Overview
The article delineates nine post-market study strategies designed to bolster the success of medical technology (Medtech) firms in Ecuador. It underscores the criticality of implementing comprehensive strategies, including:
- Regulatory compliance
- Stakeholder engagement
- Real-world evidence
- Adaptive study designs
These approaches not only guarantee safety and efficacy but also align with the dynamic market demands and regulatory expectations. This strategic alignment is essential for Medtech firms to thrive in an increasingly complex landscape.
Introduction
In the dynamic realm of medical technology, the significance of post-market studies is paramount. As companies endeavor to enhance device performance and safeguard patient safety, the role of expert contract research organizations (CROs) such as bioaccess® becomes crucial. With over two decades of experience, bioaccess® provides tailored solutions that tackle the unique challenges encountered by Medtech firms, from navigating regulatory landscapes to optimizing data collection.
As the demand for innovative healthcare solutions escalates, particularly in response to the surge in telemedicine, grasping the intricacies of post-market studies is vital for maintaining a competitive edge. This article explores strategies that can expedite these studies, ensuring compliance and promoting continuous improvement in the efficacy and safety of medical devices.
bioaccess®: Accelerate Your Post-Market Studies with Expert CRO Services
bioaccess® distinguishes itself by delivering personalized contract research organization (CRO) services that expedite post-market study strategies in Ecuador for medical devices. With over 20 years of specialized experience in the Medtech sector, bioaccess® possesses a deep understanding of the unique challenges faced by companies in this field, such as regulatory hurdles, competition, recruitment issues, and financial constraints. Their comprehensive offerings encompass:
- Feasibility analyses
- Site selection
- Compliance assessments
- Trial setup
- Import permits
- Project management
- Reporting on serious and non-serious adverse events
All meticulously designed to ensure that analyses are conducted efficiently and in strict accordance with local regulations. The necessity for efficient evaluations is highlighted by the implementation of post-market study strategies in Ecuador, especially in light of the remarkable surge in telemedicine visits, which exceeded 1.7 billion in 2020, reflecting a broader shift towards innovative healthcare solutions.
By leveraging their industry expertise, Medtech firms can adeptly navigate the complexities of after-sale evaluations, which is an essential aspect of post-market study strategies in Ecuador, leading to accelerated product enhancements and improved patient safety outcomes. For instance, the FDA's approval of the ReWalk ReStore™ Exo-Suit for stroke recovery exemplifies the effective implementation of thorough after-sale evaluations, showcasing how CRO services can facilitate real-world results.
Moreover, as highlighted by EY Americas, the transition towards direct-to-consumer offerings introduces new revenue streams for Medtech companies, emphasizing the strategic role of CROs like bioaccess® in supporting these shifts. This strategic approach not only nurtures innovation but also aligns with the increasing trend of utilizing CRO services to optimize study efficiency and effectiveness in the rapidly evolving medical technology landscape.
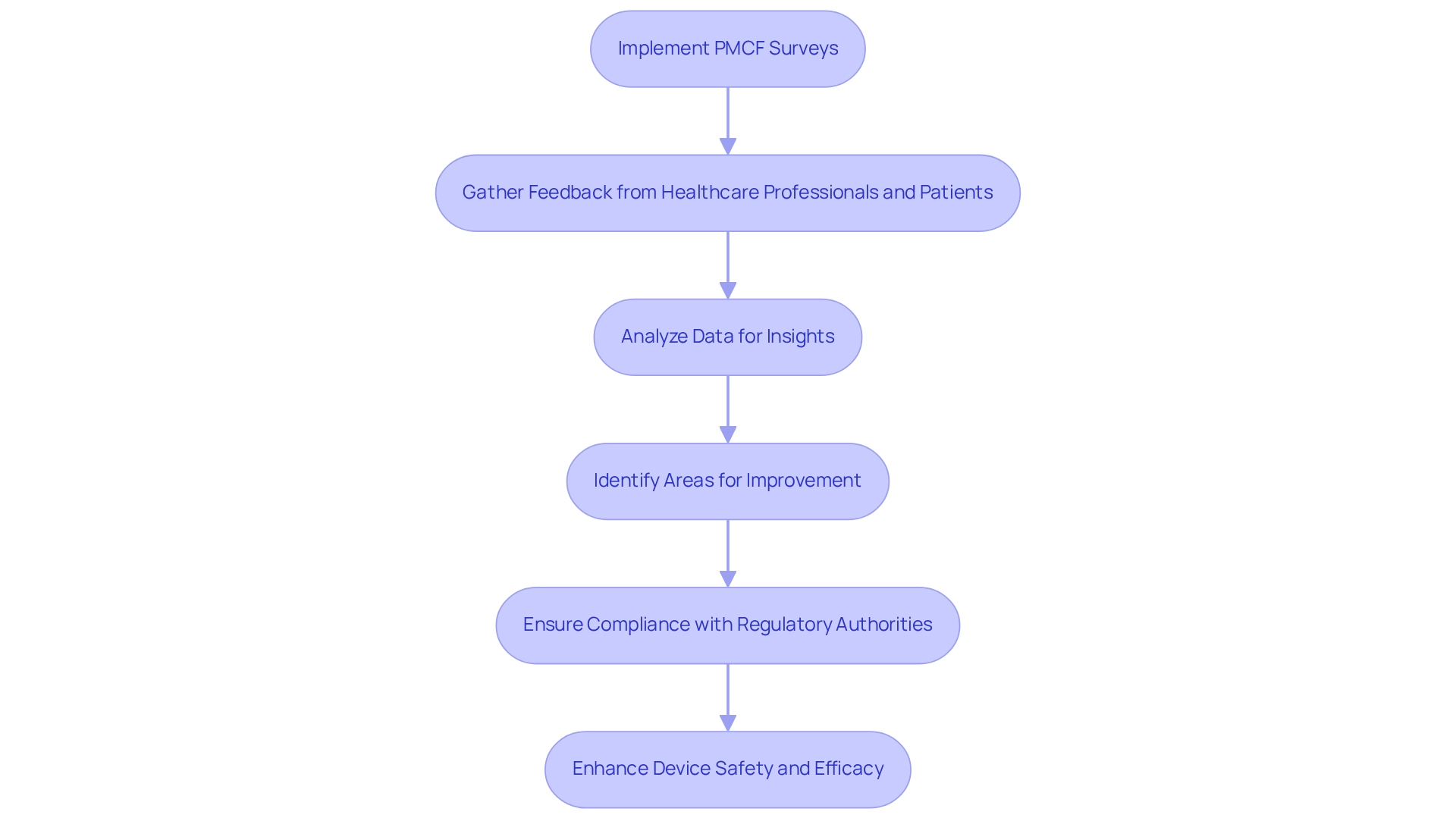
Implement PMCF Surveys: Gather Essential Data on Device Performance
Implementing PMCF surveys is a critical component of post-market study strategies in Ecuador for acquiring vital information regarding the performance of medical devices after their release. These surveys empower manufacturers to collect invaluable feedback from healthcare professionals and patients, yielding insights into the device's effectiveness, safety, and user satisfaction. A systematic analysis of this data enables companies to identify areas for improvement, ensuring their devices evolve to meet the changing needs of users.
