communication in successful training programs. As the landscape of medical research evolves, ongoing education remains foundational for achieving high standards in study execution and information reliability. A dedicated task force is outlining recommendations for metrics to be presented to NCATS, ensuring that training programs meet essential compliance standards.
Implementing Continuous Monitoring for Trial Success
Ongoing observation throughout the clinical study process is essential for ensuring participant safety and maintaining information integrity. This involves:
- Regular evaluations of experiment progress
- Rigorous data quality checks
- Comprehensive safety assessments
Leveraging over 20 years of Medtech expertise, the system employs advanced monitoring methods to provide real-time insights into study operations, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH). Such capabilities enable prompt interventions when necessary, allowing experiments to adapt to emerging challenges. By prioritizing continuous monitoring, bioaccess® not only strengthens the integrity of the collected data but also plays a crucial role in the success of clinical trials. This is particularly evident in their collaboration with Caribbean Health Group, aimed at establishing Barranquilla as a premier destination for clinical trials in Latin America.
Conclusion
The innovative approaches adopted by bioaccess® in clinical trial design highlight the organization's unwavering commitment to enhancing health outcomes in Ecuador. By adeptly navigating regulatory hurdles through strategic partnerships and meticulous planning, bioaccess® guarantees that clinical trials are not only compliant but also efficient. The focus on patient-centric methodologies significantly bolsters recruitment efforts, fostering a diverse participant pool that accurately reflects the demographics of the local population.
Cultural sensitivity is a fundamental aspect of bioaccess®’s strategy; engaging community leaders and comprehending local customs greatly enhances participant trust and involvement. This cultural competence, paired with advanced technological solutions for data management, solidifies bioaccess®'s position as a leader in the region's clinical research landscape.
Furthermore, fostering collaboration among stakeholders enriches the research ecosystem, propelling economic growth and advancing healthcare. As the demand for ethical and diverse clinical trials escalates, bioaccess® consistently sets a benchmark for excellence in the Medtech sector. The organization’s steadfast dedication to ethical practices, continuous training, and innovative strategies not only addresses immediate challenges but also lays the groundwork for future advancements in clinical research in Ecuador and beyond.
Frequently Asked Questions
What is bioaccess® known for in Ecuador?
Bioaccess® is recognized as a leader in research design in Ecuador, with over 15 years of experience in the Medtech field, specializing in tailored solutions for various study types.
What types of studies does bioaccess® specialize in?
Bioaccess® specializes in several types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Trials, and Post-Market Clinical Follow-Up Studies (PMCF).
How does bioaccess® ensure the relevance and effectiveness of its trials?
Bioaccess® tailors its approach to align with regulatory requirements and address the specific needs of the local population, emphasizing patient-focused methodologies and genuine patient involvement.
What processes does bioaccess® offer in its research services?
Bioaccess® provides a comprehensive process that includes Feasibility Studies, Site Selection, Compliance Reviews, Trial Setup, Import Permits, Project Management, and Reporting.
What is the significance of the GCSA certification framework?
The GCSA certification framework, developed with industry leaders, aims to transform clinical trial capabilities and enhance research sites, improving the outcomes of medical studies conducted in the area.
What strategies can help navigate the regulatory challenges in Ecuador?
Key strategies include early collaboration with local regulatory authorities, meticulous documentation preparation, and staying informed about legislative changes.
How does bioaccess® approach participant recruitment for research studies?
Bioaccess® employs local media, community outreach, partnerships with healthcare providers, and effective use of social media to enhance visibility and foster trust, ensuring diverse and sufficient participant enrollment.
What is the goal of the partnership between bioaccess® and Caribbean Health Group?
The partnership aims to position Barranquilla as a leading hub for medical studies in Latin America, enhancing recruitment efforts and stimulating the local economy through job creation and international collaboration in clinical research.




