Introduction
In the complex world of clinical trials, regulatory affairs play a pivotal role in ensuring the safety and efficacy of research involving human subjects. As the landscape of regulations evolves, understanding the intricacies of compliance becomes essential for successful trial management.
This article delves into the critical components of regulatory affairs, exploring the responsibilities of professionals in this field, the challenges they face, and the future trends that are shaping clinical research.
With insights from industry experts and real-world case studies, it highlights the importance of navigating the regulatory submission process and maintaining adherence to guidelines, ultimately safeguarding participant rights and enhancing the integrity of clinical data.
Understanding Regulatory Affairs in Clinical Trials
Regulatory matters in clinical trial regulatory affairs are essential elements that include the policies, guidelines, and legal requirements overseeing investigations involving human participants. Grasping key regulations, including FDA requirements, ICH guidelines, and Good Clinical Practice (GCP) standards, is essential for effective management of clinical trial regulatory affairs in research studies. Our service capabilities encompass:
- Comprehensive feasibility studies
- Meticulous selection of research sites
- In-depth compliance reviews
- Thorough feedback on study documents to ensure adherence to country requirements
- Setup
- Import permits
- Project management
- Detailed reporting processes
These frameworks are designed to safeguard the safety, rights, and well-being of participants while upholding the integrity of the data collected. Significantly, sponsors must retain a Certificate of Analysis (CoA) to record the identity, purity, and strength of the investigational products (IPs) used in the research trial, which is essential for compliance with regulations. The FDA requires that all ethics committees (ECs) examining clinical investigations must register electronically with the HHS Office for Human Research Protections (OHRP), a process that underscores the significance of compliance with regulations.
Registration is effective for three years upon approval and is part of the broader effort to maintain oversight in human subjects research. Recent updates indicate that all ethics committees must comply with this electronic registration requirement, highlighting the current oversight landscape. According to the OHRP, 'The OHRP helps ensure this by providing clarification and guidance, developing educational programs and materials, maintaining oversight, and providing advice on ethical and compliance issues in biomedical and social-behavioral research.'
