Introduction
In the highly regulated and rapidly evolving field of medical device development, effective project management is not just an asset; it is a necessity. As organizations strive to innovate while adhering to stringent regulatory standards, understanding the fundamental components of project management becomes paramount. From defining project scope and ensuring regulatory compliance to implementing robust risk management practices, each element plays a vital role in guiding projects toward successful outcomes.
This article delves into the essential techniques, best practices, and strategic approaches that empower project managers to navigate the complexities of medical device projects, ultimately enhancing efficiency and fostering innovation in a sector that is critical to public health.
Fundamentals of Project Management in Medical Device Development
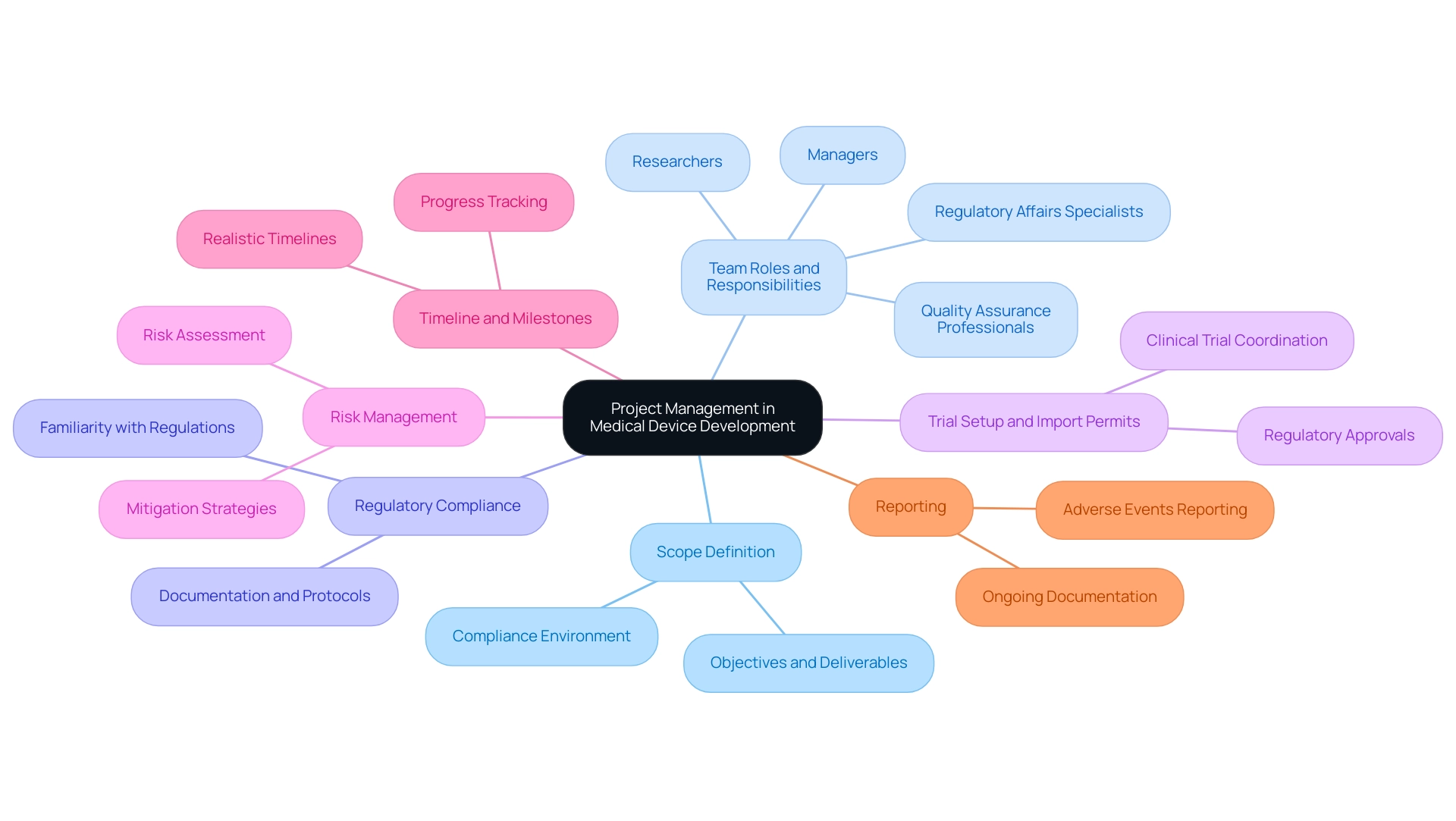
In the realm of medical device development, effective management of endeavors hinges on several essential components:
- Scope Definition: It is imperative to delineate the initiative’s objectives, deliverables, and constraints clearly. This process entails a thorough comprehension of the compliance environment, ensuring that the initiative aligns with industry standards and organizational objectives. Proper scope definition lays the groundwork for all subsequent activities.
- Team Roles and Responsibilities: Clearly identifying and assigning specific roles to team members is crucial. This includes managers, researchers, Regulatory Affairs specialists, and quality assurance professionals. Each team member must have a clear understanding of their responsibilities to foster effective collaboration and communication across the project.
