Introduction
In the intricate landscape of medical device development, each phase from concept to post-market surveillance plays a pivotal role in ensuring that products not only meet regulatory standards but also fulfill user needs.
As the industry evolves, understanding the comprehensive process becomes essential for manufacturers seeking to navigate the complexities of compliance and innovation.
This article delves into the critical steps involved in designing and developing medical devices, emphasizing the importance of:
- User research
- Regulatory engagement
- Iterative prototyping
It also highlights the necessity of robust post-market surveillance systems to maintain product efficacy and safety.
By exploring these interconnected elements, stakeholders can better position themselves to deliver effective medical solutions that enhance patient outcomes while adhering to stringent regulatory requirements.
Overview of the Medical Device Development Process
The product development medical device process includes several essential phases:
- Concept development
- Feasibility assessment
- Design and development
- Testing
- Submission
- Post-market activities
Each phase is distinct yet interconnected, necessitating meticulous planning and execution to ensure a successful outcome. A thorough understanding of the regulatory framework is essential, as it outlines the necessary measures required at every stage.
This includes knowledge of the services offered, such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
- Reporting
These services are essential for navigating the complexities of clinical trials. Familiarity with international standards, such as ISO 13485, alongside the FDA's Quality System Regulation (QSR), is imperative for confirming that the product meets stringent safety and efficacy criteria. Additionally, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is vital, as it functions as a Level 4 health authority by PAHO/WHO, overseeing medical device classification and regulation in the region.
Recent studies have highlighted the worldwide aspect of adherence to regulations, with five studies coming from the United States, four from the United Kingdom, one from Canada, one from Thailand, and one from Portugal. This emphasizes the varied compliance challenges encountered by manufacturers globally. Moreover, post-market investigations are crucial as they gather real-world evidence data to support reimbursement strategies for healthcare products, ensuring ongoing adherence and safety.
As Adnan Ashfaq aptly states, 'Make sure you know the regulatory requirements for your product, as well as the regulatory and safety requirements of the country/region you plan to sell in.' This understanding is crucial in managing the intricacies of healthcare equipment regulations, especially as the sector develops in 2024. Additionally, the case study on Matrix Requirements for product development medical device illustrates how a streamlined approach to documentation and risk management can facilitate compliance efforts, enhancing collaboration and traceability from concept to market.
To learn more about our comprehensive services and how we can assist you, BOOK A MEETING.
Key Steps in Designing and Developing Medical Devices
The product development medical device process involves several essential steps:
- Identifying user needs
- Defining product specifications
- Creating design inputs
- Engaging in iterative prototyping
Initiating the process with comprehensive user research is vital, as it allows developers to gather crucial insights and requirements directly from end-users. This information is then translated into explicit specifications that drive the design process.
The significance of user research in healthcare product development medical device cannot be exaggerated; it acts as the basis for creating effective solutions that meet compliance standards and user expectations. Significantly, Julian Grove, Systems Engineer Functional Manager at StarFish Medical, stresses that comprehending user requirements is essential for creating successful healthcare products. Moreover, it is crucial that these advancements conform to the guidelines established by INVIMA, Colombia's National Food and Drug Surveillance Institute, which supervises the safety, efficacy, and quality of healthcare products.
INVIMA is accountable for recommending technical standards for production, promotion, monitoring, and quality assurance of healthcare products, which is essential for ensuring adherence during both pre- and post-market phases. Additionally, employing iterative prototyping is key, enabling teams to test and gather feedback at each stage of development. This methodology not only aligns with the Central Limit Theorem, which supports the use of samples even when the population distribution is unknown, but also facilitates a design process that is responsive to user needs.
Recent trends in 2024 indicate a shift towards more user-centered designs, as evidenced by the case study titled 'Analysis of Studies and Literature,' which illustrates how systematic literature research helps developers identify user needs and leverage existing knowledge. For instance, developers have found that a 5X stress test leads to five times as many units failing compared to normal usage conditions, highlighting the importance of rigorous testing in the design phase. By prioritizing user needs and employing strong prototyping techniques while following INVIMA's guidelines, including its supervision in the pre- and post-market stages, developers can better manage the complexities of product development medical device and ultimately improve patient outcomes.