Furthermore, with specialists like Ana Criado, Director of Compliance at Mahu Pharma, and Katherine Ruiz, who focuses on compliance for medical devices and in vitro diagnostics in Colombia, our team is prepared to handle the complexities of these rules. A relevant case analysis titled 'Military Operations and Consent Waivers' illustrates how the requirement for informed consent can be waived under certain conditions, allowing for the administration of investigational products to military personnel in urgent situations while maintaining oversight. This case analysis illustrates our capability to adjust services to address particular compliance challenges.
Failure to adhere to these guidelines in clinical trial regulatory affairs can result in serious consequences, such as delays in research, monetary fines, and jeopardized patient safety, underscoring the importance of a thorough comprehension of compliance matters for successful management of medical investigations.
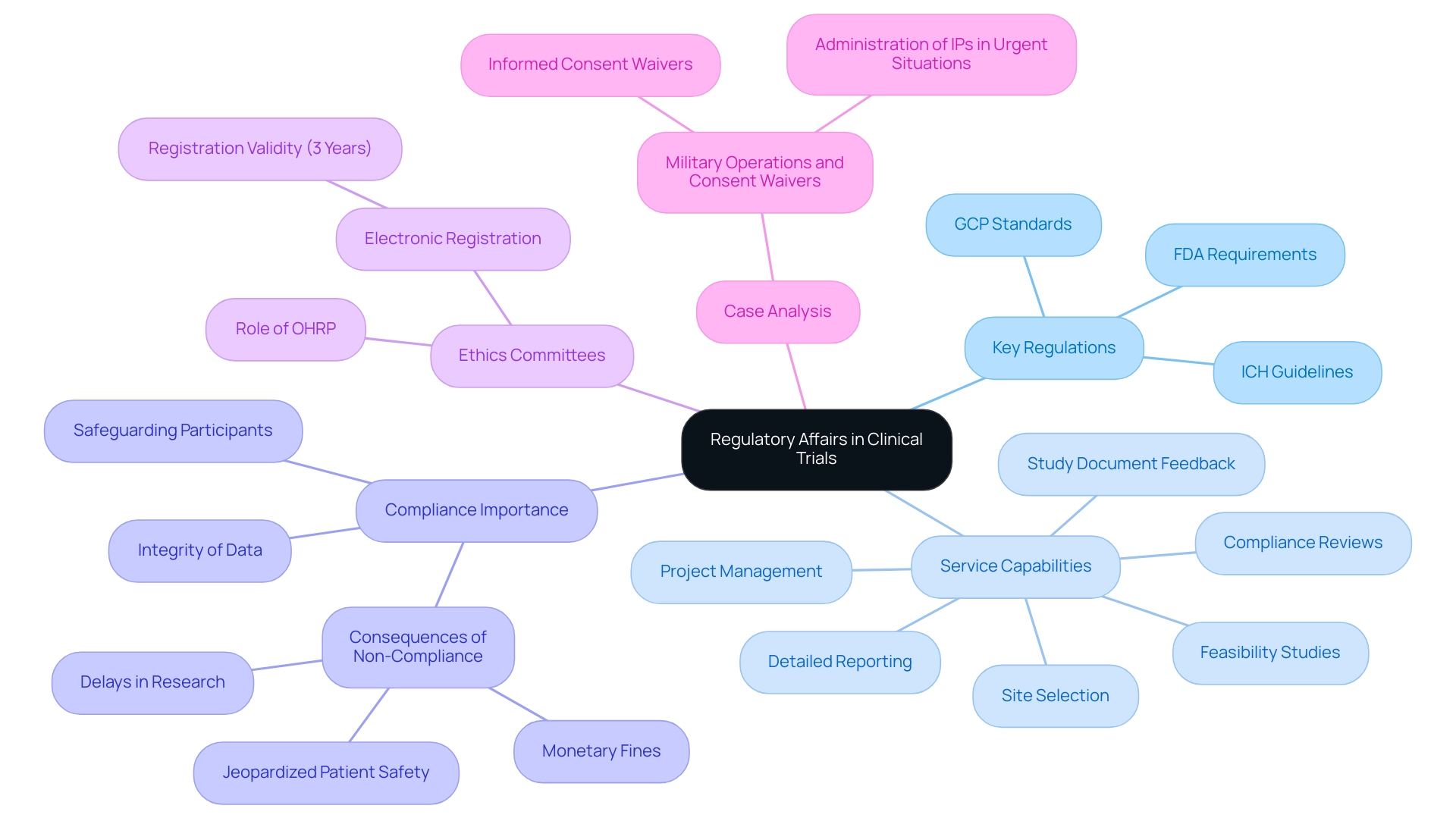
Roles and Responsibilities of Regulatory Affairs Professionals
Experts in clinical trial regulatory affairs are essential for ensuring that research studies comply with a complex network of regulations and guidelines. Their main duties involve the preparation and submission of important compliance documents essential for clinical trial regulatory affairs, which are vital for starting and sustaining research trials. Our comprehensive clinical trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Startup and approval processes (ethics committee and health ministry)
- Import permits
- Project management
- Reporting (study status, inventory, serious and non-serious adverse events)
According to recent statistics, submission success rates have improved by 15% over the past five years, highlighting the effectiveness of professionals in clinical trial regulatory affairs in navigating the approval processes. These professionals maintain ongoing communication with oversight agencies, such as the FDA, regarding clinical trial regulatory affairs, which supervises the regulation of pharmaceuticals, biologics, and medical equipment, ensuring compliance with established protocols. Furthermore, they supervise compliance audits to confirm that all activities align with these regulations.
Furthermore, compliance affairs specialists play a crucial role in clinical trial regulatory affairs by implementing informed consent procedures, ensuring that participants fully comprehend their rights and the essence of the studies they are engaged in. As Warman O’Brien notes, 'As data privacy standards tighten, the pressure to manage data securely and accurately only increases,' underscoring the heightened emphasis on data management. Additionally, they are tasked with training research staff on compliance protocols related to clinical trial regulatory affairs, reflecting the necessity for continuous education in an evolving legal landscape.