- Regulatory Compliance: A thorough familiarity with regulations governing medical equipment, such as those enforced by INVIMA in Colombia, is essential. Compliance with these regulations is a non-negotiable aspect of project management for medical devices, necessitating detailed documentation and strict adherence to established protocols. Katherine Ruiz, a specialist in compliance matters for medical instruments and in vitro diagnostics in Colombia, illustrates the significance of knowledge in maneuvering through these intricacies.
- Trial Setup and Import Permits: The setup of clinical trials and obtaining necessary import permits are critical steps in ensuring that investigational devices can be utilized effectively. This involves coordinating with regulatory bodies to secure the appropriate approvals and permits, which is essential for the smooth progression of project management for medical devices.
- Risk Management: Creating a strong risk management plan is essential for identifying potential risks throughout the initiative's lifecycle. This involves assessing the likelihood and potential impact of these risks, paired with implementing effective mitigation strategies to minimize their effects on progression.
- Timeline and Milestones: Establishing a realistic timeline that incorporates key milestones is critical. This practice aids in tracking progress and ensures that the initiative adheres to its schedule, facilitating timely deliverables.
- Reporting: Ongoing documentation of study status, inventory, and adverse events is a fundamental element of clinical trial oversight. This guarantees clarity and permits prompt decision-making during the initiative.
By mastering these essential elements and utilizing extensive project management for medical devices alongside clinical trial oversight services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, task coordination, and reporting—managers can skillfully navigate the intricacies inherent in medical device development. This significantly increases the likelihood of successful outcomes. As indicated in industry observations, the significance of regulatory adherence is emphasized by an anticipated 33% growth rate in job opportunities related to this field in India by 2027, showcasing the increasing demand for skilled professionals in this area.
This growth highlights the essential role that effective management plays in ensuring compliance and successful execution. Furthermore, as automation continues to streamline routine tasks and enhance efficiency, management processes are set for substantial improvements, aligning with industry advancements. A notable example of expertise in this field is Peter Sebelius, acknowledged for his contributions to the medical equipment industry.
His participation in updating ISO standards and creating innovative medical instruments has earned numerous awards and acknowledgment, demonstrating the influence of talented experts in improving results within this sector.
Essential Techniques and Best Practices for Medical Device Project Management
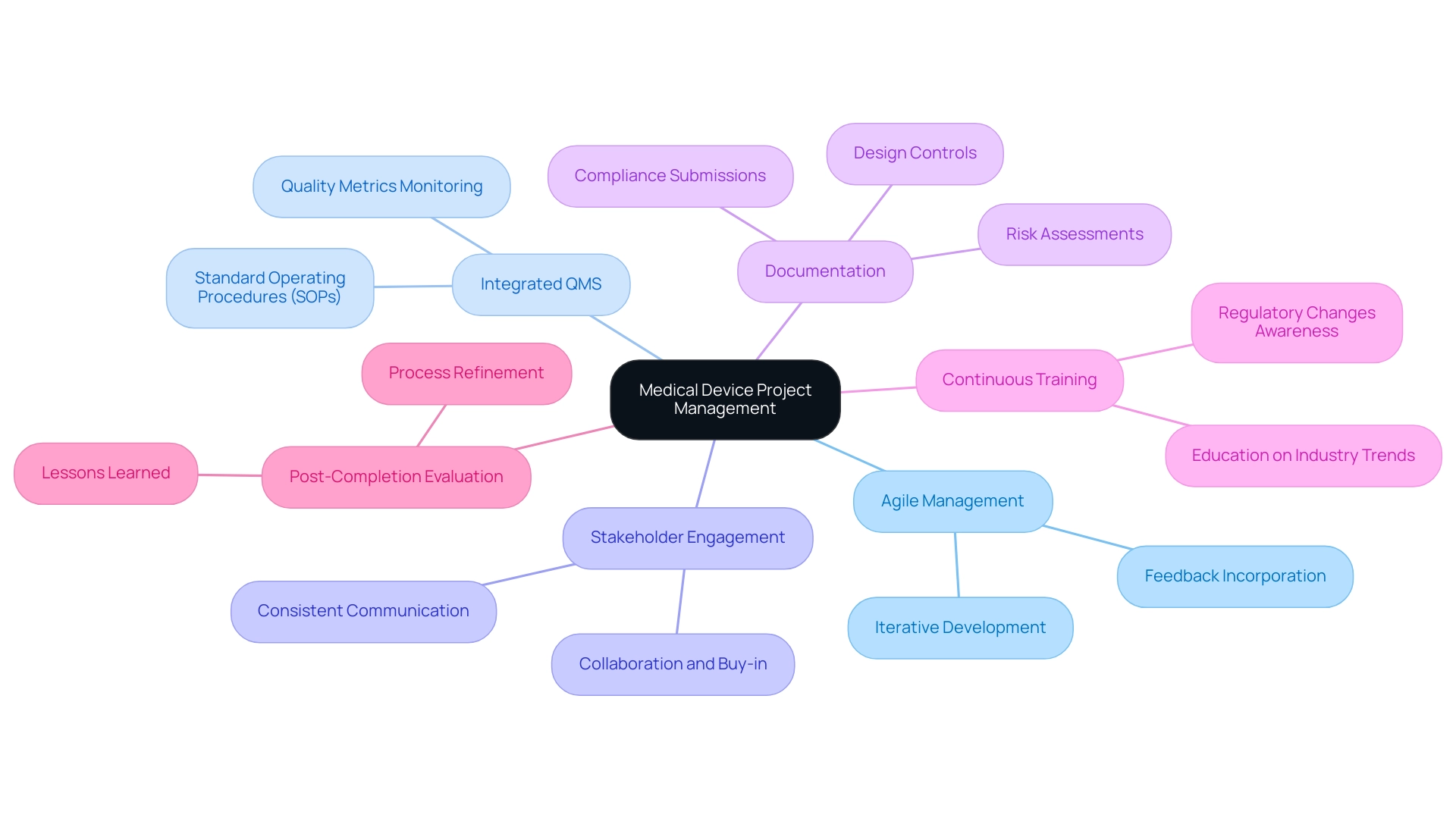
To effectively manage medical device initiatives, particularly in the context of clinical trials in Latin America, it is crucial to adopt essential techniques and best practices that ensure compliance and innovation:
- Agile Management: Embracing agile methodologies enhances flexibility and responsiveness within teams. Agile techniques facilitate iterative development, allowing teams to quickly adapt to changes and incorporate feedback, which is vital in a rapidly evolving industry, especially for studies managed by experienced teams like bioaccess®, which boasts over 20 years of experience in Medtech.
