Overview
Best practices for clinical trial logistics in Latin America are crucial for ensuring compliance and efficiency, particularly in the face of regulatory hurdles and resource fragmentation. This article underscores the significance of strategic partnerships, local expertise, and innovative solutions. These elements are essential for streamlining processes and enhancing patient engagement, ultimately facilitating successful research outcomes in the region. By addressing these challenges, stakeholders can significantly improve the landscape of clinical research, paving the way for more effective trials and better patient experiences.
Introduction
In the rapidly evolving landscape of clinical research, Latin America emerges as a pivotal player, particularly for Medtech companies seeking to conduct successful trials. This region, characterized by a diverse patient population and a cost-effective environment, offers unique advantages. However, it also presents a myriad of challenges. Stakeholders must navigate complex regulatory frameworks and overcome logistical hurdles, necessitating strategic approaches to ensure efficiency and compliance.
As partnerships flourish and innovative solutions arise, the importance of robust clinical trial logistics becomes increasingly clear, paving the way for enhanced research capabilities and improved patient outcomes. Understanding these dynamics is essential for any organization aiming to thrive in this promising yet intricate market.
The Significance of Clinical Trial Logistics in Latin America
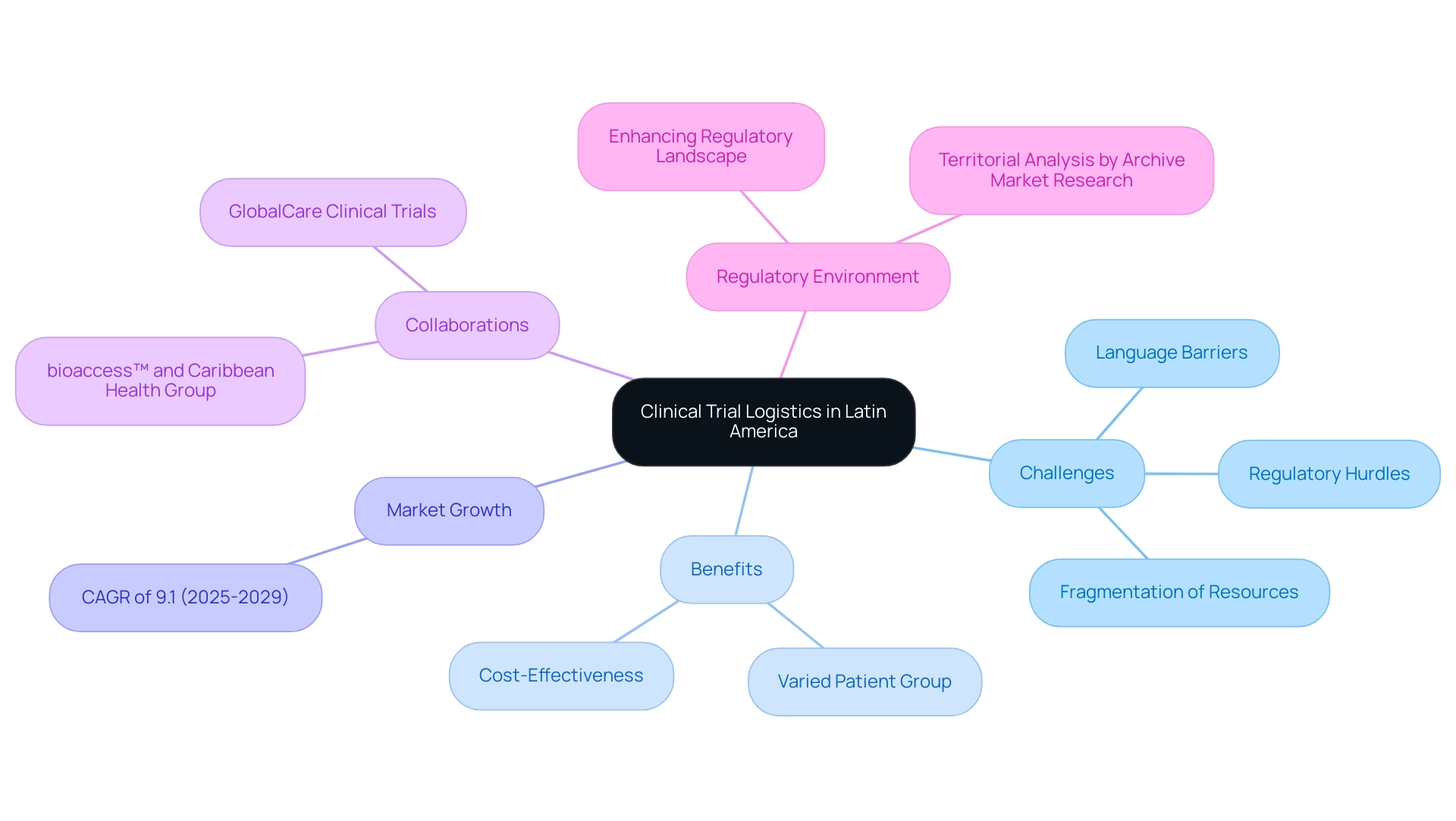
For the successful implementation of medical studies, particularly for Medtech firms, understanding clinical trial logistics in Latin America is essential as they navigate this evolving environment. These companies face multifaceted challenges, including regulatory hurdles, language barriers, and fragmentation of resources, complicating their operations in the region. The varied patient group enhances the general applicability of study outcomes, while its cost-effectiveness offers a notable benefit for research studies.
As the supply and distribution market is anticipated to expand at a compound annual growth rate (CAGR) of 9.1% from 2025 to 2029, the significance of effective distribution becomes even more evident. Prompt delivery of medical supplies, including investigational medicinal products (IMPs), is crucial for preserving the integrity of studies. Delays in clinical trial logistics in Latin America can jeopardize timelines and outcomes, making it imperative for stakeholders to grasp the intricacies of clinical trial logistics in the region. The collaboration between bioaccess™ and Caribbean Health Group exemplifies a strategic partnership aimed at positioning Barranquilla as a leading destination for medical research in South America, supported by Colombia's Minister of Health.