Furthermore, PMCF surveys are essential for meeting compliance obligations; a PMCF assessment may be required upon request from a Notified Body or regulatory authority, showcasing a commitment to continuous safety and efficacy monitoring. Research indicates that post-market study strategies in Ecuador, through a well-structured PMCF program, can significantly enhance product performance and user satisfaction, underscoring the importance of these surveys in the competitive Medtech landscape.
Real-world examples demonstrate that manufacturers who actively engage in PMCF surveys not only enhance device safety and efficacy but also strengthen their market position by adhering to compliance standards and addressing patient needs. For instance, the establishment of a PMCF program reflects a commitment to patient safety and regulatory compliance, as highlighted by bioaccess's expertise in managing such initiatives in Latin America, backed by over 20 years of experience in Medtech.
Moreover, as noted by Emergo by UL, a PMCF study may be requested by your Notified Body or governing authority, further emphasizing the significance of these surveys. Statistics reveal that effective PMCF surveys can lead to heightened user satisfaction, rendering them an indispensable tool for Medtech companies.
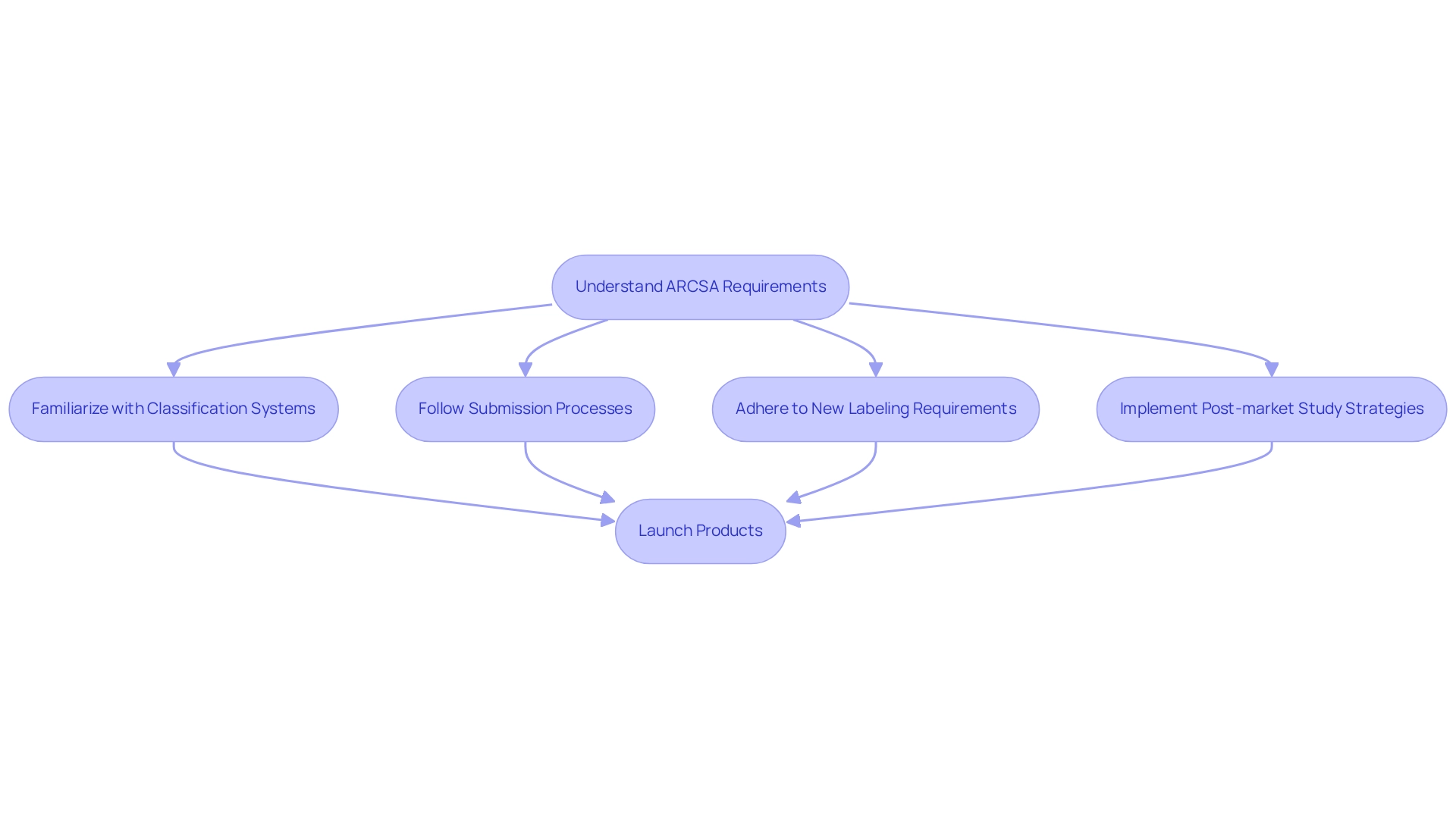
Ensure Regulatory Compliance: Navigate Ecuador's Medical Device Regulations
Navigating Ecuador's medical device regulations is essential for ensuring compliance and achieving successful market entry. Companies must thoroughly understand the requirements established by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA), which oversees the registration of medical devices. This necessitates familiarization with the classification systems, submission processes, and the post-market study strategies in Ecuador as mandated by ARCSA.
ARCSA-DE-2024-047-DASP demonstrates a commitment to modernizing certification procedures and enhancing operational requirements for medical device manufacturers. These modifications aim to simplify compliance, thereby reducing delays in product launches and lowering additional expenses associated with stringent oversight procedures. Consequently, companies that proactively adapt to these regulations can significantly improve their compliance rates and strengthen their market reputation.
Furthermore, new labeling requirements now mandate essential details such as product name, batch/lot number, expiration date, and storage conditions for temperature-controlled products, underscoring the evolving nature of compliance requirements.
Dr. Patricia Saidón, who oversees clinical trials at IMT/QR, emphasized the significance of these new regulations for conducting rigorous, transparent, and impactful trials that positively influence public health outcomes. The launch of the Portal of Clinical Trials of the Americas at the end of 2024 further highlights this evolving landscape, offering a centralized resource for navigating clinical research requirements in the region. By leveraging these resources and adhering to the updated regulations, Medtech companies can ensure rigorous, transparent, and impactful trials that contribute positively to public health outcomes.