- Integrated Quality Management Systems (QMS): Implementing a comprehensive QMS is paramount for maintaining quality throughout the lifecycle. This involves establishing robust standard operating procedures (SOPs) and continuously monitoring quality metrics to ensure compliance with regulatory standards. Organizations that score higher on the Organizational Competence Score (OCS) tend to demonstrate better compliance and innovation in their projects.
- Stakeholder Engagement: Actively involving stakeholders throughout the initiative lifecycle is essential. Consistent communication and updates foster collaboration and buy-in, which are essential components for achieving success. Engaged stakeholders provide valuable insights that can drive enhancements.
- Documentation: Maintaining meticulous records of all activities—including design controls, risk assessments, and compliance submissions—is vital. Proper documentation aids audits and guarantees adherence to legal obligations, protecting the initiative's integrity and enabling successful navigation through the compliance landscape, such as that overseen by INVIMA in Colombia.
- Continuous Training: Investing in education and development for team members is essential to keep them informed about industry trends, regulatory changes, and best practices in management. A knowledgeable team is better prepared to navigate challenges and improve results.
- Post-Completion Evaluation: Conducting a thorough assessment after completion allows teams to identify lessons learned and areas for improvement. This reflective practice is crucial for refining processes and strategies for future initiatives. For instance, grasping typical traps in clinical data oversight can assist clinical trial supervisors in evading expensive errors and enhancing overall data handling strategies.
Recent advancements, such as Peter Sebelius's creation of a mechanical chest compression apparatus and an ex vivo perfusion machine for lungs, exemplify the innovative spirit within the medical equipment industry. By implementing these best practices, managers can significantly enhance their effectiveness in project management for medical devices initiatives in Latin America. Such diligence not only leads to enhanced patient outcomes but also contributes to progress in medical technology, supported by comprehensive clinical trial oversight services like those offered by bioaccess®.
As emphasized in the PMBOK, ultimately, the job of a good manager is to bring the product to the market by any ethical and legal means they can. This dedication to integrity and quality supports successful management in the medical equipment sector.
Effective Communication Strategies in Project Management
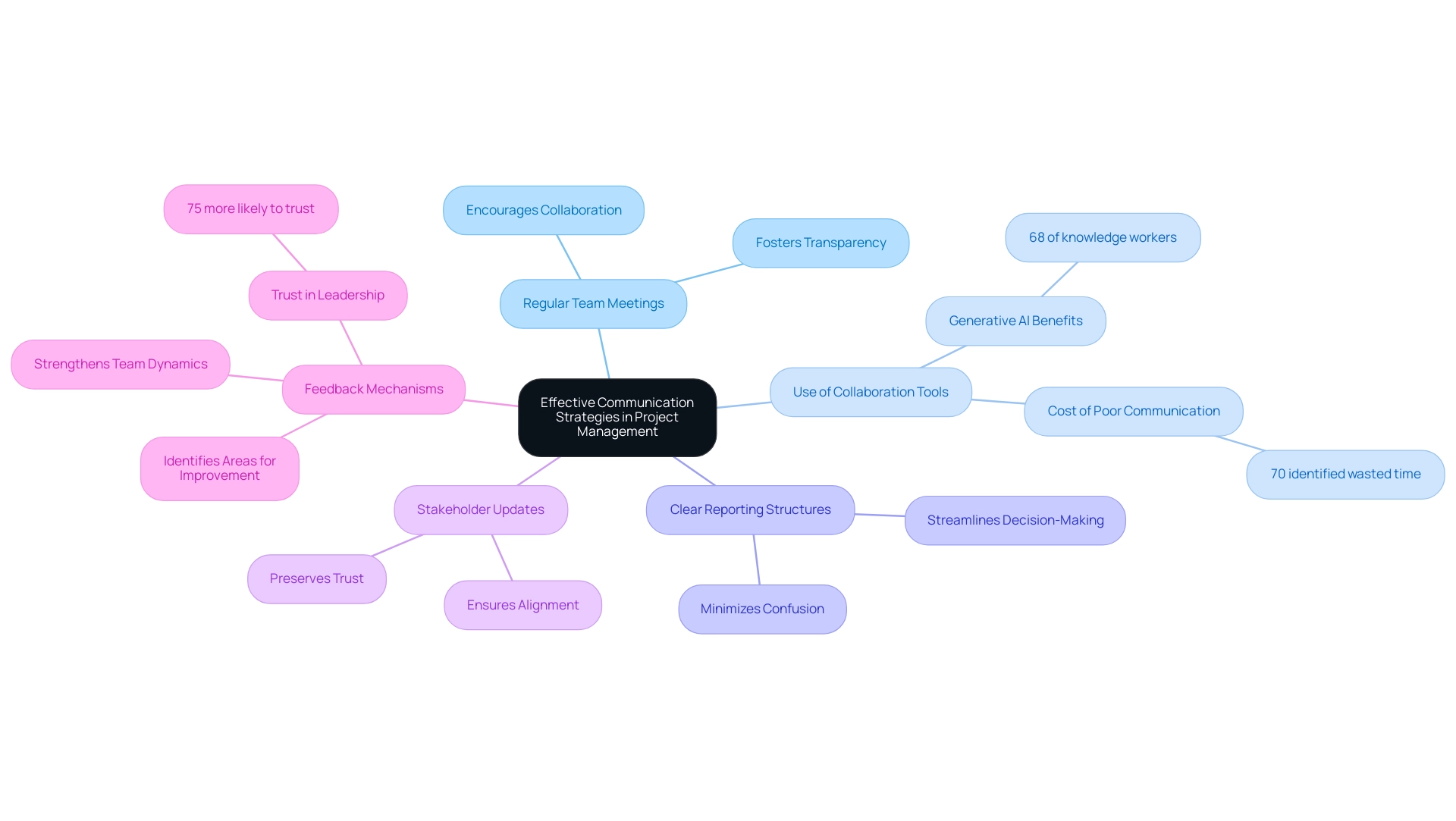
Implementing effective communication strategies is critical to enhancing success in project management for medical devices. Here are several key approaches:
- Regular Team Meetings: Scheduling routine meetings enables teams to discuss progress, address challenges, and outline next steps. This practice not only fosters transparency but also encourages team members to share insights and updates, promoting a collaborative atmosphere.
- Use of Collaboration Tools: Utilizing task coordination software and collaboration platforms, such as Slack or Trello, enables real-time communication and effective information sharing among team members. Notably, 68% of knowledge workers state that generative AI can help them communicate more effectively at work, suggesting that incorporating technology can mitigate inefficiencies. Furthermore, with 70% of survey participants identifying wasted time as a cost of poor business communication, effective tools can significantly enhance management outcomes.
- Clear Reporting Structures: Establishing well-defined reporting lines ensures that team members know whom to approach for specific issues. This clarity minimizes confusion, streamlines decision-making processes, and contributes to a more efficient workflow.
- Stakeholder Updates: Offering consistent updates to stakeholders, including sponsors and oversight agencies, is crucial for preserving trust and ensuring alignment with objectives. Keeping stakeholders informed fosters a sense of partnership and accountability.
- Feedback Mechanisms: Encouraging feedback from both team members and stakeholders is paramount. A structured feedback system not only helps identify areas for improvement but also strengthens team dynamics. Research indicates that 52% of survey respondents associate communication with added stress, underscoring the need for effective communication strategies. Additionally, a case study titled "Feedback Mechanisms in Organizations" reveals that employees who feel their feedback is valued are 75% more likely to trust their leadership, highlighting the significance of responsive communication strategies.
By prioritizing effective communication in project management for medical devices, managers can cultivate a collaborative atmosphere that drives success, ultimately resulting in more innovative and compliant medical products.
Navigating Regulatory Challenges in Medical Device Projects
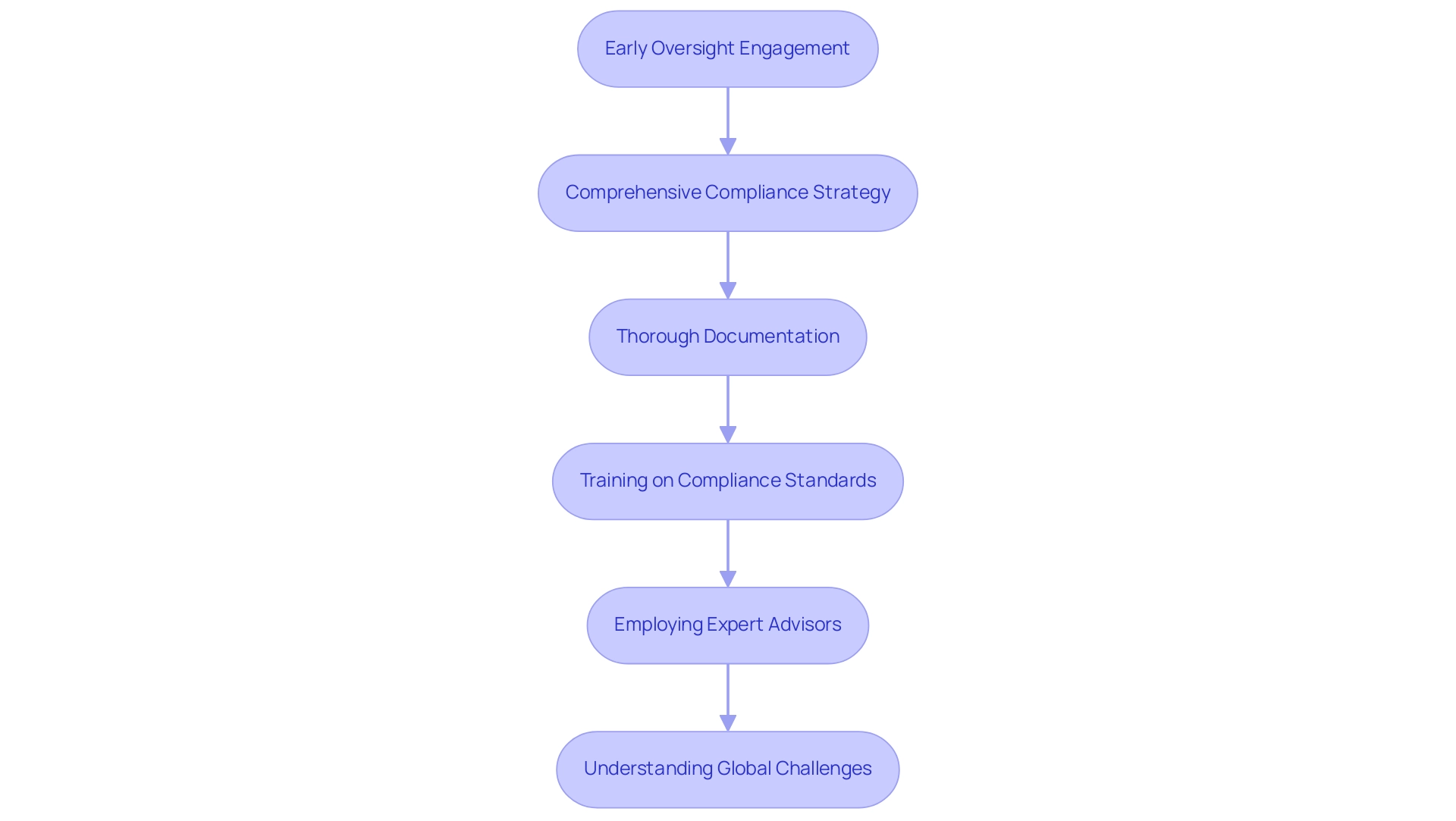
Navigating compliance challenges is essential in project management for medical devices initiatives. A proactive approach can significantly reduce barriers and streamline processes. Here are key strategies to consider:
-
Early Oversight Engagement: Initiating dialogue with oversight bodies, such as INVIMA in Colombia, at the project's outset is essential. This engagement allows for the clarification of requirements and expectations, ultimately preventing costly delays in the future. A Freedom of Information Act request revealed that the FDA has denied only two PMA applications since 1980, underscoring the importance of understanding the oversight landscape early on.
