Overview
The advantages of multi-site trials in Argentina for Medtech success are significant, encompassing:
- Enhanced patient diversity
- Accelerated patient recruitment
- Reduced operational costs
- Improved data collection efficiency
These trials effectively leverage Argentina's diverse demographics, efficient regulatory environment, and extensive healthcare networks. Such strategic utilization optimizes research processes and outcomes, ultimately facilitating faster innovation and market entry for medical technologies.
Introduction
In the rapidly evolving landscape of medical technology, the significance of conducting multi-site clinical trials is paramount. These trials not only enhance patient diversity but also streamline processes, reduce operational costs, and accelerate the time-to-market for groundbreaking innovations. By tapping into Argentina's rich demographic tapestry and robust healthcare infrastructure, researchers can gain invaluable insights that drive the efficacy and safety of medical devices.
This article explores the multifaceted benefits of multi-site trials, highlighting how they foster collaboration among research teams, improve patient engagement, and navigate regulatory advantages. Ultimately, this positions Argentina as a key player in the Medtech sector. As the demand for innovative healthcare solutions grows, grasping the dynamics of multi-site trials will be crucial for companies aiming to stay ahead in this competitive field.
Enhance Patient Diversity Across Multiple Locations
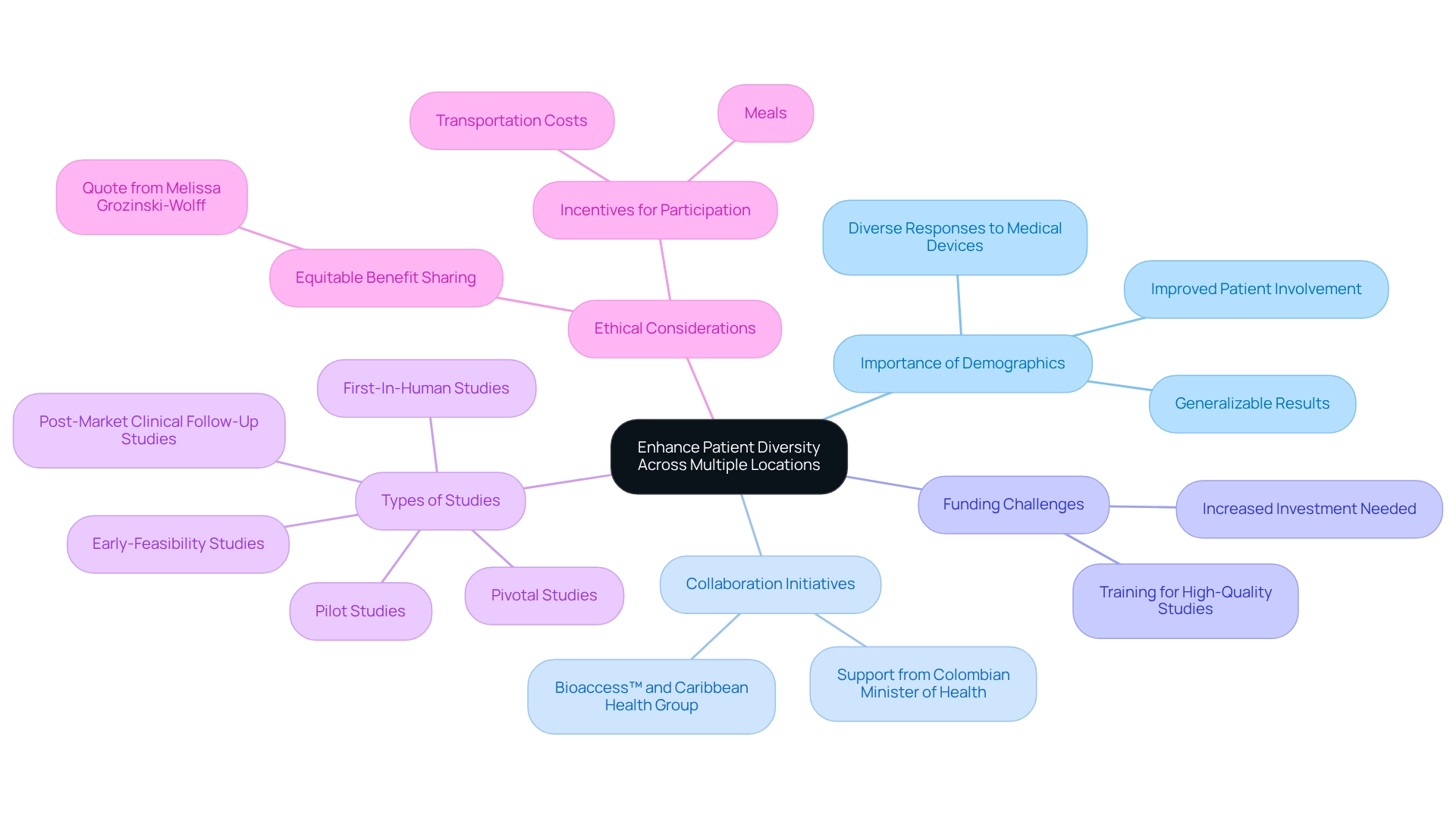
Conducting multi-site trials in Argentina provides researchers with access to a diverse array of demographics, which is crucial for comprehensively understanding the varying responses to medical devices. This diversity not only enriches the data collected but also enhances the reliability of outcomes, as it accounts for the myriad genetic, environmental, and lifestyle factors influencing health. Engaging multiple locations ensures that findings are pertinent to a broader population, ultimately leading to improved safety and efficacy of medical devices across different demographic groups.