Such initiatives create opportunities for improved communication and resource sharing between South American hospitals and US research clients, bridging gaps in medical research and innovation. Moreover, the enhancing regulatory landscape in South America encourages this expansion, promoting a more efficient procedure for executing research studies. The geographical proximity to the United States facilitates collaboration and resource sharing, further enhancing overall efficiency. The recent collaboration with GlobalCare Clinical Trials demonstrates how bioaccess™ is actively striving to improve trial ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and impressive 95% retention rates.
Furthermore, Archive Market Research's territorial analysis, which considers political, economic, social, and legislative effects on markets, highlights the intricacy of the supply chain environment in Latin America. Understanding these factors is essential for Medtech companies to navigate potential challenges while leveraging opportunities in the region. Specialist perspectives underscore the importance of supply chain management in medical research. As noted by a US TPS Business Development Manager, 'The response was good, and I got what I was looking for as far as the report. Thank you for that.' This statement illustrates the essential function that dependable supply chain management serves in gaining practical insights and sustaining operational effectiveness in research studies.
In summary, understanding clinical trial logistics in Latin America is crucial for stakeholders aiming to conduct research successfully in this area, ensuring adherence and efficiency while capitalizing on the distinct benefits it provides. A solution-driven approach is vital to bridge the gaps between innovation and execution, addressing the specific challenges faced by Medtech companies in the region.
Navigating the Challenges of Clinical Trial Logistics in Latin America
Conducting research studies in Latin America necessitates navigating the intricate logistics of clinical trials, characterized by unique obstacles such as diverse regulatory frameworks, cultural subtleties, and logistical challenges. The complexity of regulatory compliance is heightened by the differing legal frameworks across various nations, often leading to confusion and delays in initiating the process. As research expert Jennifer Mendoza points out, "Due to varying update cycles, statistics can display more up-to-date data than referenced in the text," underscoring the critical need for continuous monitoring of regulatory changes.
Moreover, socio-economic factors significantly influence the logistics of medical studies. Infrastructure limitations and transportation issues can severely hinder the timely delivery of medical supplies. For instance, certain regions may lack reliable transport networks, complicating access to remote patient populations.