This statistic highlights that with proper engagement, the likelihood of denial is minimal, reinforcing the need for proactive measures.
-
Comprehensive Compliance Strategy: Formulate a detailed compliance strategy that delineates the adherence path, including timelines for submissions and approvals. Considering the intricacy of the compliance landscape, particularly in Colombia where local knowledge, such as that offered by experts like Ana Criado, can be essential, this strategic framework guarantees alignment among all team members concerning compliance objectives, enabling more efficient execution.
Incorporating service capabilities such as trial setup and study project management into this strategy is crucial for effective implementation.
-
Thorough Documentation: Maintain meticulous documentation of all processes and decisions related to compliance. Such documentation is invaluable during audits and reviews, providing transparency and clarity in the compliance journey.
This should include records from feasibility studies and compliance reviews to ensure comprehensive oversight.
-
Training on Compliance Standards: It is crucial to equip team members with a solid understanding of relevant compliance standards, such as ISO 13485 and FDA 21 CFR Part 820. A well-trained team is better positioned to meet compliance expectations and navigate complexities effectively.
-
Employing Expert Advisors: In situations where complexities occur, involving consultants with specialized expertise in medical equipment can be advantageous. Authorities such as Ana Criado, who possesses extensive experience in affairs and compliance, can offer insights to help tackle complex issues and accelerate the approval process.
-
Understanding Global Challenges: Consider the case study of 'Medical Equipment Lag in Japan,' which illustrates the delays in the approval and adoption of new medical technologies compared to the EU and USA.
This delay has been linked to strict oversight procedures and growing equipment complexity, highlighting the necessity for thorough oversight strategies that adjust to diverse international environments.
By proactively tackling these oversight challenges and incorporating essential project management for medical devices capabilities such as trial setup, planning, and reporting into their strategies, leaders can significantly improve the chances of initiative success and guarantee adherence, thus positioning their organizations advantageously in a highly controlled landscape. The ongoing updates in FDA regulations for 2024 further highlight the need for comprehensive regulatory strategies, as the landscape continues to evolve.