The case study titled 'The Future of Medical Data Management' illustrates the increasing significance of compliance in clinical trial regulatory affairs, emphasizing the need for skilled professionals to ensure adherence to regulations. With experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, we ensure that our clients benefit from a wealth of knowledge and experience. By skillfully handling these duties in clinical trial regulatory affairs, compliance professionals promote the smooth advancement of research studies while protecting the rights and safety of participants, thus strengthening the integrity of the research process.
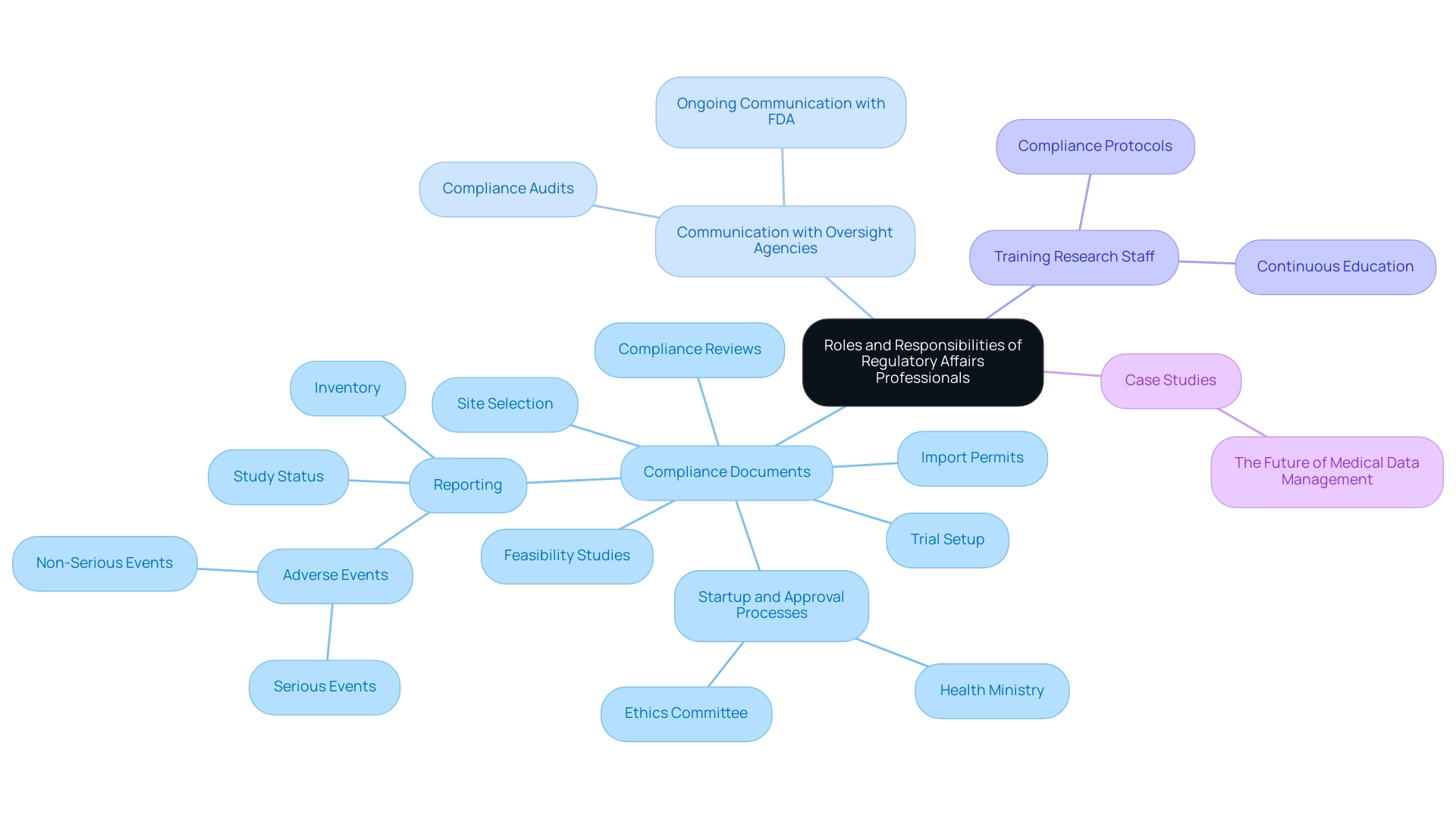
Navigating the Regulatory Submission Process
Navigating the clinical trial regulatory affairs submission process for clinical studies is a multifaceted endeavor that demands meticulous attention to detail. Initially, researchers must formulate a comprehensive protocol that clearly delineates the study's design, objectives, and methodologies. This foundational document serves as a roadmap for the entire process.