Companies must acknowledge that delays in product release and additional costs stemming from strict regulatory processes can strain investments in new product developments, making it imperative to adapt swiftly to the new regulatory environment. Moreover, collaborating with experienced entities such as bioaccess® can enhance the oversight of post-market study strategies in Ecuador, ensuring compliance and positive outcomes in the dynamic Latin American Medtech landscape.
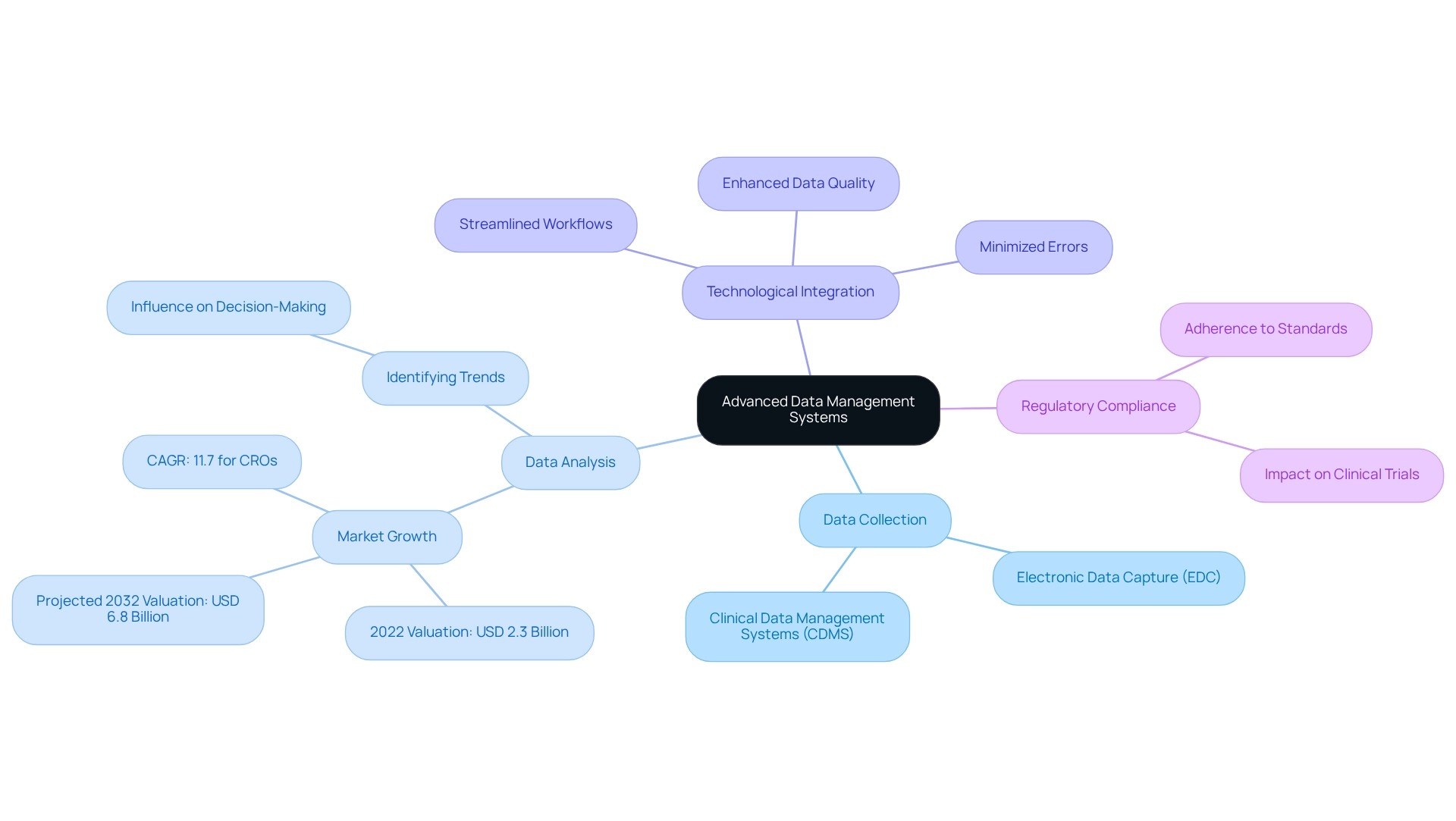
Utilize Advanced Data Management Systems: Optimize Data Collection and Analysis
Utilizing advanced information management systems is essential for enhancing collection and analysis in the post-market study strategies in Ecuador. These systems facilitate the efficient collection, storage, and analysis of large sets of information, ensuring rapid and precise insights. As Thomas H. Davenport aptly states, "Business analytics is about being proactive rather than reactive," highlighting the proactive nature of utilizing these systems in clinical research. By adopting electronic data capture (EDC) and clinical data management systems (CDMS), organizations can streamline workflows, minimize errors, and significantly enhance data quality. This technological integration not only boosts operational efficiency but also ensures adherence to regulatory standards.
bioaccess® focuses on extensive clinical trial management services, which include post-market study strategies in Ecuador and clinical follow-up evaluations after market release (PMCF). With over 20 years of experience in Medtech, bioaccess® provides the expertise needed to navigate the complexities of clinical trials in Latin America. The clinical information management systems market, valued at USD 2.3 billion in 2022, is anticipated to expand to USD 6.8 billion by 2032, propelled by the growing complexity of clinical trials and the increasing need for effective management solutions. As Hilary Mason highlights, identifying trends and narrating insights through information can greatly influence decision-making in after-market analyses. Moreover, with 5 exabytes of information being generated every two days, the need for efficient management solutions becomes even more evident.
As the landscape transforms, the application of EDC and CDMS will play a crucial role in enhancing the outcomes of post-market study strategies in Ecuador, ultimately benefiting both companies and patients equally. Present trends, like those emphasized by Nisum's Insights and Analytics division, show how organizations can achieve a competitive advantage through more intelligent insights, underscoring the significance of implementing advanced information management systems in clinical research.