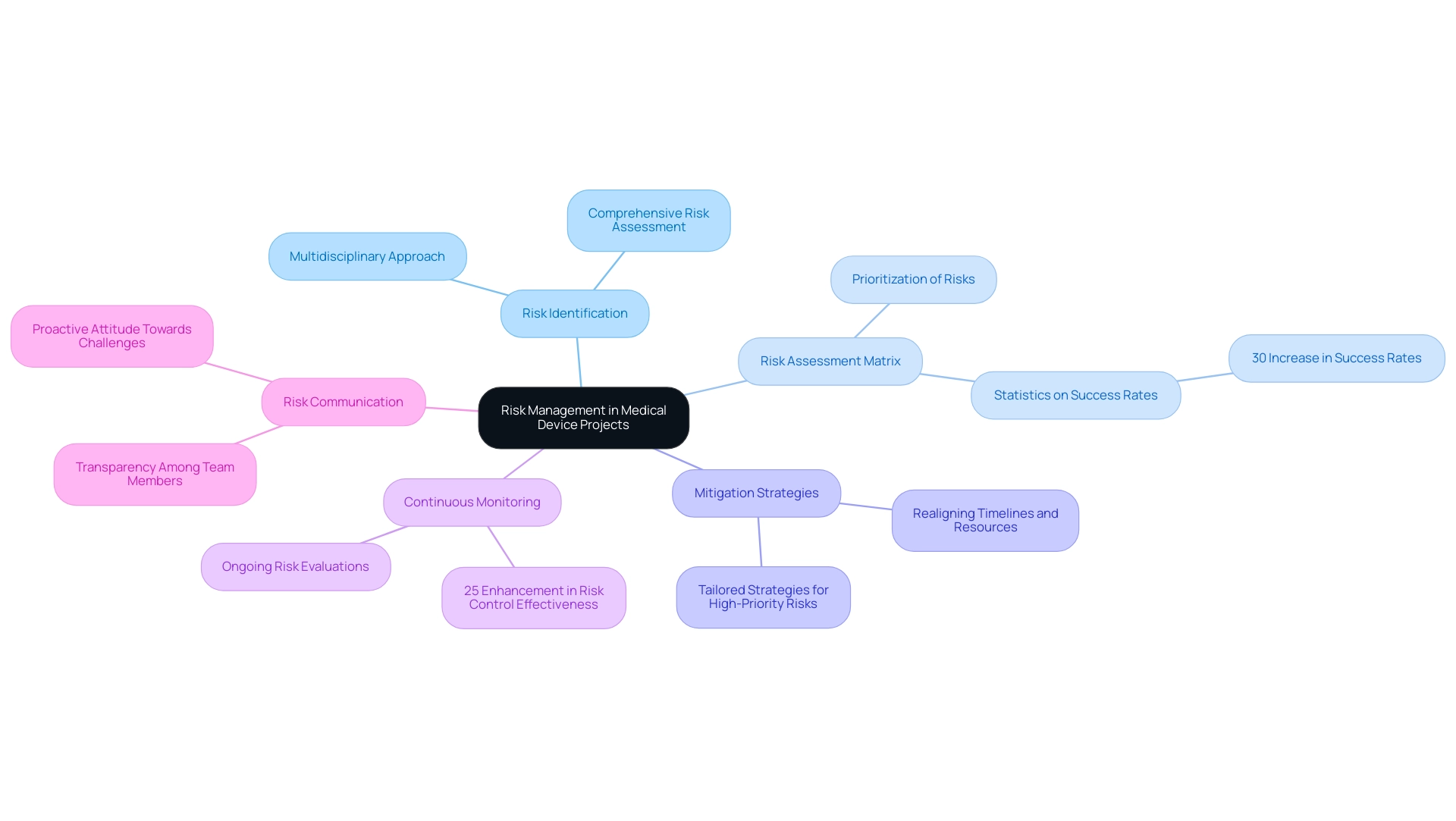
Risk Management in Medical Device Projects
To effectively navigate the complexities of risk management in medical device initiatives, the following strategies are essential:
- Risk Identification: Initiate a comprehensive risk assessment at the beginning, engaging team members from diverse disciplines. This multidisciplinary approach is crucial for uncovering a wide array of potential challenges that may impact project management for medical devices.
- Risk Assessment Matrix: Implement a risk assessment matrix to systematically evaluate the likelihood and impact of identified risks. This tool allows teams to prioritize risks based on their potential effects on outcomes, enabling focused resource allocation where it is most needed. Recent studies indicate that organizations utilizing risk assessment matrices report a 30% increase in success rates, emphasizing their importance in management.
- Mitigation Strategies: Formulate and execute tailored mitigation strategies for high-priority risks. This may involve realigning timelines, securing extra resources, or reassessing the scope to reduce exposure to identified threats.
- Continuous Monitoring: Establish a robust framework for the ongoing monitoring of risks throughout the lifecycle. Frequent evaluations and revisions to the risk strategy are essential for adjusting to new threats and preserving oversight. Continuous monitoring has been shown to enhance risk control effectiveness by 25%, according to industry statistics.
- Risk Communication: Foster an environment of transparency by ensuring that all team members are informed about existing risks and the corresponding mitigation strategies. Efficient communication regarding risks fosters a proactive attitude, enabling teams to tackle challenges rapidly.
By embracing these thorough risk handling practices in project management for medical devices, managers can greatly reduce uncertainties, thereby improving the likelihood of successful outcomes. The effectiveness of risk assessment matrices and the significance of ongoing monitoring are highlighted by recent statistics showing that organizations using systematic risk strategies achieve better outcomes. Moreover, as emphasized by specialists at Square-1 Engineering, the team at Square-1 Engineering consists of a range of technical and project professionals who are subject matter experts in the fields of NPD, Quality, Compliance, and Manufacturing Engineering.