Navigating Regulatory Requirements for Medical Devices
Navigating compliance requirements for health equipment necessitates a thorough understanding of the guidelines set by the FDA and EMA. An essential initial step is identifying the suitable categorization for your medical apparatus, which affects the compliance route. Following this, preparing the necessary documentation—such as pre-market notification (510(k)) or pre-market approval (PMA)—is essential.
Conducting thorough risk assessments is also vital in product development of medical devices to identify potential challenges early in the development process. Interacting with oversight bodies, including the FDA and EMA, at the outset can clarify expectations and streamline the submission process, ultimately facilitating a smoother approval journey. As noted by Uwe Scherf from the Center for Devices and Radiological Health,
We believe this action will also enhance patients' access to beneficial innovative devices.
Furthermore, it's significant that CBER has awarded 12 Breakthrough Device designations, emphasizing the importance of proactive interaction with oversight agencies. The recent amendment of 21 CFR part 866 further emphasizes the evolving nature of compliance requirements. Insights from experts like Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, reinforce the significance of in-depth knowledge in navigating these regulations.
For example, Ana's extensive experience at INVIMA enables her to guide companies through intricate compliance landscapes, while Katherine's expertise in vitro diagnostics assists in tackling specific adherence challenges. Furthermore, case studies on emerging technologies, such as AR/VR in healthcare, illustrate that while these innovations increase access to care and improve treatment preparation, they also pose risks such as cybersickness and privacy concerns. By sustaining continuous dialogue with governing bodies, developers can maneuver the evolving framework of health product regulation in product development of medical devices more efficiently.
The Importance of Prototyping and Testing in Development
Prototyping in product development medical device is fundamentally an iterative process, essential for refining product design and ensuring usability. Initially, teams should develop low-fidelity prototypes, which are cost-effective for early-stage validation, to explore various concepts and gather preliminary insights. As development progresses, transitioning to high-fidelity prototypes that closely mimic the final product is crucial.
This phase allows for comprehensive usability testing with real users, gathering valuable feedback on both functionality and design. Moreover, thorough safety and performance evaluations must be conducted in the context of product development medical device, following established standards, including adherence to INVIMA's rules for health products in Colombia, to ensure conformity and safeguard patient outcomes. Mitch Maiman aptly notes, 'The difference in the medtech world is that the implications of such testing can affect the safety and efficacy of the products in ways that can impact patient outcomes.'
Incorporating iterative prototyping can lead to final models that are 15% more attainable, further supporting the argument for its effectiveness in achieving better design outcomes. Furthermore, meticulous documentation of all findings during the product development medical device process is essential, not only to support compliance with regulatory bodies like INVIMA but also to facilitate continuous improvements to the product, ensuring a high-quality, reliable solution ready for market introduction. In addition, the integration of prototyping within the broader framework of clinical trial management services is vital.
This includes:
- Conducting feasibility studies to identify suitable research sites and principal investigators
- Ensuring that trial setups comply with local regulations
- Managing project timelines effectively
Reporting mechanisms must also be established to monitor study status, inventory, and any adverse events, thereby enhancing the overall success of clinical trials.