In addition to the protocol, essential documents must be compiled for clinical trial regulatory affairs, including:
- Investigator brochures
- Informed consent forms
- Safety monitoring plans
All of which are critical for ensuring participant safety and regulatory compliance. At this stage, leveraging comprehensive clinical study management services can significantly enhance the process. Our service capabilities encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup—including obtaining ethics committee and health ministry approvals
- Project management
- Detailed reporting on study status, inventory, and adverse events
These services are directed by professionals such as Katherine Ruiz, who focuses on compliance matters for medical devices and in vitro diagnostics in Colombia. Once all materials are meticulously prepared, they must be submitted to the relevant regulatory authority for thorough review. Understanding submission timelines is vital in clinical trial regulatory affairs, as research indicates that the average duration from the first patient to the last patient randomized in clinical trials spans approximately 17.0 months (ranging from 6.5 to 21.7 months).
Familiarity with these timelines can help mitigate delays that may impede study initiation. After the initial submission, researchers should be poised to address any inquiries or requests for additional information from oversight agencies, which can necessitate modifications to the protocol. The intricacies of preparing these official documents are highlighted by recent discoveries from the Tufts Center for the Study of Drug Development, which showed that FDA-approved medications and biologics spent an average of 89.8 months in clinical studies between 2014 and 2018—an increase from 83.1 months in the previous five years.
This highlights the evolving landscape of clinical trial regulatory affairs and emphasizes the importance of proactive communication with regulatory bodies. Additionally, the case study titled 'Accelerating Drug Development' demonstrates that the average duration to introduce a drug to the market surpasses 10 years, highlighting the importance of swift reactions, as observed in the COVID-19 vaccine studies. This suggests that regulators are increasingly open to innovative approaches that can expedite the approval process.
Furthermore, the participation of specialty doctors in cancer research, as evaluated by Klabunde CN et al., highlights the cooperative endeavors required to address these challenges successfully. Therefore, being knowledgeable about the submission process not only simplifies the route to starting studies but also improves the chances for positive results in research.