Their insights reflect the necessity of integrating specialized knowledge into risk approaches to meet the evolving demands of the medical technology landscape. Furthermore, the case analysis on the Global Harmonization of Medical Equipment Regulation demonstrates how standardized risk oversight practices can improve safety and adherence in the medical equipment sector.
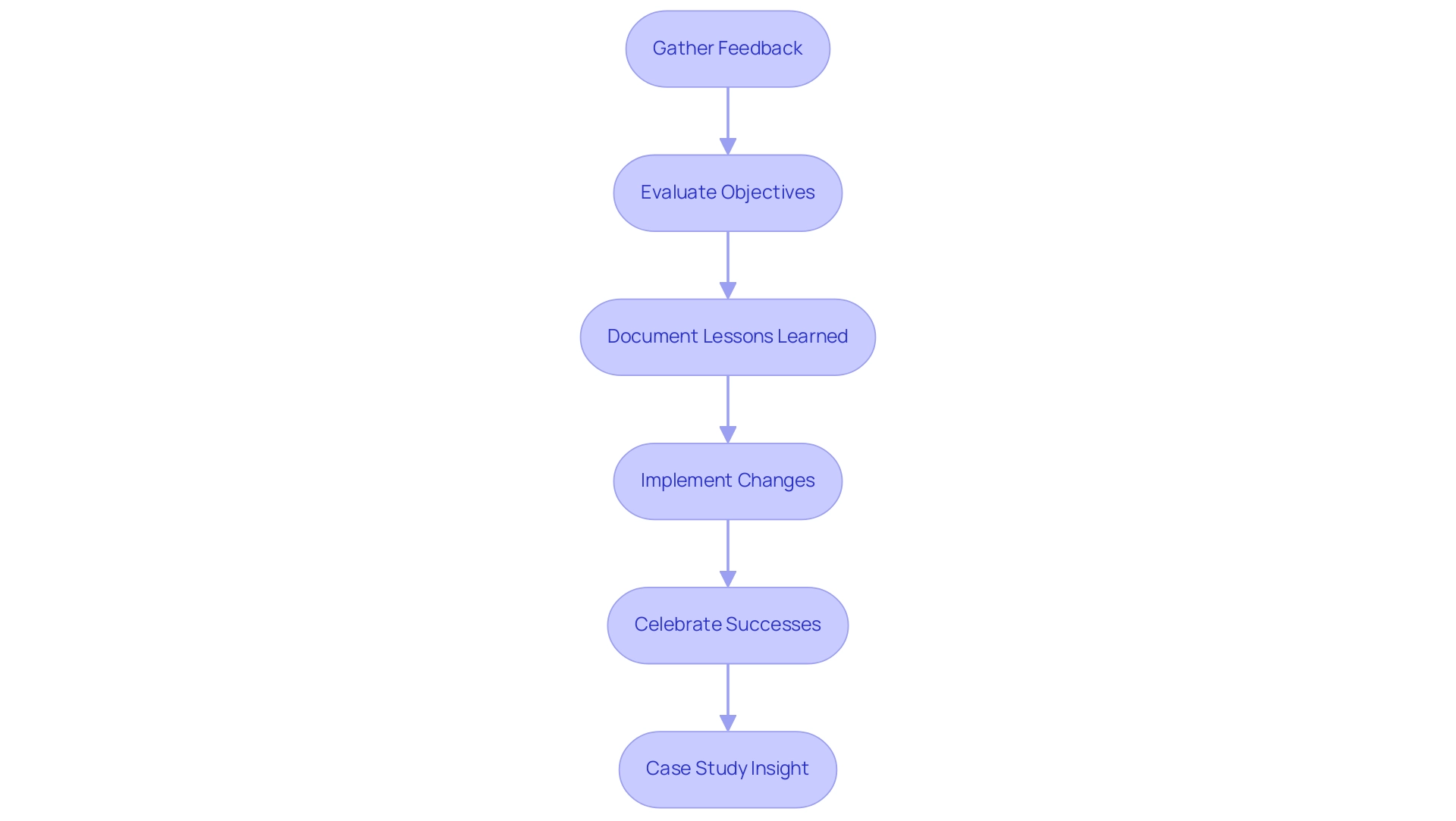
Post-Project Analysis and Continuous Improvement
Post-analysis serves as an essential component of effective project management for medical devices, particularly in the sector where US spending on medical devices accounted for 5.2% of total national health expenditures in 2016, highlighting the financial significance of effective management. To ensure a comprehensive analysis, consider the following key steps:
-
Gather Feedback: Actively collect input from all team members and stakeholders involved in the initiative.
This step is crucial, as diverse perspectives illuminate what functioned effectively and identify areas for improvement. The importance of feedback is underscored by recent trends in continuous improvement methodologies, which emphasize iterative learning.
-
Evaluate Objectives: Conduct a thorough assessment of whether the initiative achieved its initial aims and deliverables.
This evaluation should pinpoint discrepancies and delve into the reasons behind any shortcomings. Insights from the FDA's Medical Device Performance Goals, particularly from the MDUFA IV CDRH Performance Data accessed in 2018, can serve as a valuable benchmark during this assessment, linking regulatory standards directly to results.
-
Document Lessons Learned: Create a detailed document encapsulating the lessons learned throughout the undertaking.