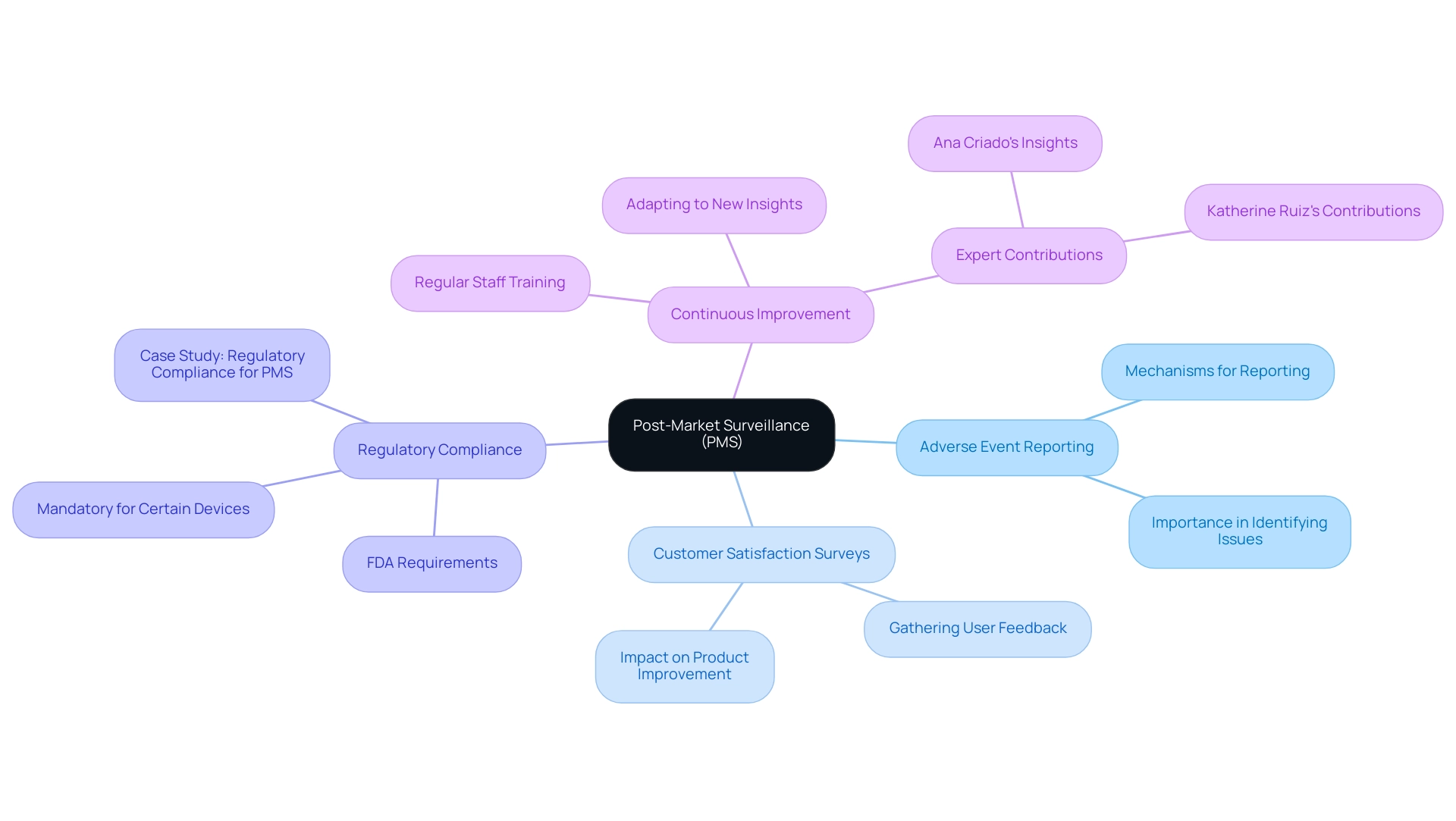
Post-Market Surveillance and Continuous Improvement
After the introduction of a healthcare apparatus, creating a strong post-market surveillance (PMS) system is essential for tracking performance and gathering user input. This system should incorporate:
- Adverse event reporting mechanisms
- Customer satisfaction surveys
These elements are instrumental in identifying potential issues that may arise after deployment. The gathered information must be meticulously examined to guide ongoing enhancement efforts, ensuring that any required modifications to the equipment or its usage instructions are made promptly.
Furthermore, regular evaluations of product performance not only assist in ensuring adherence to regulatory mandates established by authorities such as the FDA but also uphold the high standards of safety and efficacy anticipated in the healthcare equipment sector. As the FDA emphasizes, 'Adherence to these regulations is essential for manufacturers to demonstrate their commitment to patient safety and meet the FDA’s requirements and ongoing performance monitoring of the equipment.' This adherence is further supported by statistics showing that Sternum's security solution can cut security patch management costs by as much as 60%, highlighting the cost-effectiveness of implementing PMS systems.