Statistics indicate that diverse groups significantly influence research outcomes, with studies showing that investigations incorporating varied demographics yield more generalizable results. For instance, a recent examination highlighted that multi-site trials in Argentina, encompassing a wide range of ethnic backgrounds, demonstrated improved patient involvement and retention rates.
Moreover, the collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a key hub for research activities in Latin America, with support from Colombia's Minister of Health. This initiative not only bolsters the region's research capacity but also stimulates economic growth and job creation through international collaboration in Medtech. Case analyses reveal the challenges faced by South American nations in developing local research capabilities, particularly in cancer research.
The case study titled "Challenges in Local Cancer Research Capacity" underscores the need for increased funding in research infrastructure and training to enhance the region's ability to conduct high-quality medical studies. By promoting diversity in medical research, researchers can address health inequalities and ensure equitable benefit distribution among the communities involved. As Melissa Grozinski-Wolff, Senior Director of Clinical Operations, articulates, "As we strive for equitable benefit sharing with the communities we serve, we can actively address the disparities in access to healthcare."
This approach aligns with ethical research practices and elevates the overall quality and relevance of findings in healthcare. Furthermore, providing incentives such as transportation expenses and meals can encourage participation from a diverse group, further strengthening the case for enhancing participant diversity in research studies. Additionally, bioaccess™ specializes in various clinical studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
These studies are essential for propelling Medtech innovations.
Accelerate Patient Recruitment and Streamline Processes
Multi-site trials in Argentina significantly enhance participant recruitment by leveraging the extensive resources and networks available across various locations. With a population exceeding 45 million, Argentina presents substantial potential for study participants in these trials. Researchers can effectively collaborate with local healthcare providers and community organizations to swiftly identify and enroll eligible patients.