This is particularly relevant given that 78% of biotech firms operate without income, relying solely on venture capital, which highlights the urgency for effective management of experiments.
In this context, bioaccess® emerges as a dependable Contract Research Organization (CRO) dedicated to accelerating medical device evaluations in Latin America. Through its partnership with the Caribbean Health Group, bioaccess is actively positioning Barranquilla as a premier destination for medical studies in the region, garnering the support of Colombia's Minister of Health. This initiative not only enhances the appeal of clinical research in Colombia but also leverages local expertise to streamline processes.
Furthermore, bioaccess is committed to addressing client concerns via its Grievance Officer, ensuring that any queries or issues regarding data protection are managed in accordance with applicable law. This commitment is reflected in their Privacy Policy, emphasizing the importance of safeguarding client information.
GlobalCare Clinical Trials has partnered with bioaccess™ to enhance ambulatory services, achieving over a 50% reduction in recruitment time and impressive 95% retention rates. This collaboration exemplifies how strategic partnerships can alleviate logistical challenges, ensuring that evaluations are conducted smoothly and efficiently. Specific metrics from these initiatives illustrate the effectiveness of bioaccess's approach in enhancing outcomes.
The expanding pediatric and elderly populations in Latin America present both opportunities and challenges for medical research. As the region positions itself as a top location for medical studies, the evolving regulatory frameworks and diverse patient demographics enhance research capacities. However, overcoming logistical barriers remains essential to ensure that clinical trial logistics in Latin America are conducted smoothly and efficiently.