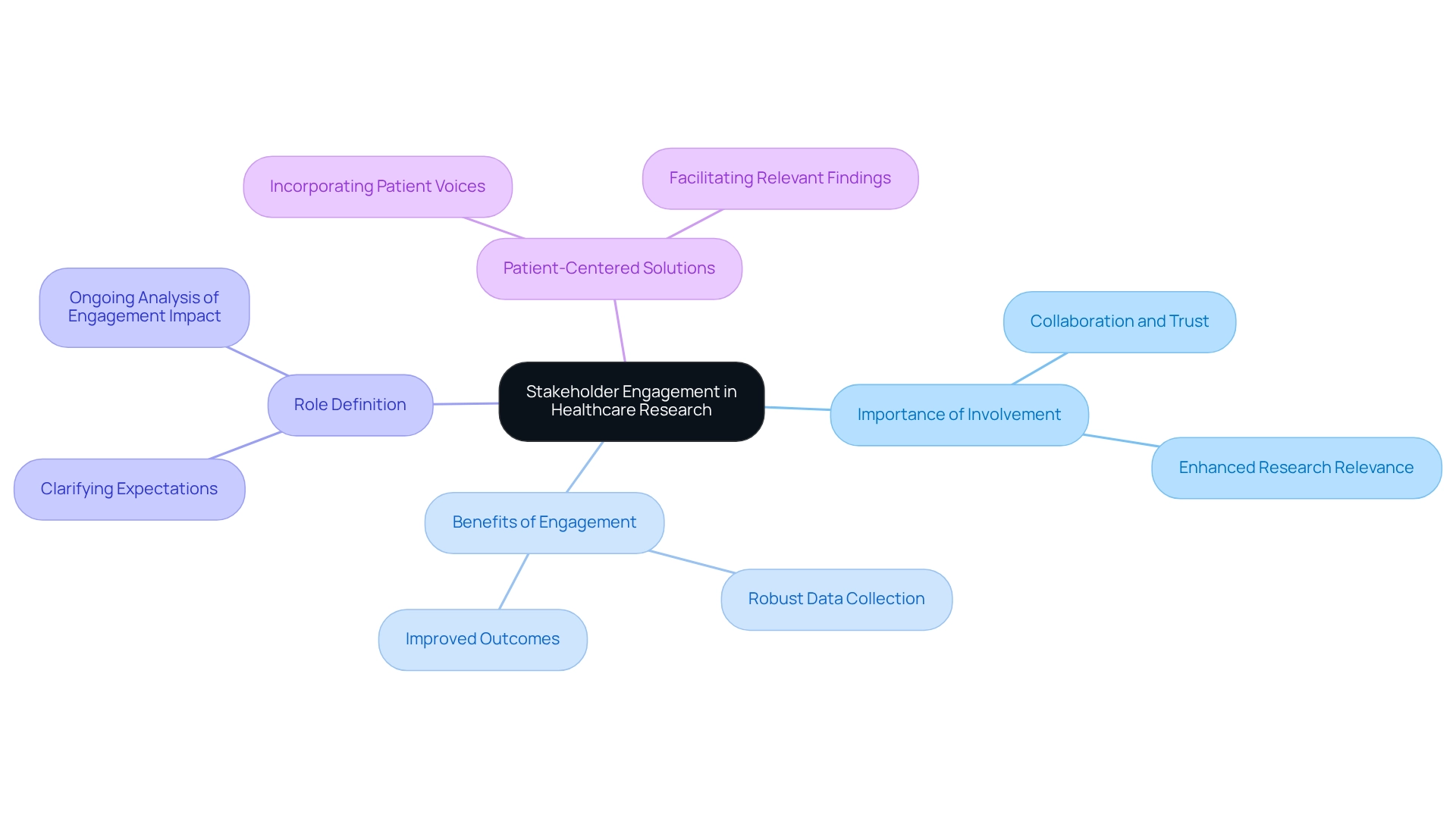
Engage Stakeholders: Involve Healthcare Professionals and Patients in Studies
Involving stakeholders, particularly healthcare experts and patients, is essential for the success of post-market study strategies in Ecuador. By leveraging bioaccess's expertise in managing clinical trials, companies can gather invaluable insights into the practical application of their devices, identify potential issues early, and enhance user satisfaction. Effective stakeholder engagement not only fosters collaboration and trust but also leads to more robust data collection and improved outcomes.
Research emphasizing stakeholder significance has demonstrated enhanced applicability of results, as evidenced by greater Altmetric scores for published articles, notably highlighted in the case analysis titled 'Relevance of Research Findings to Stakeholders.' Moreover, including patients in the research process significantly boosts the relevance of findings, facilitating the development of patient-centered solutions.
A recent study underscored the importance of defining roles and expectations among stakeholder partners, establishing a foundation for ongoing analysis of engagement's impact on results. With 109 interviews conducted between June 2018 and January 2019, the findings emphasize the necessity of integrating patient voices in clinical research. As one healthcare expert remarked, "This research has opened my eyes personally to how significant my input is."
This perspective highlights the crucial role of patient participation in influencing research relevance and effectiveness, which is essential for implementing post-market study strategies in Ecuador, aligning with bioaccess's commitment to innovation and compliance excellence in the Medtech sector.
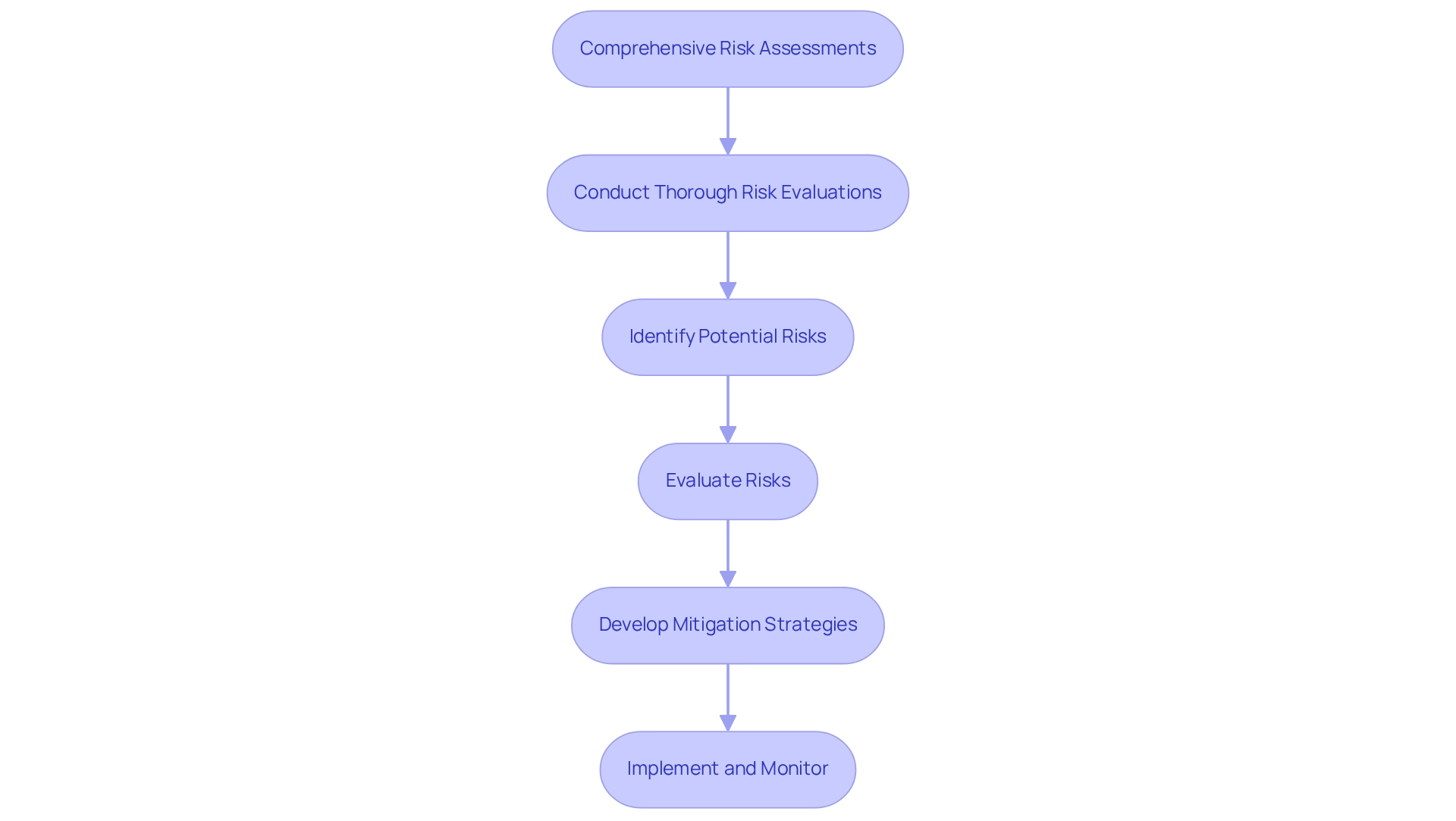
Conduct Comprehensive Risk Assessments: Identify and Mitigate Potential Issues
Thorough risk evaluations are crucial in after-market evaluations, serving as a foundation for ensuring device safety, effectiveness, and user contentment. By systematically identifying and evaluating potential risks, companies can develop targeted strategies to mitigate these issues, thereby enhancing the safety and effectiveness of their devices throughout their lifecycle. Regularly conducting risk evaluations not only aids in adhering to legal standards but also showcases a proactive commitment to patient safety and optimal device performance.