This strategic approach not only shortens recruitment durations but also enhances the overall effectiveness of the study process, enabling faster data gathering and evaluation.
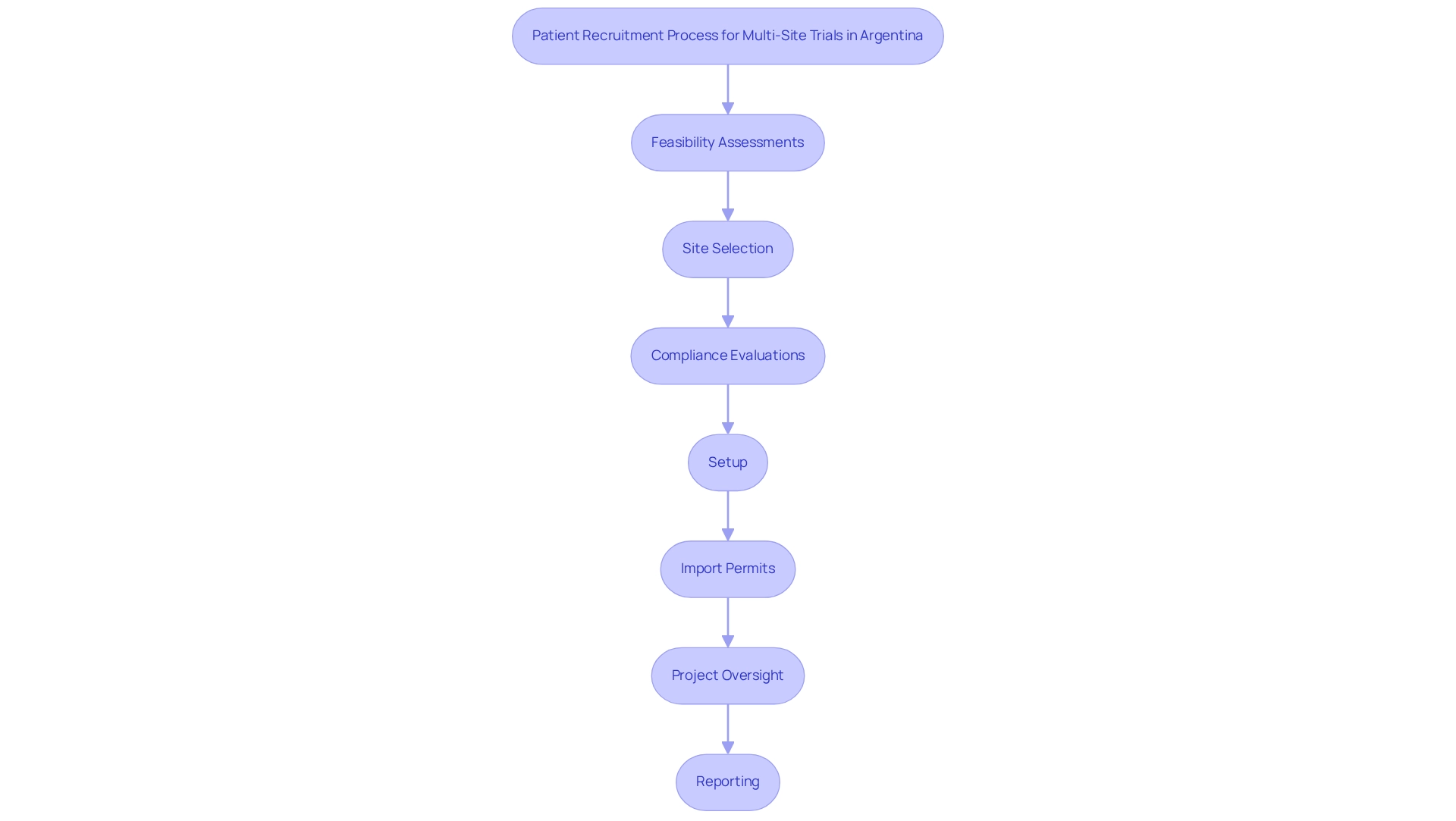
bioaccess® specializes in overseeing studies in Latin America, drawing on more than 20 years of experience in the Medtech industry. Our comprehensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
This tailored method ensures that Medtech firms can adeptly navigate the complexities of the research environment.
For instance, the implementation of nature-inspired metaheuristic algorithms has proven successful in optimizing participant recruitment strategies for global studies. These algorithms adeptly adapt to complex constraints, ensuring high-quality solutions that streamline recruitment plans. As a result, multi-site trials in Argentina are projected to accelerate in 2025, underscoring the nation’s readiness for groundbreaking exploration.
Expert perspectives indicate that as Argentina navigates the intricacies of the research landscape, it fosters an environment conducive to multi-site trials, thereby enhancing scientific progress and global cooperation. By leveraging multi-site trials in Argentina, Medtech firms can expedite patient enrollment, ultimately leading to quicker and more effective research.
Reduce Operational Costs and Optimize Resource Allocation
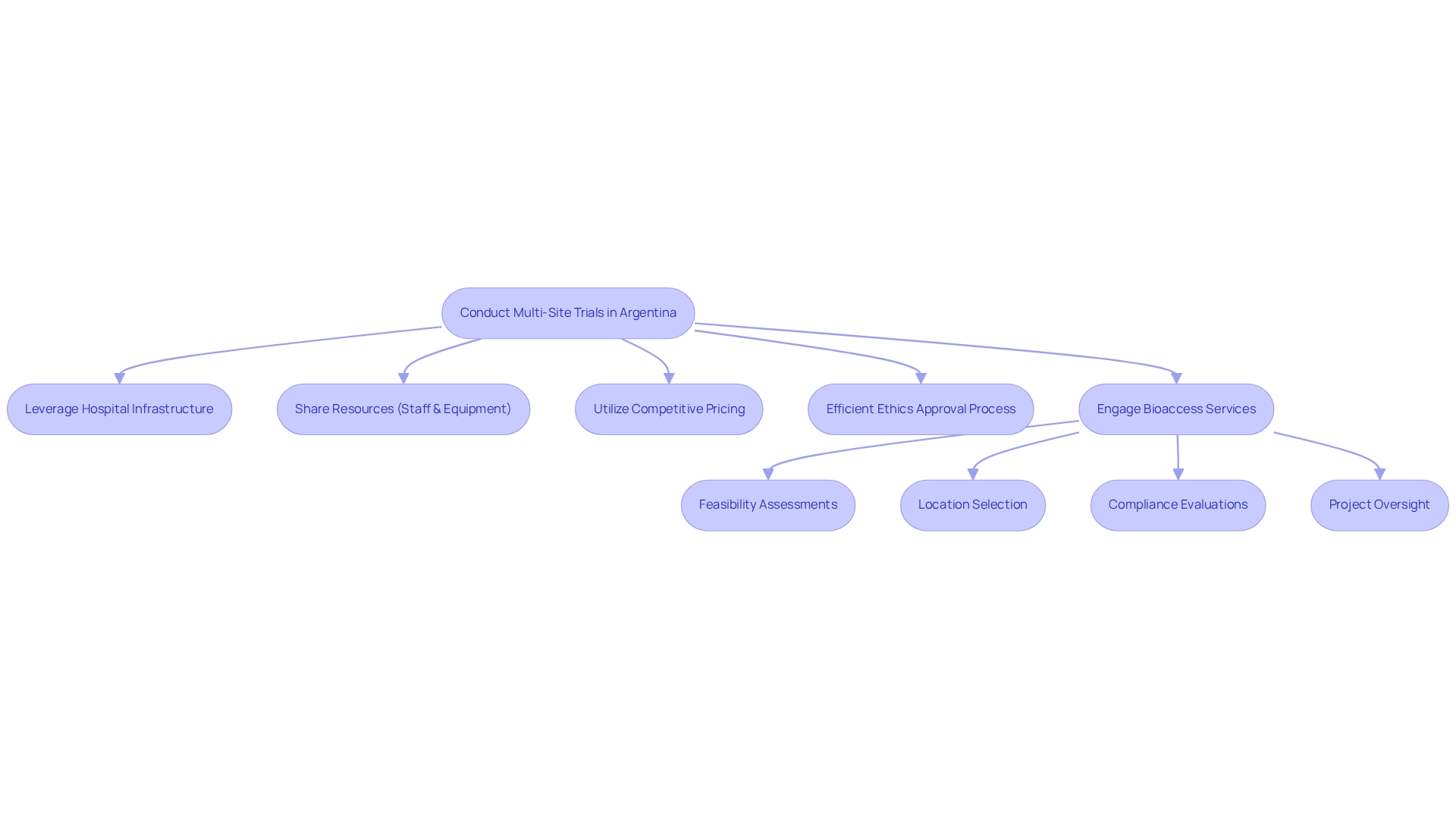
Conducting multi-site trials in Argentina presents substantial opportunities for reducing operational expenses through effective resource distribution. By leveraging the country's robust hospital infrastructure, particularly in the greater Buenos Aires metropolitan area, organizations can share resources such as staff and equipment, effectively minimizing redundancies and lowering overall expenses. This cooperative approach not only optimizes processes but also enhances the quality of medical research.
Argentina's competitive pricing for medical services further amplifies the cost-efficiency of studies. As noted by Julio G. Martinez-Clark, CEO, Argentina now averages approximately 200 new studies each year, positioning the nation as the second largest recipient of foreign-sponsored research in Latin America per million inhabitants, trailing only Chile. This trend underscores the growing appeal of Argentina as a destination for Medtech firms aiming to conduct studies without sacrificing quality.