Firms that embrace innovative solutions, such as leveraging local partnerships and investing in sustainable supply chain practices, can gain a competitive advantage in this dynamic environment. By integrating sustainability into their logistics strategies, companies can not only address logistical challenges but also tap into the potential new drug market represented by the growing patient populations.
Ensuring Regulatory Compliance in Clinical Trials
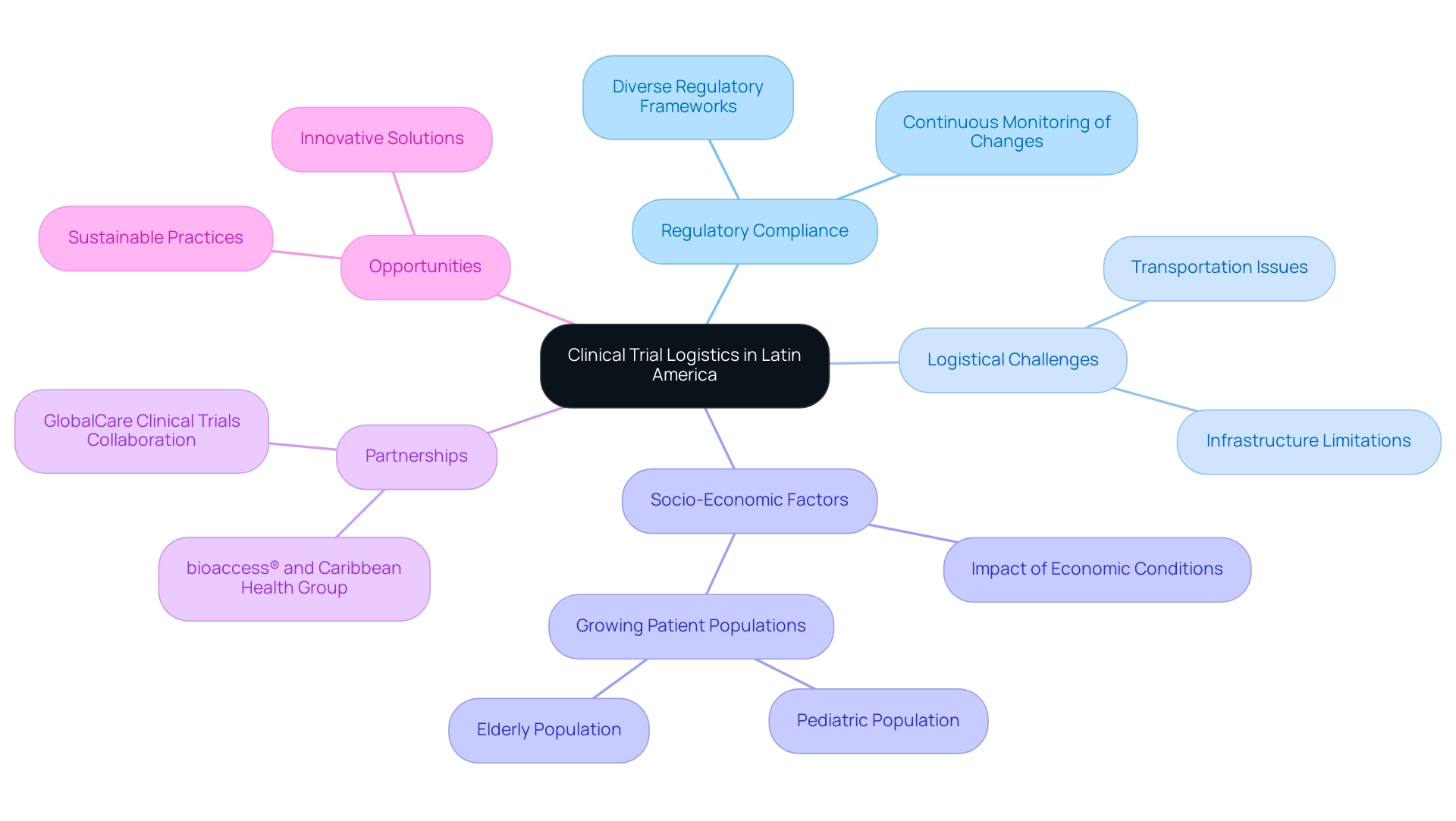
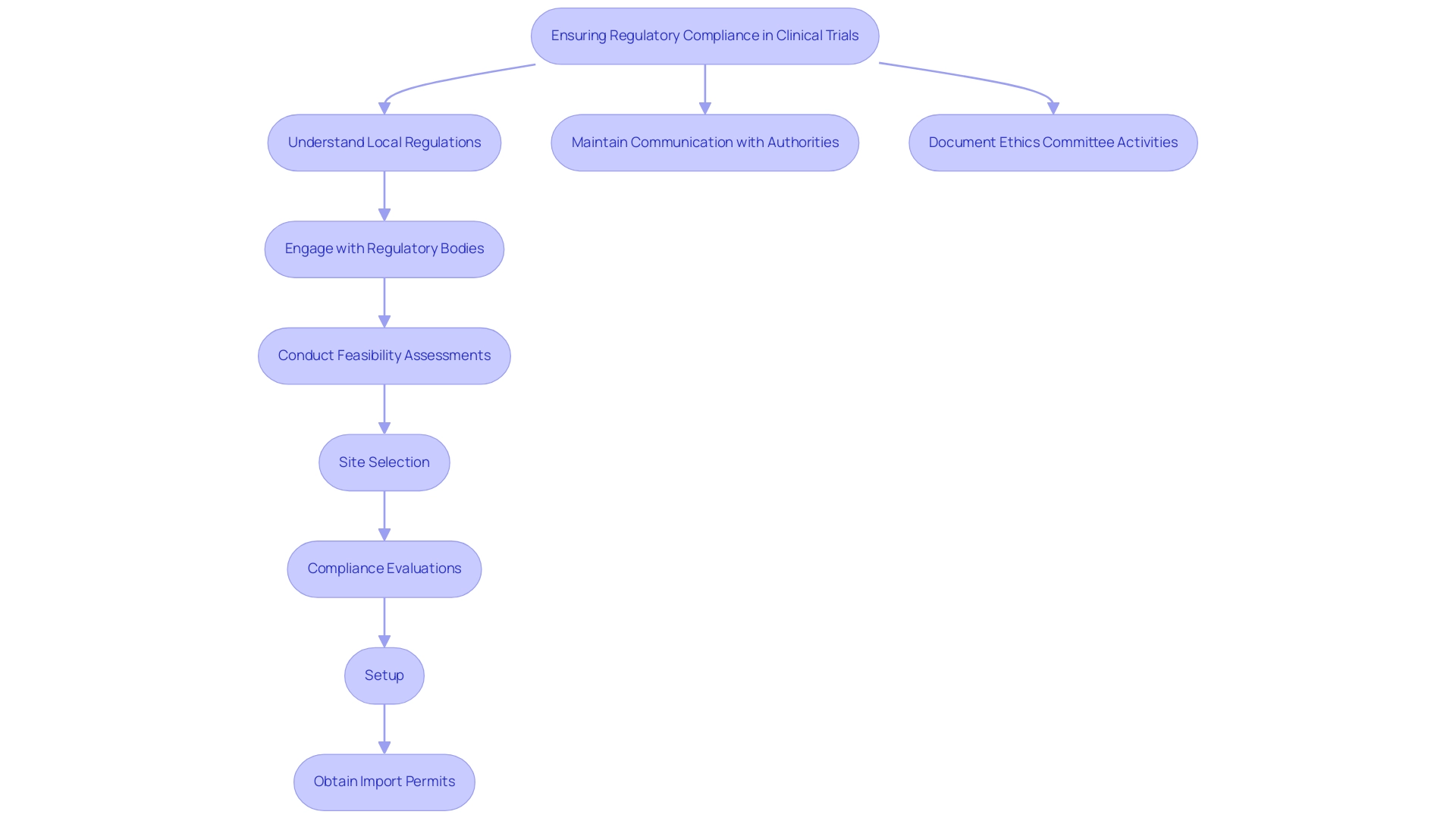
Ensuring regulatory adherence is vital for the success of clinical trial logistics in Latin America. Stakeholders must thoroughly understand local regulations established by national health authorities, such as INVIMA in Colombia. These regulations are crucial for clinical trial logistics in Latin America, where INVIMA serves as a Level 4 health authority recognized by PAHO/WHO. Engaging with regulatory bodies early in the planning phase can clarify requirements and expedite the approval process for clinical trial logistics in the region.
For instance, Brazil has recently enacted legislative modifications designed to simplify the approval process for research studies. This highlights the importance of understanding clinical trial logistics in Latin America for sponsors.
As a prominent Contract Research Organization, bioaccess® provides extensive management services for research studies. These services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Nationalization of investigational devices