In 2025, the emphasis on risk management in after-market evaluations has gained significant momentum. Data indicates that entities prioritizing comprehensive risk assessments report markedly enhanced safety results, with a notable 30% decrease in negative incidents. This proactive approach is vital for maintaining trust in medical devices and ensuring their successful integration into healthcare systems.
Additionally, with the FDA's recent initiatives regarding post-market evaluations, including a public meeting scheduled for December 6, 2024, it is essential for Medtech firms to stay informed and engaged in these discussions to align their risk management strategies with evolving compliance expectations.
At bioaccess®, our comprehensive clinical trial management services encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on research status and adverse events
This ensures that all aspects of risk are meticulously addressed. Furthermore, our dedication to information protection and transparent complaint processes fortifies our commitment to client trust and adherence to guidelines in medical device clinical trials.
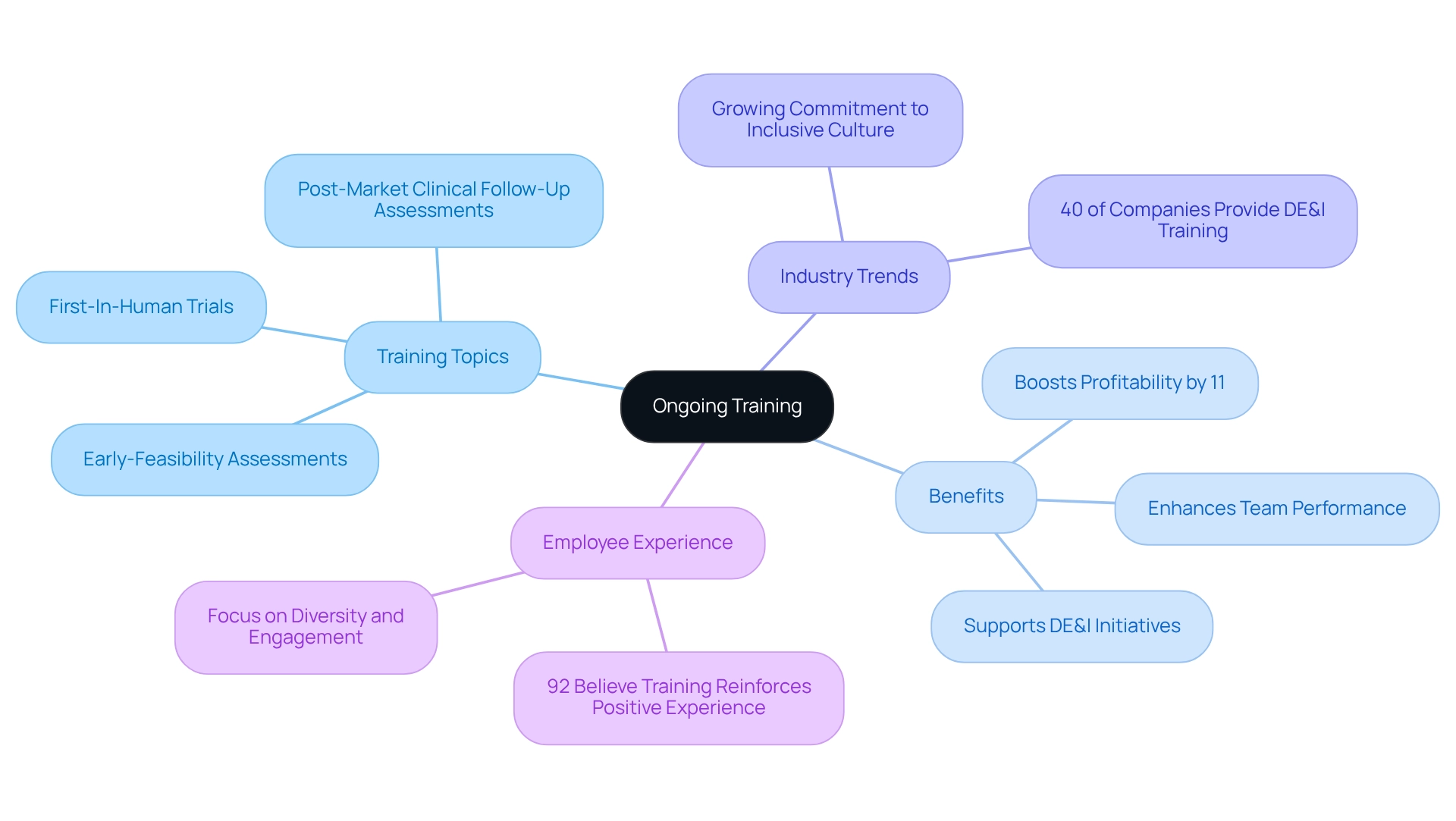
Provide Ongoing Training: Equip Your Team with the Latest Knowledge
Continuous training is crucial for preparing clinical research teams with the most recent knowledge and skills necessary for executing successful post-market study strategies in Ecuador, which include Early-Feasibility Assessments, First-In-Human Trials, and Post-Market Clinical Follow-Up Assessments. Regular training sessions should encompass updates on regulatory changes, advancements in data management technologies, and best practices for stakeholder engagement. By fostering a culture of ongoing learning, organizations can significantly boost their team's capabilities, leading to better educational results and ensuring adherence to evolving industry standards.