This documentation should encompass successes, challenges, and actionable recommendations for future endeavors. Given the expertise of bioaccess® in project management for medical devices, which includes managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), such documentation becomes vital for enhancing future initiatives, especially considering the lack of available estimates on the cost of bringing medical devices to market, which hampers policy-making and innovation.
-
Implement Changes: Utilize the insights gained from the post-initiative analysis to make necessary adjustments in processes and practices for future endeavors.
This could involve refining management methodologies or enhancing communication strategies, ultimately aiming for a more streamlined approach in future initiatives.
-
Celebrate Successes: Acknowledge and celebrate the accomplishments of the team.
Recognizing successes not only boosts morale but also fosters a culture of continuous improvement, vital in a sector where innovation is paramount.
-
Case Study Insight: For instance, the approval of the Implant, Resorbable for Articular Osteochondral Repair in March 2022 showcases the application of Bayesian analysis in improving clinical outcomes.
Examining such case studies, like those handled by bioaccess®, can offer further insights into effective execution and post-analysis practices. Furthermore, a custom tabulation from a proprietary database estimating clinical study costs from 2004 to 2012 can underscore the financial implications of project management for medical devices and the importance of thorough documentation. By thoroughly conducting post-project analysis and implementing changes based on documented lessons learned, project management for medical devices can significantly enhance effectiveness and drive future project success within the dynamic landscape of medical device development in Latin America.
With over 20 years of experience in the Medtech sector, bioaccess® is well-positioned to navigate the complexities of clinical studies, contributing to local economies through job creation and healthcare improvements.
Conclusion
Effective project management is indispensable in the medical device development landscape, where innovation must coexist with stringent regulatory requirements. By focusing on critical components such as:
- Project scope definition
- Team dynamics
- Regulatory compliance
- Risk management
project managers can lay a robust foundation for successful outcomes. This comprehensive approach not only enhances efficiency but also fosters a culture of continuous improvement and innovation.
The adoption of best practices, including:
- Agile methodologies
- Integrated quality management systems
further empowers teams to navigate the complexities of medical device projects. Engaging stakeholders, maintaining meticulous documentation, and investing in continuous training are integral to ensuring that projects not only meet regulatory standards but also achieve their intended objectives.
Moreover, the importance of effective communication cannot be overstated. Regular updates and feedback mechanisms cultivate a collaborative environment that drives project success. Proactively addressing regulatory challenges and implementing systematic risk management strategies further bolster the likelihood of favorable project outcomes.
In conclusion, the medical device sector's rapid evolution necessitates a commitment to excellence in project management. By embracing these fundamental principles and practices, organizations can position themselves favorably in a competitive market, ultimately leading to advancements that significantly impact public health. The future of medical device development hinges on the ability to blend innovation with compliance, ensuring that new technologies can be brought to market efficiently and safely.
Frequently Asked Questions
What are the essential components for effective management in medical device development?
The essential components include scope definition, team roles and responsibilities, regulatory compliance, trial setup and import permits, risk management, timeline and milestones, and reporting.
Why is scope definition important in medical device projects?
Scope definition is important as it clearly outlines the initiative’s objectives, deliverables, and constraints, ensuring alignment with industry standards and organizational goals, which is crucial for subsequent activities.
How should team roles and responsibilities be managed in medical device projects?
Team roles and responsibilities should be clearly identified and assigned to ensure that each member understands their tasks, fostering effective collaboration and communication within the project team.
What is the significance of regulatory compliance in medical device development?
Regulatory compliance is essential as it involves adhering to regulations governing medical equipment, which requires detailed documentation and strict adherence to protocols to ensure the project's success.
What steps are involved in trial setup and obtaining import permits?
Setting up clinical trials and obtaining necessary import permits involves coordinating with regulatory bodies to secure appropriate approvals and permits for effective use of investigational devices.
How can risk management be effectively implemented in medical device projects?
Effective risk management involves creating a strong plan to identify potential risks, assess their likelihood and impact, and implement mitigation strategies to minimize their effects throughout the project lifecycle.
Why is establishing a timeline and milestones important?
Establishing a timeline with key milestones is critical for tracking progress and ensuring adherence to the project schedule, which facilitates timely deliverables.
What role does reporting play in clinical trial oversight?
Ongoing documentation of study status, inventory, and adverse events is fundamental for clinical trial oversight, ensuring clarity and enabling prompt decision-making during the initiative.
What methodologies can enhance project management in medical device initiatives?
Agile management, integrated quality management systems, stakeholder engagement, meticulous documentation, continuous training, and post-completion evaluation are key methodologies that enhance project management effectiveness.
How does stakeholder engagement contribute to the success of medical device projects?
Actively involving stakeholders throughout the initiative fosters collaboration and buy-in, providing valuable insights that can drive enhancements and contribute to the project's success.
Why is continuous training important for team members in medical device development?
Continuous training keeps team members informed about industry trends, regulatory changes, and best practices, preparing them to navigate challenges and improve project outcomes.
What is the purpose of post-completion evaluation in project management?
Post-completion evaluation allows teams to identify lessons learned and areas for improvement, refining processes and strategies for future initiatives.
How do recent advancements in medical technology impact project management?
Recent advancements, such as innovative medical devices, exemplify the importance of implementing best practices in project management to enhance patient outcomes and progress in medical technology.