- Project oversight
- Reporting on study status, inventory, and serious and non-serious adverse events
Research indicates that maintaining open lines of communication with regulatory authorities and conducting comprehensive due diligence can significantly mitigate the risk of non-compliance and the penalties that may arise. Notably, sensitive personal data processing may occur without consent in cases of compliance with legal obligations or protection of life, underscoring the importance of understanding these legal frameworks.
Moreover, both Brazilian and U.S. regulations mandate that ethics committees maintain adequate documentation of their activities, including research proposals and meeting minutes, for a minimum of three years post-study completion. This requirement encourages transparency and accountability in the ethics review process, aiding in regulatory compliance and oversight, ultimately improving the integrity of research studies. Insights from Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics, emphasize the critical nature of adherence to regulatory standards to ensure public health safety and compliance.
Strategies for Enhancing Efficiency in Clinical Trial Logistics
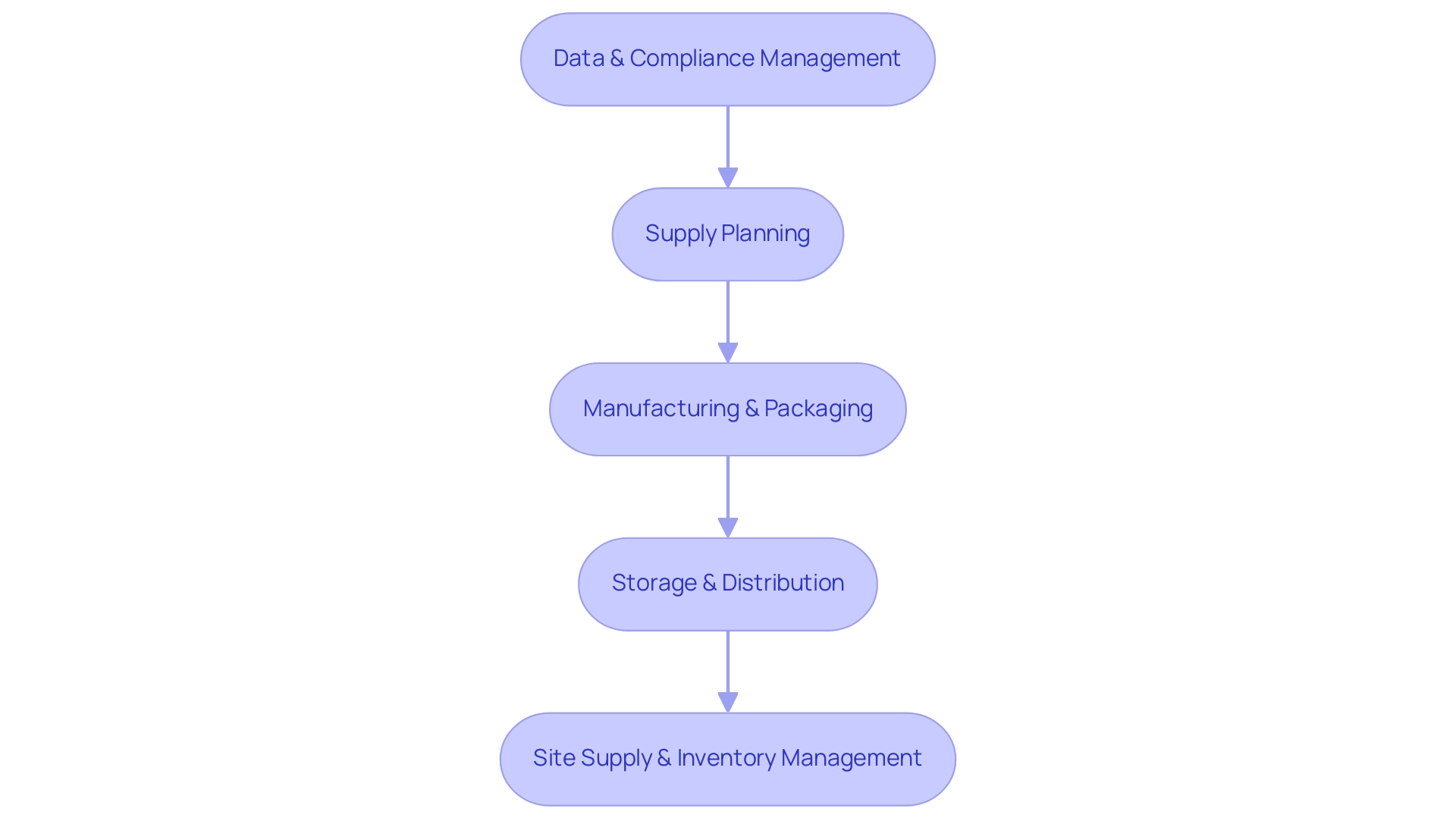
To enhance efficiency in research coordination, stakeholders must adopt various best practices, particularly when collaborating with specialized partners like bioaccess®. Establishing robust partnerships with local logistics providers is crucial for optimizing clinical trial logistics in Latin America. These collaborations streamline the supply chain and ensure the timely delivery of medical supplies. The research supply chain comprises five phases:
- Data & Compliance Management
- Supply Planning
- Manufacturing & Packaging
- Storage & Distribution
- Site Supply & Inventory Management
Comprehending these stages is vital for sustaining momentum and ensuring compliance.
Bioaccess® offers extensive study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
These services significantly enhance operational efficiency. The integration of technology, such as real-time tracking systems and advanced inventory management software, substantially improves visibility and mitigates the risk of stockouts. Moreover, creating a centralized supply chain plan that accommodates regional variations is essential for effective clinical trial logistics in Latin America and resource management.
This strategic approach to clinical trial logistics allows for tailored strategies that consider local conditions and regulatory requirements, ultimately leading to smoother operations. Consistently evaluating and modifying supply chain strategies in response to test advancements and emerging challenges will further enhance operational efficiency. This ensures that studies remain on schedule and comply with industry standards. As Bree Burks, Vice President of Site Strategy at Veeva, aptly noted, "Sponsors will step up to solve site capacity issues," highlighting the significance of clinical trial logistics in Latin America and the necessity for proactive logistics management in the evolving landscape of research studies. Furthermore, the development of electronic Patient Reported Outcomes (ePRO) systems exemplifies how technology adapts to meet the evolving expectations of participants, improving engagement and retention throughout the process.