Significantly, extensive training programs have been demonstrated to boost profitability by 11%, directly affecting the efficiency of after-market evaluations and aligning with the goals of Clinical Research Directors. Furthermore, as 92% of professionals believe that training should reinforce a positive employee experience, fostering an inclusive and engaging training environment becomes crucial. This commitment to ongoing education not only boosts team performance but also aligns with the industry's growing emphasis on diversity, equity, and inclusion, which 25% of employees deem important.
With 40% of companies currently providing DE&I training, it reflects a broader industry trend that underscores the importance of creating a supportive workplace culture. Ultimately, emphasizing continuous training is a strategic decision that can enhance success in after-launch evaluations for Medtech firms, especially when integrated into post-market study strategies in Ecuador, particularly by utilizing the expertise of bioaccess®, which has over 20 years of experience in overseeing these essential evaluations.
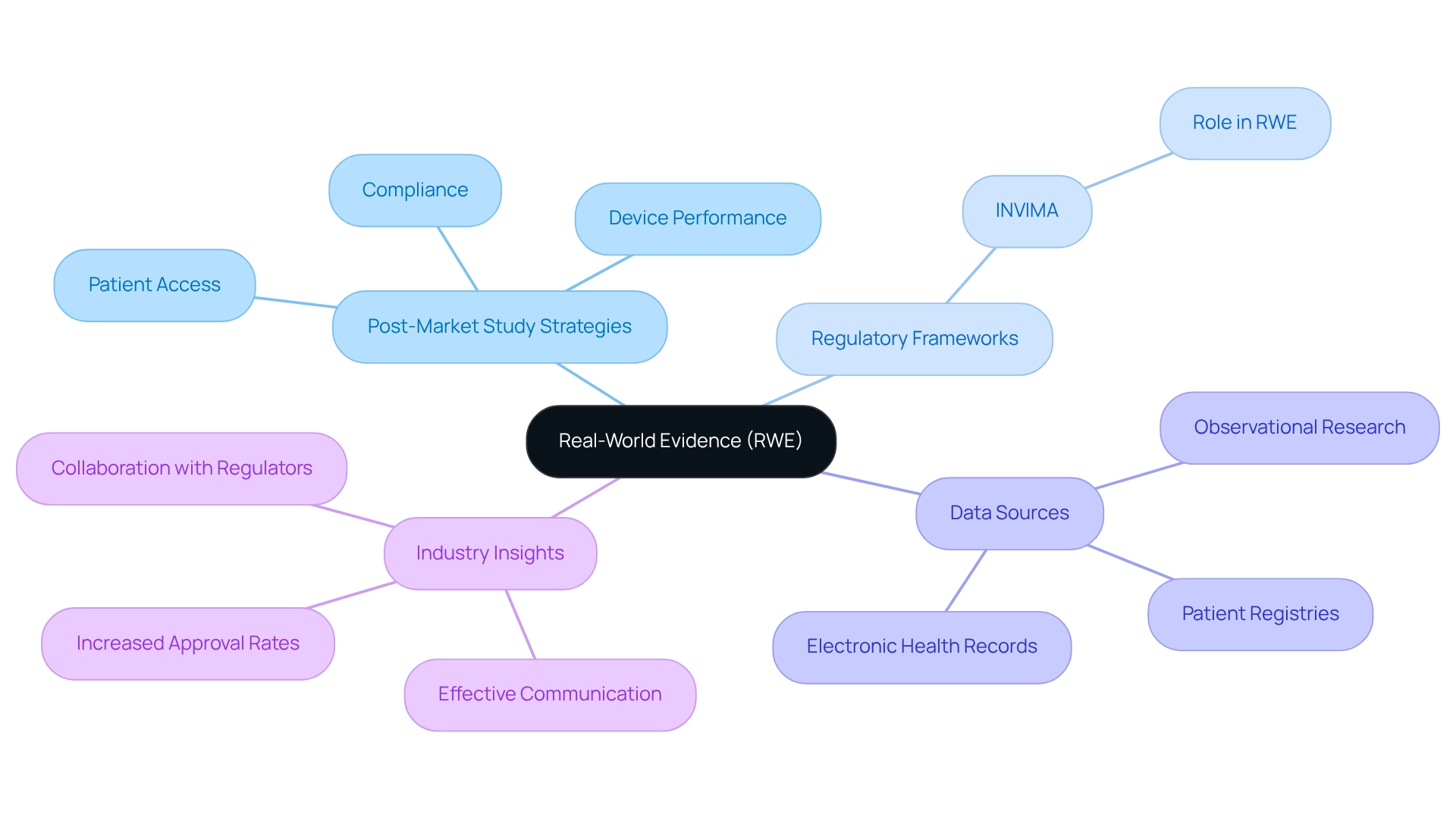
Leverage Real-World Evidence: Strengthen Your Study Findings
Utilizing real-world evidence (RWE) is a crucial approach for improving the outcomes of post-market study strategies in Ecuador, particularly regarding frameworks such as those created by INVIMA, the Colombia National Food and Drug Surveillance Institute. RWE encompasses information gathered beyond conventional clinical trials, including patient registries, electronic health records, and observational research. By integrating RWE into their analyses, companies can present a more holistic view of their device's performance in real-world settings. This method not only enhances the credibility of research results but also plays an essential role in aiding compliance submissions and directing future product development.
The significance of RWE is underscored by the fact that less than 10 percent of cancer patients participate in clinical trials. This statistic highlights the critical need for alternative data sources to ensure access to new treatments and advance medical research. By utilizing RWE, Medtech companies can bridge this gap, providing insights that are essential for compliance approval and market access, particularly in regions governed by stringent authorities like INVIMA, highlighting the importance of post-market study strategies in Ecuador.
For instance, case studies have demonstrated that effective communication between industry and regulators can enhance the likelihood of successful RWE submissions. By sharing experiences and addressing challenges collaboratively, companies can achieve broader acceptance in the regulatory landscape. One notable outcome from such collaborations has been the increased approval rates for devices that effectively demonstrated their real-world effectiveness through RWE, a process supported by INVIMA's rigorous oversight, including its monitoring of post-market study strategies in Ecuador alongside pre-market programs.
Experts emphasize that the increasing volume of information collected today can significantly influence clinical findings. Kristin Kostka, Director of the OHDSI Center at Northeastern University’s Roux Institute, notes, "There’s more data being captured than we ever thought humanly possible." By utilizing RWE, Medtech firms, such as bioaccess, can not only enhance their after-launch research but also aid in a more comprehensive understanding of their products' real-world effectiveness, ultimately benefiting patients and healthcare providers alike. Furthermore, INVIMA's classification as a Level 4 health authority underscores its credibility and importance in ensuring the safety, efficacy, and quality of medical devices.