Moreover, the approval process from independent ethics committees in Argentina can be completed in as few as 15 working days, facilitating faster project initiation. This efficiency is bolstered by the importance of obtaining import permits for investigational devices, which bioaccess expertly manages as part of its comprehensive study management services. These services include:
- Feasibility assessments
- Location selection
- Compliance evaluations
- Test preparation
- Project oversight
- Reporting
All crucial for enhancing the testing process.
As challenges in developed nations drive a shift towards low- and middle-income regions for medical studies, Argentina stands out as an ideal choice for Medtech companies seeking to maximize their research investments while upholding high standards of medical practice. By adopting multi-site trials in Argentina, organizations can achieve significant operational cost reductions and improve the overall efficiency of their research efforts. Additionally, the ongoing partnership highlighted by CAEME is essential in ensuring that innovations reach those in need swiftly, emphasizing the importance of collaboration in successful medical studies.
Facilitate Faster Data Collection and Analysis
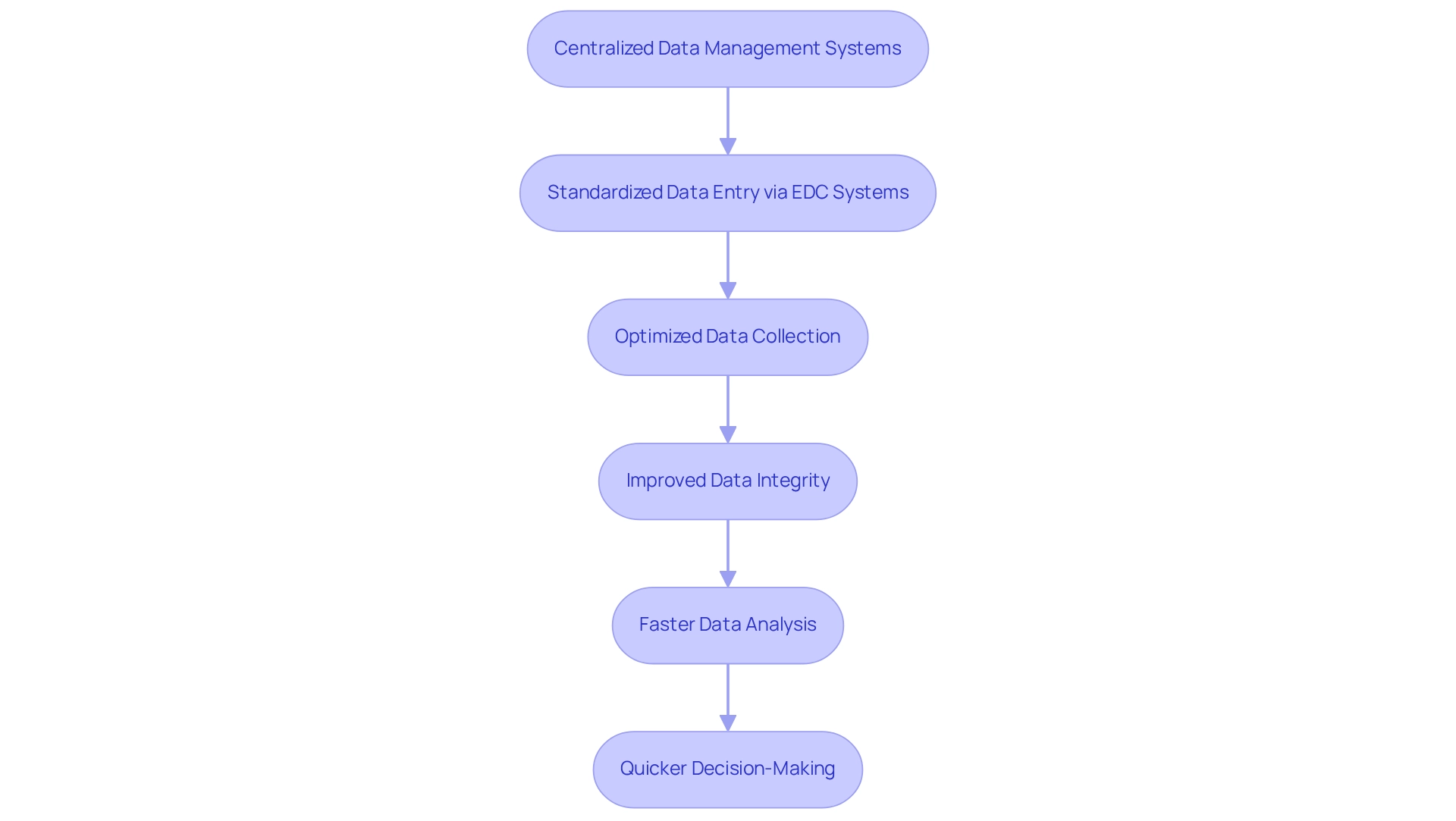
Multi-site studies leverage centralized data management systems to optimize data collection and analysis, significantly improving study efficiency. By standardizing data entry through electronic data capture (EDC) systems, researchers achieve uniformity across all participating sites. This standardization not only accelerates the data analysis process but also strengthens data integrity, facilitating quicker decision-making and more timely reporting of results.
For instance, the implementation of EDC systems has been shown to increase the likelihood of regulatory approval by 23% for pharmaceutical companies, underscoring the critical role of effective data management in research. Furthermore, platforms like REDCap allow for customizable Case Report Forms (CRFs), tailored to specific research needs, thereby enhancing data accuracy and collection speed in multi-site investigations. As bioaccess offers comprehensive clinical study management services—including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting—these capabilities significantly boost the overall efficiency of multi-site trials in Argentina.