This focus is particularly relevant for bioaccess®, which specializes in managing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH). It connects innovative medtech companies with opportunities to engage in clinical trial logistics in Latin America. This emphasis not only drives job creation and economic growth but also contributes to healthcare improvement through international collaboration.
The Role of Partnerships in Streamlining Clinical Trial Logistics
Collaborations are crucial for enhancing clinical trial logistics in Latin America, particularly in Colombia, which presents notable competitive benefits for first-in-human studies. Partnering with local organizations, logistics providers, and regulatory experts not only streamlines processes but also enhances execution in clinical trial logistics in Latin America with invaluable insights and resources. For instance, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research, backed by Colombia's Minister of Health.
This initiative underscores the significance of local partnerships in improving patient recruitment and retention—essential elements for the success of clinical trial logistics in Latin America.
Colombia offers cost savings exceeding 30 percent compared to trials in North America or Western Europe, highlighting the advantages of clinical trial logistics in Latin America with a regulatory review timeline of just 90-120 days for IRB/EC and MoH (INVIMA) approvals. Furthermore, the country boasts a high-quality healthcare system, ranked #22 by the World Health Organization and recognized by various publications for its excellence in Latin America.
Moreover, collaborations with transportation companies are essential for guaranteeing effective clinical trial logistics in Latin America. With the anticipated expansion of the in vitro diagnostics (IVD) market from USD 106.9 billion in 2023 to USD 202.9 billion by 2032, the necessity for flexible and responsive supply chain management is more evident than ever. Innovations in pharmaceutical logistics, such as smarter packaging and agile supply routes, are set to play a pivotal role in 2025, addressing the complexities of global healthcare demands.
Involving stakeholders early in the process fosters a collaborative environment that proactively addresses potential logistical challenges in clinical trial logistics in Latin America. As Kevin Coker, Innovation Strategist and Head of MedTech Development at MD Anderson Cancer Center, aptly noted, "Conducting studies across various countries sounds great but navigating different regulations, cultures, and standards is no small feat." This highlights the importance of local collaborations to effectively manage the intricacies involved in clinical trial logistics in Latin America.
Furthermore, utilizing AI in medical studies can lead to reduced approval expenses, quicker market entry, and enhanced patient outcomes. Effective collaborations in clinical research operations have already demonstrated their value, with pharmaceutical firms establishing strategic alliances to leverage AI in drug development, such as those between Amgen and Amazon Web Services, and Roche and Unlearn.AI. By prioritizing partnerships, stakeholders can significantly enhance overall outcomes and optimize clinical trial logistics in Latin America.
Moreover, investments in science, technology, and innovation projects in Colombia are supported by R&D tax incentives, including a 100% tax deduction and various financial advantages, further increasing the attractiveness of conducting experiments in the region.
Future Trends in Clinical Trial Logistics in Latin America
The research landscape in Latin America is undergoing substantial transformation, driven by various emerging trends. A particularly significant development is the rise of decentralized research studies (DCTs), which leverage technology to bring studies closer to patients. This innovative approach not only enhances patient engagement but also alleviates the logistical burdens typically associated with traditional site visits. By minimizing the need for patients to travel, DCTs can lead to higher retention rates and improved data quality.
Moreover, advancements in data analytics and artificial intelligence are poised to revolutionize supply chain management within research studies. These technologies facilitate more accurate forecasting and resource allocation, ensuring that the right materials are available at the right time. As a leader in Medtech research, bioaccess provides comprehensive services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
Each of these services is crucial in adapting to the advancements of DCTs, ensuring that studies remain compliant and efficient.
Looking ahead to 2025, the emphasis on decentralized research studies is likely to intensify, with strategic planning evolving to accommodate this shift. Key areas in clinical trial logistics within Latin America, particularly in Brazil, are essential for understanding the dynamics of clinical research. Companies are increasingly pursuing partnerships with local delivery service providers to enhance their operational efficiency and ensure compliance with regional regulations.