Additionally, adherence to PMS regulations is mandatory for certain classes of medical equipment, as illustrated in the case study titled 'Regulatory Compliance for PMS.' This case study shows that adherence is not just a formal requirement but a dedication to patient safety and efficient monitoring of equipment performance. Experts like Ana Criado, Director of Regulatory Affairs and a professor with extensive experience in biomedical engineering and health economics, emphasize that effective post-market surveillance necessitates continuous staff training on PMS procedures.
This proactive strategy cultivates a setting of continuous enhancement, aligning with the best methods for performance monitoring in a swiftly changing compliance landscape. Katherine Ruiz, a specialist in oversight matters for health equipment and in vitro diagnostics in Colombia, also contributes to this area by offering insights into local laws and compliance strategies, further enhancing the effectiveness of PMS initiatives. Together, Ana and Katherine's combined expertise underscores the importance of a comprehensive approach to Regulatory Affairs and PMS in ensuring the safety and efficacy of medical devices.
Conclusion
The medical device development process is a multifaceted journey that requires diligence and attention to detail at every stage, from initial concept through to post-market surveillance. Emphasizing the critical steps of:
- User research
- Regulatory engagement
- Iterative prototyping
Manufacturers are equipped to navigate the complexities inherent in this industry. Understanding user needs serves as the foundation for effective design, while compliance with regulatory standards, such as those set by INVIMA, is vital for ensuring the safety and efficacy of devices.
Furthermore, the role of prototyping and testing cannot be understated. These processes not only refine product design but also ensure usability and adherence to regulatory requirements. By employing a systematic approach to testing, developers can identify potential issues early, ultimately leading to improved patient outcomes. Continuous engagement with regulatory bodies further streamlines the approval process, facilitating a smoother path to market.
Post-market surveillance is equally crucial, as it allows for ongoing monitoring of device performance and user feedback. This proactive approach to safety and efficacy ensures that any necessary adjustments are made in a timely manner, reinforcing the commitment to patient care. As the medical device landscape evolves, embracing a comprehensive strategy that incorporates these essential elements will empower manufacturers to deliver innovative solutions that enhance healthcare outcomes while adhering to stringent regulatory expectations.
Frequently Asked Questions
What are the essential phases of the product development process for medical devices?
The essential phases include: 1. Concept development, 2. Feasibility assessment, 3. Design and development, 4. Testing, 5. Submission, and 6. Post-market activities.
Why is understanding the regulatory framework important in medical device development?
A thorough understanding of the regulatory framework is essential as it outlines the necessary measures required at every stage of the product development process to ensure compliance and successful outcomes.
What services are offered to assist in the medical device development process?
The services include feasibility studies, site selection, compliance reviews, trial setup, project management, and reporting, which help navigate the complexities of clinical trials.
What international standards should be familiar in medical device development?
Familiarity with international standards such as ISO 13485 and the FDA's Quality System Regulation (QSR) is imperative for confirming that the product meets stringent safety and efficacy criteria.
What is the role of INVIMA in medical device regulation?
INVIMA, the Colombia National Food and Drug Surveillance Institute, oversees medical device classification and regulation, functioning as a Level 4 health authority by PAHO/WHO.
How do global compliance challenges affect medical device manufacturers?
Studies indicate varied compliance challenges encountered by manufacturers globally, emphasizing the need for adherence to regulations across different countries.
What is the significance of post-market investigations in medical device development?
Post-market investigations are crucial for gathering real-world evidence data to support reimbursement strategies for healthcare products, ensuring ongoing adherence and safety.
What is the importance of user research in healthcare product development?
Comprehensive user research is vital as it allows developers to gather insights and requirements directly from end-users, forming the basis for creating effective solutions that meet compliance standards and user expectations.
How does iterative prototyping contribute to the development process?
Iterative prototyping enables teams to test and gather feedback at each stage of development, ensuring the design process is responsive to user needs and improving the overall product quality.
What recent trends are influencing medical device development in 2024?
There is a shift towards more user-centered designs, with an emphasis on understanding user needs and employing strong prototyping techniques while adhering to INVIMA's guidelines throughout the development phases.