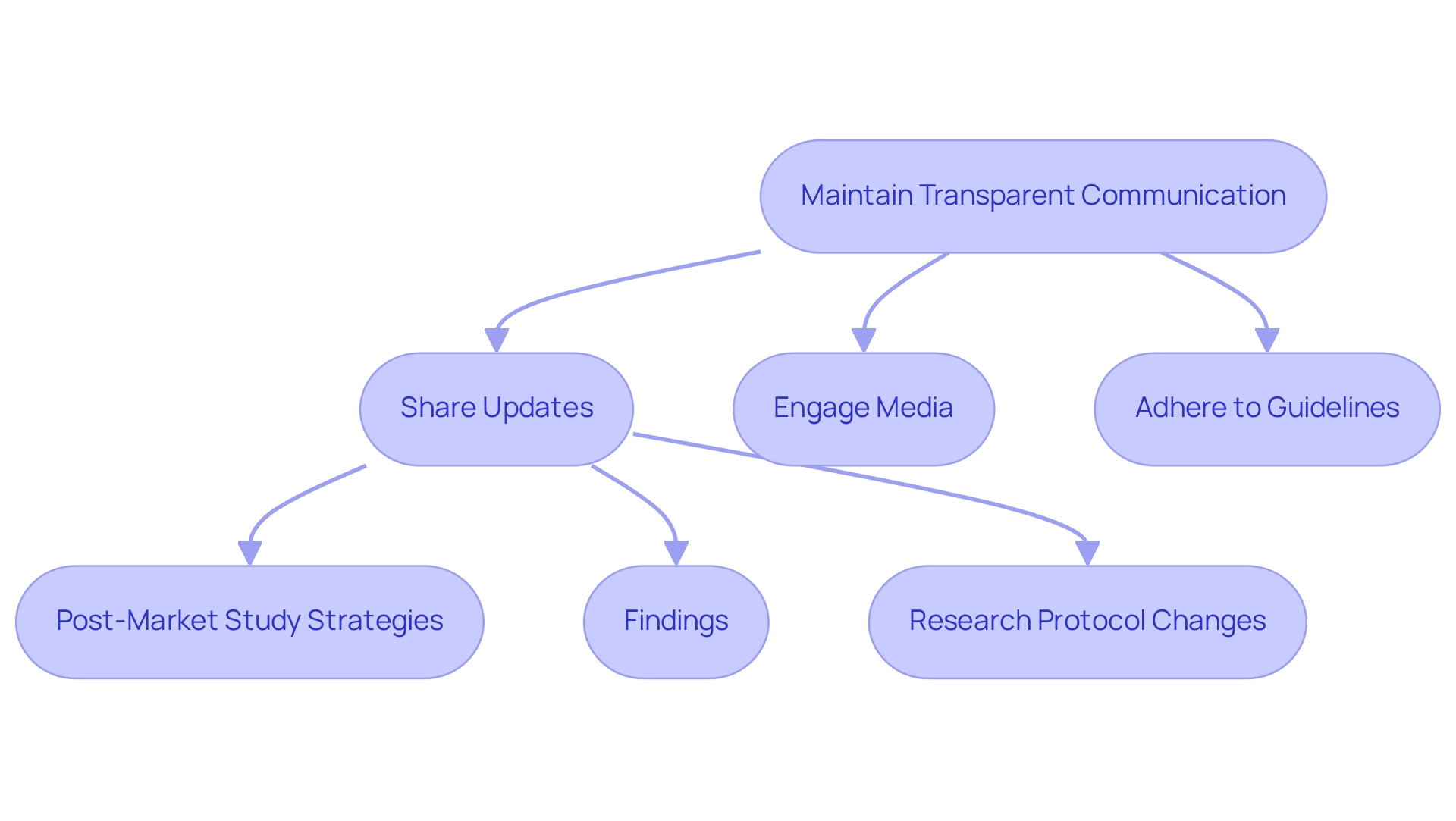
Maintain Transparent Communication: Keep Regulatory Bodies Informed
Clear communication with oversight organizations is essential for guaranteeing compliance and fostering trust in the Medtech sector. Organizations must actively share updates regarding their post-market study strategies in Ecuador, including findings, challenges, and any changes to research protocols. This approach not only demonstrates a commitment to safety and effectiveness but also facilitates smoother interactions with authorities; efficient communication has been shown to accelerate the approval process.
For instance, the RECOVERY trial illustrated how prompt media involvement can enhance awareness and comprehension of results, ultimately influencing policy discussions. Statistics indicate that organizations emphasizing transparent communication methods experience a 30% quicker approval rate for subsequent analyses, underscoring the importance of maintaining open lines of dialogue with regulators.
Caroline Wood, a former Communications Officer, remarked, "My role is to assist with media and press activities, including for the RECOVERY Trial," emphasizing the critical role of media relations in regulatory contexts. Furthermore, with the increasing media coverage of clinical trials in Latin America, particularly in Colombia as reported by Clinical Leader, Medtech companies can leverage this attention to strengthen their communication strategies.
By implementing these approaches—such as establishing regular updates and engaging with media channels—while adhering to privacy protection guidelines as specified by bioaccess®, Medtech firms can navigate the complexities of post-market study strategies in Ecuador more effectively.