The adoption of EDC systems is on the rise, as evidenced by their increasing utilization among researchers at institutions such as the University of Missouri, where this transition has led to improved research efficiency. This trend highlights the importance of these systems in addressing the challenges faced by Directors of Clinical Research in managing multi-site trials in Argentina.
Test Innovations in Diverse Clinical Environments
Multi-site trials in Argentina enable researchers to assess the performance of medical devices across diverse healthcare environments. This approach provides essential insights into how local healthcare practices, individual demographics, and resource availability influence device efficacy. For instance, the necessity for adequate participant numbers in specific subgroups is crucial for significant analysis, as highlighted by recent studies.
By conducting multi-site trials in Argentina, Medtech companies can evaluate innovations in varied settings, allowing them to identify potential challenges and opportunities while deepening their understanding of market dynamics. Furthermore, the performance of medical devices can vary significantly based on local practices, underscoring the importance of evaluating these devices in real-world conditions. Such assessments not only guide product development but also align with the growing emphasis on enhancing diversity in clinical studies, which is vital for ensuring that medical devices meet the needs of all patient groups.
Significantly, 67% of healthcare organizations acknowledge interoperability as their most substantial obstacle to effective device data use, which can impact the results of these studies. As Diana Zuckerman from the National Center for Health Research noted, 'The main limitation to this study is the small number of devices that was examined.' This underscores the challenges encountered in evaluating medical devices across diverse settings.
Moreover, the FDA's strategy for enhancing diversity in studies highlights the broader context of ensuring that medical research reflects the varied populations that will ultimately utilize these devices. In partnership with Caribbean Health Group, bioaccess™ is positioning Barranquilla to emerge as a prominent hub for medical studies in Latin America, supported by the Colombian Minister of Health. This initiative not only enhances the capacity to conduct various experiments but also contributes to local economic development through job creation and improved healthcare outcomes.