Feedback from clients, including analysts and executives, underscores the importance of high-quality reports that bolster decision-making processes. One US TPS Business Development Manager remarked, "The response was good, and I got what I was looking for as far as the report. Thank you for that," exemplifying how bioaccess's compliance reviews and monitoring services contribute to client satisfaction and informed decision-making.
As the sector continues to embrace these trends, understanding the impact of decentralized studies on operations will be vital for maintaining a competitive edge in the research field. By proactively adapting to these changes and leveraging bioaccess's extensive service capabilities, including compliance reviews and monitoring, organizations can ensure compliance and efficiency in clinical trial logistics across Latin America. This ultimately contributes to job creation, economic growth, healthcare improvement, and international collaboration.
Conclusion
The landscape of clinical trials in Latin America presents a unique blend of opportunities and challenges. The region's diverse patient population and cost-effective environment render it an attractive destination for Medtech companies. However, navigating complex regulatory frameworks, logistical hurdles, and infrastructure limitations is critical for the successful execution of clinical research.
Strategic partnerships, such as those formed between bioaccess™ and local health organizations, are essential for enhancing operational efficiency and overcoming these challenges. These collaborations not only streamline logistics but also foster better communication and resource sharing, leading to improved patient recruitment and retention rates. Moreover, as the regulatory environment continues to evolve, staying informed and engaged with local authorities is paramount to ensuring compliance and expediting trial processes.
Looking ahead, the emergence of decentralized clinical trials and advancements in technology will reshape the clinical trial landscape in Latin America. By embracing innovative logistics strategies and leveraging partnerships, stakeholders can enhance their research capabilities and ultimately drive better patient outcomes. The commitment to integrating sustainability and technology into logistics practices will position Latin America as a leader in clinical research, fostering economic growth and healthcare improvement in the region.
In conclusion, understanding the intricacies of clinical trial logistics in Latin America is vital for any organization aiming to thrive in this promising market. By adopting a solution-driven approach and prioritizing strategic collaborations, Medtech companies can navigate the complexities of this dynamic landscape, ensuring that their clinical trials are not only efficient but also impactful in advancing healthcare solutions.
Frequently Asked Questions
Why is understanding clinical trial logistics important for Medtech firms in Latin America?
Understanding clinical trial logistics is essential for Medtech firms in Latin America to navigate regulatory hurdles, language barriers, and resource fragmentation, which complicate their operations in the region.
What are the benefits of conducting clinical trials in Latin America?
The varied patient group enhances the general applicability of study outcomes, and the cost-effectiveness of conducting research studies in the region offers notable advantages.
What challenges do Medtech companies face in clinical trial logistics in Latin America?
Medtech companies face challenges such as regulatory compliance complexities, language barriers, infrastructure limitations, and transportation issues that can hinder timely delivery of medical supplies.
How is the supply and distribution market expected to grow in Latin America?
The supply and distribution market in Latin America is anticipated to expand at a compound annual growth rate (CAGR) of 9.1% from 2025 to 2029.
What role does timely delivery of medical supplies play in clinical trials?
Prompt delivery of medical supplies, including investigational medicinal products (IMPs), is crucial for preserving the integrity of studies and ensuring that clinical trial timelines and outcomes are not jeopardized.
What is the significance of the partnership between bioaccess™ and Caribbean Health Group?
This partnership aims to position Barranquilla as a leading destination for medical research in South America and improve communication and resource sharing between South American hospitals and US research clients.
How has bioaccess™ improved trial ambulatory services in Colombia?
Through its collaboration with GlobalCare Clinical Trials, bioaccess™ has achieved over a 50% reduction in recruitment time and impressive 95% retention rates.
What factors influence the logistics of medical studies in Latin America?
Political, economic, social, and legislative factors significantly influence the logistics and supply chain environment for medical studies in the region, creating both challenges and opportunities.
How does bioaccess™ ensure compliance with data protection regulations?
bioaccess™ addresses client concerns through its Grievance Officer, ensuring that any queries regarding data protection are managed according to applicable law, as outlined in their Privacy Policy.
What strategies can Medtech firms adopt to overcome logistical challenges in Latin America?
Medtech firms can embrace innovative solutions, such as leveraging local partnerships and investing in sustainable supply chain practices, to gain a competitive advantage and address logistical challenges effectively.