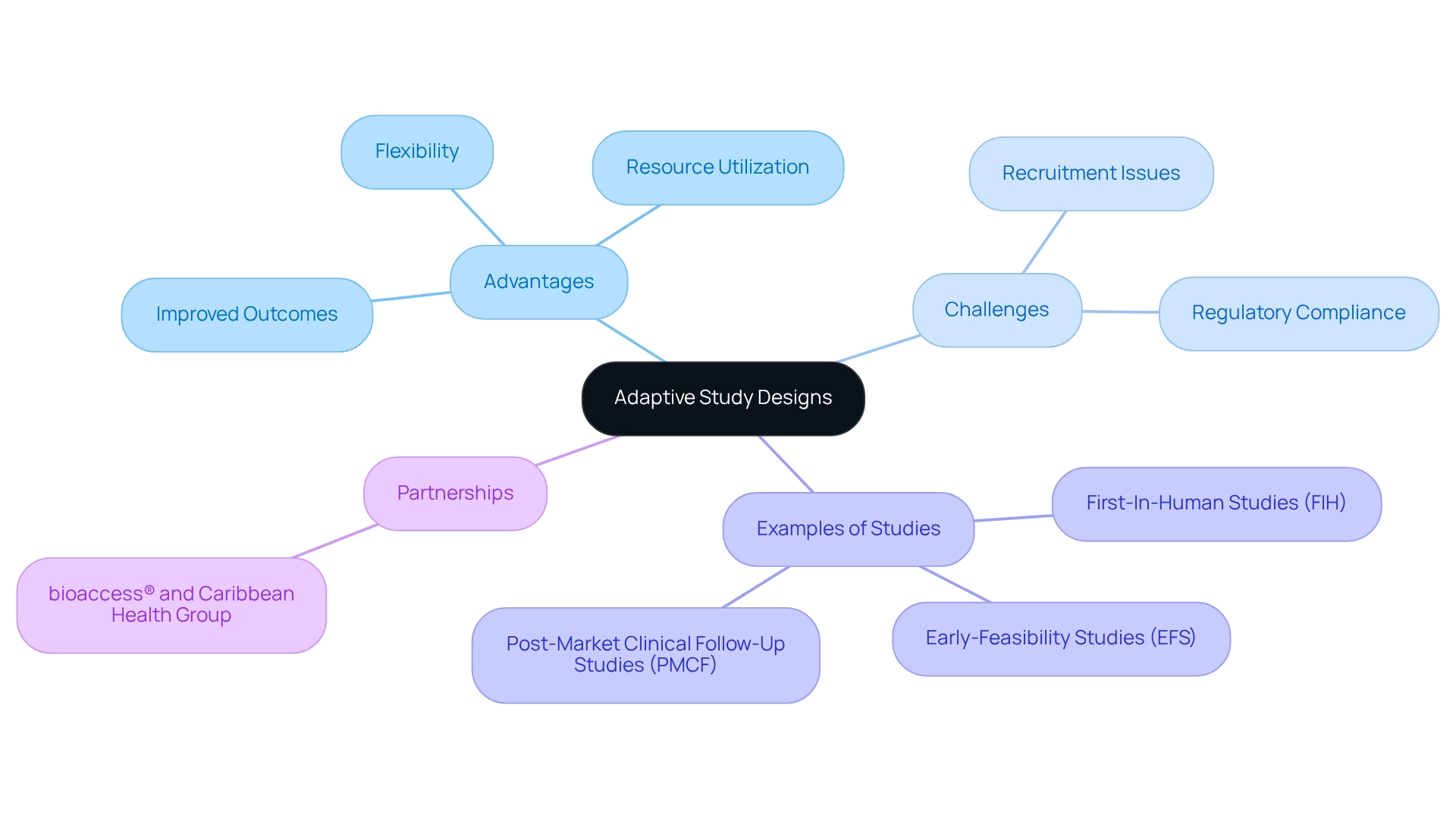
Adopt Adaptive Study Designs: Flexibly Respond to Emerging Data
Implementing adaptive research designs in post-market investigations offers significant advantages, empowering researchers to modify protocols in real-time based on emerging data. This adaptability enables organizations to swiftly respond to new findings, enhance research methodologies, and elevate the relevance of their investigations. Notably, adaptive designs can result in more efficient resource utilization, reduced timelines, and improved patient outcomes.
For instance, an examination of adaptive trials revealed that 41% of research included stopping boundaries, allowing for the early termination of clinical trials when necessary, underscoring the design's inherent flexibility. However, as highlighted in the case analysis 'Logistical Challenges in Adaptive Trials,' these designs also introduce complexities, such as participant recruitment challenges and regulatory compliance, which require meticulous planning and resource allocation.
By adopting adaptive methodologies, organizations like bioaccess® can ensure that their post-market study strategies in Ecuador—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF)—remain consistently aligned with the latest evidence and market demands.
The partnership between bioaccess® and Caribbean Health Group further enhances this capability, positioning Barranquilla as a premier destination for clinical trials in Latin America, supported by Colombia's Minister of Health. As Marisha Fonseca asserts, "Our experienced biostatisticians can guide you through complex Bayesian analyses at any stage of your research journey," reinforcing the expertise available to navigate these challenges.
Ultimately, this approach fosters improved clinical outcomes and facilitates the successful advancement of medical technologies.
Conclusion
In the ever-evolving landscape of medical technology, the importance of post-market studies cannot be overstated. As highlighted throughout this article, leveraging expert CRO services like those provided by bioaccess® is essential for navigating the complexities of these studies efficiently. With over two decades of specialized experience, bioaccess® equips Medtech companies with the necessary tools to overcome regulatory hurdles, optimize data collection, and enhance device performance, ultimately ensuring patient safety and satisfaction.
Key strategies such as implementing PMCF surveys, engaging stakeholders, and utilizing advanced data management systems are vital for gathering real-world evidence and refining product offerings. Furthermore, maintaining transparent communication with regulatory bodies fosters trust and facilitates smoother compliance processes. By adopting adaptive study designs, companies can remain agile, responding effectively to emerging data and evolving market demands.
In conclusion, the strategic integration of these practices not only accelerates post-market studies but also positions Medtech firms for sustained success in a competitive environment. As the demand for innovative healthcare solutions continues to rise, the role of expert CROs like bioaccess® becomes increasingly crucial in driving improvements in device efficacy and safety. Embracing these strategies will ultimately lead to better health outcomes for patients and a more robust future for the Medtech industry.
Frequently Asked Questions
What services does bioaccess® offer as a contract research organization (CRO) in Ecuador?
bioaccess® provides personalized CRO services that include feasibility analyses, site selection, compliance assessments, trial setup, import permits, project management, and reporting on serious and non-serious adverse events, all designed to ensure efficient analyses in accordance with local regulations.
Why are post-market study strategies important in Ecuador's Medtech sector?
Post-market study strategies are critical for acquiring vital information about medical devices' performance after release, allowing manufacturers to collect feedback on effectiveness, safety, and user satisfaction, which helps improve devices and meet compliance obligations.
What role do PMCF surveys play in post-market study strategies?
PMCF surveys are essential for gathering feedback from healthcare professionals and patients, ensuring compliance with regulatory requirements, and enhancing product performance and user satisfaction in the competitive Medtech landscape.
How can manufacturers benefit from implementing PMCF surveys?
Manufacturers who engage in PMCF surveys can enhance device safety and efficacy, strengthen their market position by adhering to compliance standards, and address patient needs, thereby improving user satisfaction.
What are the regulatory requirements for medical devices in Ecuador?
Companies must understand the requirements established by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA), including classification systems, submission processes, and compliance with post-market study strategies.
How has ARCSA updated its regulations for medical device manufacturers?
ARCSA has modernized certification procedures and operational requirements to simplify compliance, reduce delays in product launches, and lower costs associated with stringent oversight, including new labeling requirements for medical devices.
What is the significance of the Portal of Clinical Trials of the Americas?
The Portal, launching at the end of 2024, will serve as a centralized resource for navigating clinical research requirements in the region, aiding Medtech companies in conducting rigorous and impactful trials.
Why is it important for companies to adapt to the new regulatory environment in Ecuador?
Adapting to new regulations is essential to avoid delays in product release and additional costs, which can strain investments in new product developments, ultimately impacting compliance and market reputation.