Navigate Regulatory Advantages in Argentina
Argentina provides a highly advantageous regulatory environment for multi-site trials, characterized by efficient procedures that significantly expedite approval durations. The National Administration of Drugs, Food and Medical Technology (ANMAT) has introduced regulations aimed at enhancing the efficiency of study evaluations. These measures not only facilitate quicker approvals but also empower Medtech companies to accelerate their innovations to market, thereby strengthening their competitive position.
For instance, the implementation of Provision 4009/2017 has established clear requirements for approving Principal Investigators in Bioequivalence and Bioavailability Studies, alongside special conditions for Healthcare Centers conducting Phase I Clinical Studies. This regulatory change is anticipated to fortify the framework for medical studies in Argentina, facilitating multi-site trials and establishing the nation as a significant participant in Latin America, with an average of 200 new medical studies launched each year.
Furthermore, the regulatory benefits in Argentina are underscored by the country's involvement in multi-site trials, as it ranks as the second highest recipient of foreign-sponsored medical research in Latin America per million population, following Chile. This trend emphasizes the success of Anna's efforts in attracting international research.
In summary, the simplified approval procedures and regulatory assistance offered by ANMAT not only enhance the efficacy of research studies but also create a supportive atmosphere for Medtech firms to thrive, particularly in the context of multi-site trials in Argentina. To capitalize on these benefits, research directors should actively engage with ANMAT and remain informed about regulatory changes to refine their study strategies. Moreover, collaborating with bioaccess® can significantly improve the study management process, as their comprehensive services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—ensure a successful research journey.
Reach out to bioaccess® today to discover how we can assist with your clinical study requirements in this dynamic regulatory environment.

Foster Collaboration Among Research Teams
Multi-site studies inherently promote collaboration among research groups, necessitating coordination across diverse locations and stakeholders. This cooperative environment not only facilitates the sharing of knowledge and resources but also encourages the exchange of best practices, ultimately leading to improved results. Effective communication is paramount; research teams that establish robust communication channels and clearly defined roles can significantly enhance their operational efficiency.
Statistics reveal that:
- 75% of workers prefer authentic communication with colleagues.
- 80% experience stress due to inadequate communication from employers.
- 90% of remote workers rely on video conferencing.
This underscores the critical role of communication tools in fostering collaboration among research teams, especially in a multi-site context. This highlights the necessity for organizations to prioritize effective communication strategies to enhance team cohesion and mitigate stress.
By applying decision-making models, as emphasized by specialists such as Natalia Rossingol, teams can address challenges more efficiently, ensuring that the future of their endeavors is not left to chance. Successful project examples demonstrate that when research groups focus on cooperation, they achieve improved outcomes, showcasing the tangible benefits of teamwork in multi-site trials in Argentina. Moreover, bioaccess provides extensive management services for research projects, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project initiation
- Import permits
- Project oversight
- Reporting
All of which are essential for the success of these initiatives.
As Michael Jordan once said, 'I always believed that if you put in the work, the results will come,' reinforcing the value of effort and teamwork in achieving successful trial outcomes. Additionally, the impact of Medtech research studies on local economies—such as job creation, economic growth, and healthcare enhancement—underscores the broader significance of these collaborative initiatives. Customers expect fast, personalized, and seamless service, further emphasizing the need for effective collaboration in clinical research.
Increase Visibility and Credibility of Innovations
The visibility and credibility of Medtech innovations are significantly enhanced through multi-site trials in Argentina. By showcasing research across multiple reputable sites, such as the collaboration between bioaccess™ and Caribbean Health Group in Barranquilla, companies can foster trust among stakeholders, including investors, healthcare providers, and patients. This initiative, backed by Colombia's Minister of Health, seeks to establish Barranquilla as a premier location for medical studies in Latin America, which is essential in a context where 70% of the population resides two hours or more from an academic healthcare facility.
Moreover, creating effective practices for all phases of international site clinical studies (ICTs) is essential for success, as recent research stresses the significance of verification, validation, and uncertainty quantification (VVUQ) in guaranteeing the dependability of study results. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, underscoring the effectiveness of their services. By integrating these practices, Medtech companies can effectively build credibility and visibility, similar to the results achieved through multi-site trials in Argentina, ultimately driving the advancement of medical devices in the market.
To execute these strategies, companies should prioritize forming alliances with trustworthy sites and concentrate on thorough VVUQ processes to improve the reliability of their studies.
Improve Patient Engagement and Retention
Multi-site studies significantly enhance participant involvement by providing accessible venues and flexible scheduling options. Engaging patients in the study design process not only addresses their concerns but also fosters a sense of ownership and commitment, which is crucial for retention. Research indicates that when participants perceive their input as valued, they are more likely to remain engaged throughout the study.
Leveraging technology, such as mobile apps and telehealth platforms, further enhances communication and support for participants. These tools facilitate real-time updates and interactions, making it easier for participants to stay informed and connected. For instance, incorporating monitoring tools such as glucose meters and blood pressure monitors into the study can enhance participant involvement by enabling ongoing engagement and data collection, thereby strengthening their connection to the research.
In addition to these approaches, comprehensive clinical study management services are vital for ensuring the success of multi-site studies. Services including feasibility assessments, site selection, compliance evaluations, test setup, and project management are essential for establishing an efficient and effective testing environment. By ensuring that all regulatory requirements are met and that the research is organized for success from the outset, these services contribute to improved participant experiences and outcomes.
A case analysis on participant retention strategies underscores the importance of building rapport between coordinators and participants. It highlights that effective retention techniques—such as social interaction, appointment reminders, and creating a supportive environment—can significantly boost retention rates in long-term research. Expert opinions, including insights from Dr. John P. Docherty, emphasize that utilizing digital data to monitor participant behavior enables researchers to proactively adjust engagement strategies, ensuring motivation remains high.
Overall, these strategies are essential for enhancing patient retention in multi-site trials in Argentina, ultimately leading to more reliable data and successful study outcomes. Furthermore, improving participant diversity and leveraging digital platforms are crucial elements for enhancing recruitment strategies, further strengthening the overall success of clinical studies.
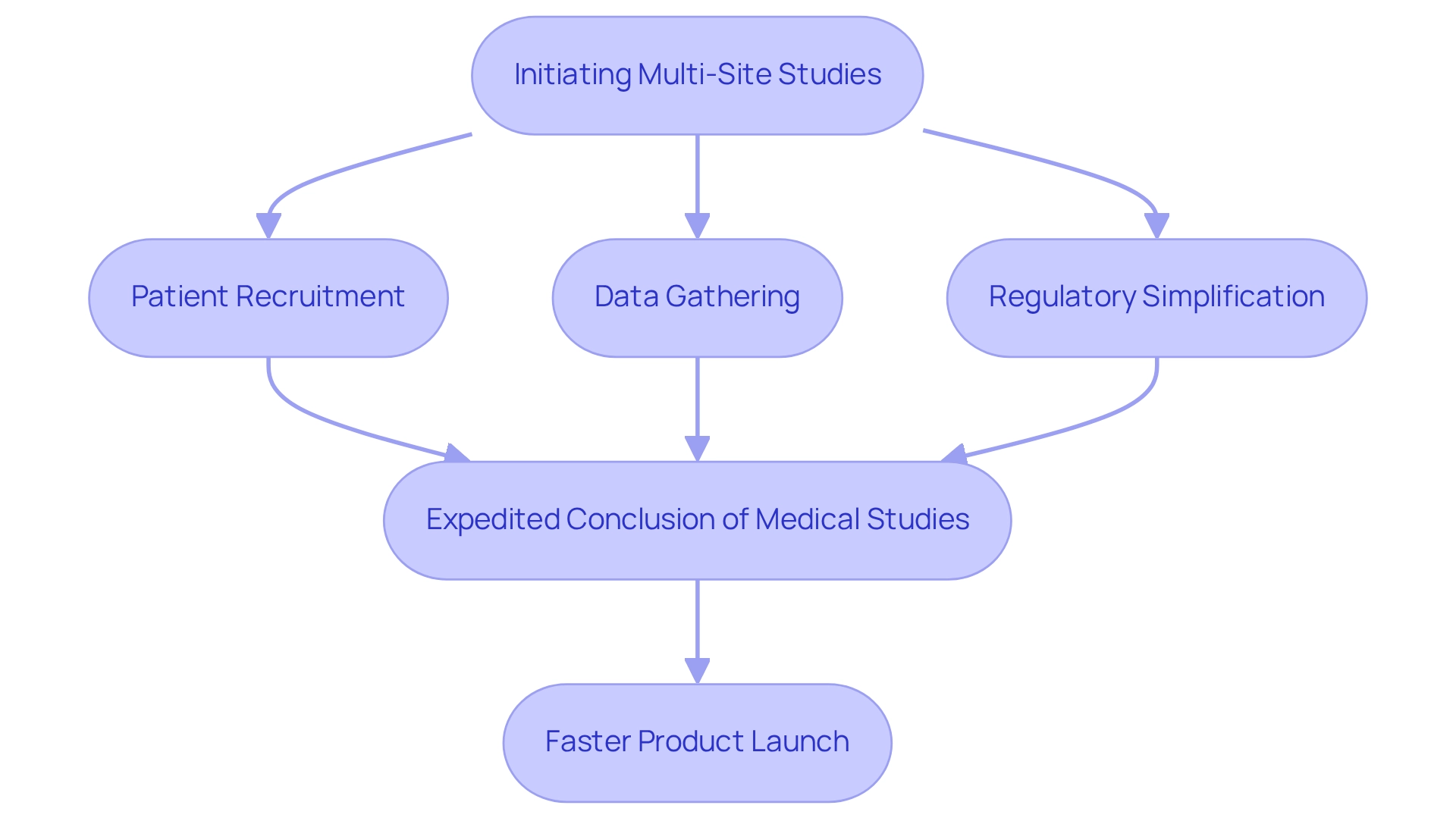
Accelerate Time-to-Market for Medtech Innovations
Leveraging the advantages of multi-site studies allows Medtech firms to significantly accelerate their time-to-market for new innovations. By enabling quicker patient recruitment across various locations, these studies enhance data gathering effectiveness and simplify regulatory procedures. This method not only expedites the conclusion of medical studies but also empowers companies to introduce their products more rapidly, thereby enhancing their competitive advantage in the marketplace.
As bioaccess® emphasizes, their expertise in overseeing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) ensures that clients can navigate the complexities of clinical research efficiently. Consequently, patients gain timely access to groundbreaking and potentially life-saving technologies. In fact, statistics indicate that multi-site studies can reduce product launch timelines by up to 30%, underscoring their critical role in the Medtech landscape.
With the cardiovascular devices industry projected to reach $80 billion by 2030 and the growing demand for surgical tools driven by advancements in minimally invasive procedures and an aging population, the urgency for rapid progress in medical studies has never been more pronounced. Organizations that embrace this approach, particularly with the support of bioaccess's comprehensive management services for research, are better equipped to tackle market complexities and meet the rising demand for innovative healthcare solutions. Furthermore, the increasing adoption of telehealth technology in hospitals reflects a broader trend toward technological integration in healthcare, further reinforcing the case for multi-site trials as a modern approach to clinical research.
Conclusion
The exploration of multi-site clinical trials in Argentina unveils a multitude of benefits poised to significantly enhance the Medtech landscape. By leveraging Argentina's diverse patient demographics, researchers can amass comprehensive data that reflects varied responses to medical devices, ultimately bolstering safety and efficacy. This diversity not only enriches clinical findings but also addresses health disparities, ensuring equitable benefit sharing with local communities.
Furthermore, the advantages of accelerated patient recruitment and streamlined processes are paramount. With a substantial population and extensive healthcare networks, Argentina presents an ideal environment for efficient trial management, thereby reducing timelines for data collection and analysis. Coupled with diminished operational costs through shared resources and a favorable regulatory landscape, Argentina stands out as a premier location for conducting high-quality clinical trials.
The collaborative essence of multi-site trials fosters teamwork among research teams, propelling innovation and enhancing trial outcomes. By harnessing technology and effective communication, organizations can elevate patient engagement and retention, ensuring that trials yield reliable and actionable results. Ultimately, the capacity to navigate these dynamics positions Medtech companies to expedite their time-to-market for groundbreaking innovations, addressing the urgent demand for new healthcare solutions.
In conclusion, embracing multi-site clinical trials in Argentina not only fortifies research capabilities but also contributes to a more responsive and effective healthcare system. As the Medtech sector continues to evolve, comprehending and implementing these strategies will be essential for organizations striving to remain at the forefront of medical innovation.
Frequently Asked Questions
What are the benefits of conducting multi-site trials in Argentina?
Multi-site trials in Argentina provide access to a diverse array of demographics, enriching data collected and enhancing the reliability of outcomes. This diversity accounts for various genetic, environmental, and lifestyle factors influencing health, leading to improved safety and efficacy of medical devices across different demographic groups.
How does participant diversity influence research outcomes?
Statistics indicate that diverse groups significantly influence research outcomes. Studies show that investigations incorporating varied demographics yield more generalizable results, leading to improved patient involvement and retention rates.
What initiatives are being taken to enhance research activities in Latin America?
The collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a key hub for research activities in Latin America. This initiative is supported by Colombia's Minister of Health and seeks to bolster the region's research capacity while stimulating economic growth and job creation through international collaboration in Medtech.
What challenges do South American nations face in local cancer research?
A case study titled 'Challenges in Local Cancer Research Capacity' highlights the need for increased funding in research infrastructure and training to enhance the region's capability to conduct high-quality medical studies.
How does promoting diversity in medical research address health inequalities?
By promoting diversity in medical research, researchers can address health inequalities and ensure equitable benefit distribution among the communities involved, aligning with ethical research practices and improving the overall quality and relevance of healthcare findings.
What types of clinical studies does bioaccess™ specialize in?
Bioaccess™ specializes in various clinical studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
How does Argentina facilitate participant recruitment for multi-site trials?
Argentina's extensive resources and networks across various locations enable effective collaboration with local healthcare providers and community organizations, leading to swift identification and enrollment of eligible patients, which shortens recruitment durations.
What advantages does Argentina offer for conducting medical studies?
Argentina offers substantial opportunities for reducing operational expenses through effective resource distribution, competitive pricing for medical services, and a quick approval process from independent ethics committees, which can be completed in as few as 15 working days.
What services does bioaccess® provide for research management?
Bioaccess® provides comprehensive research management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
Why is Argentina considered an ideal choice for Medtech companies?
Argentina is seen as an ideal choice for Medtech companies due to its growing appeal for conducting studies without sacrificing quality, as it is the second largest recipient of foreign-sponsored research in Latin America per million inhabitants, and it offers significant operational cost reductions and improved research efficiency.