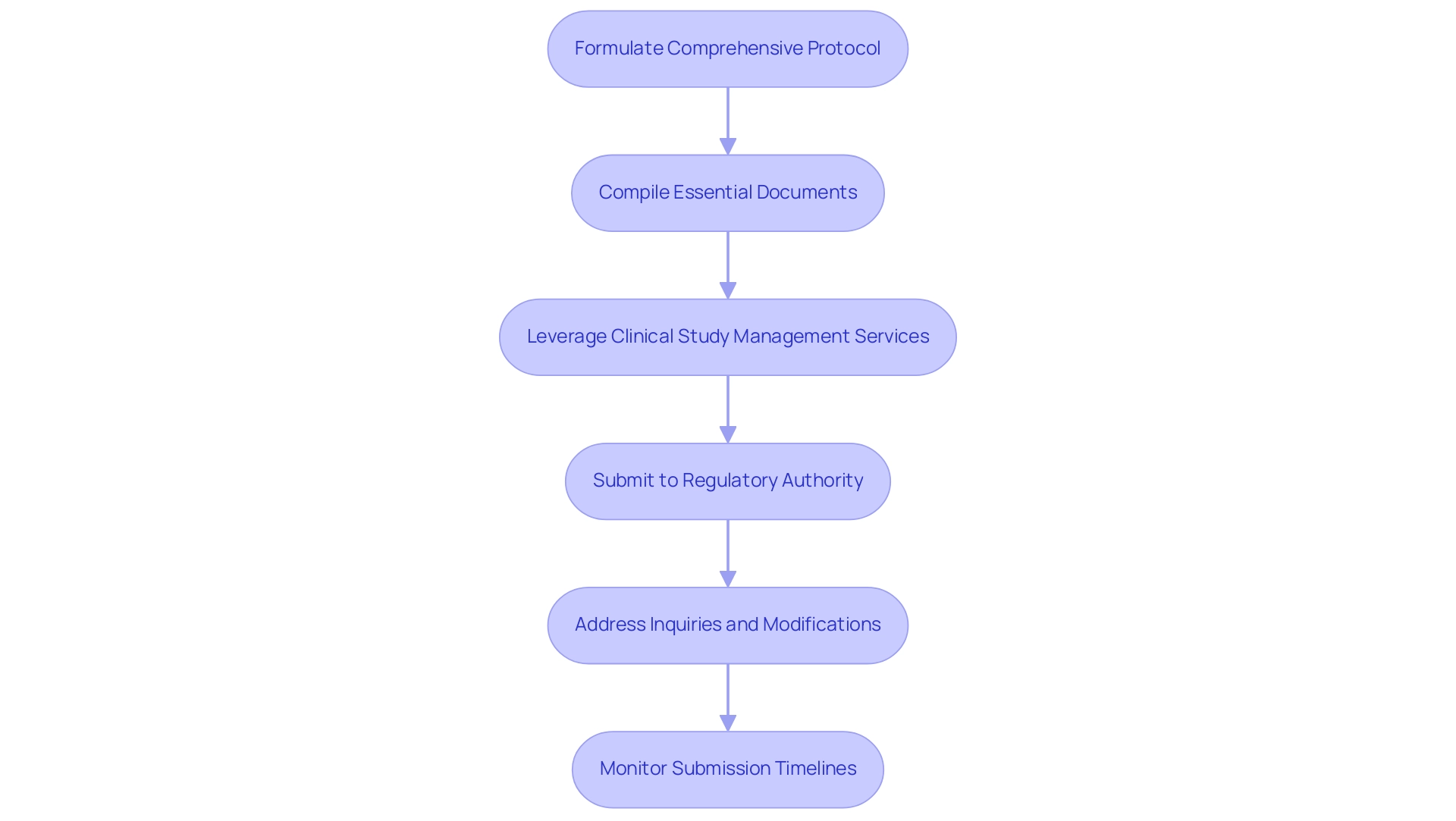
Challenges in Regulatory Affairs for Clinical Research
Navigating clinical trial regulatory affairs in medical research presents numerous challenges, particularly in Latin America, where compliance across multiple jurisdictions can be intricate. Our comprehensive services in clinical trial regulatory affairs include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures a streamlined process from start to finish. This encompasses review and feedback on research documents to comply with country requirements and detailed reporting on research status, inventory, and both serious and non-serious adverse events.
Researchers often face complexities in clinical trial regulatory affairs due to diverse regulations and the need to adapt to rapidly evolving guidelines. A significant hurdle in clinical trial regulatory affairs is obtaining timely approvals, which can delay the initiation of studies and impact project timelines. Furthermore, ensuring that all team members receive adequate training on compliance matters related to clinical trial regulatory affairs poses logistical difficulties, particularly in multi-jurisdictional settings.
According to recent findings, electronic data capture (EDC) systems can achieve up to a 90% reduction in data errors compared to traditional paper-based methods, emphasizing the need for effective technological solutions. Additionally, the FDA has established a framework for evaluating decentralized and virtual trials, which can provide valuable insights into navigating compliance in a more flexible manner. Experts like Katherine Ruiz, who specializes in compliance affairs for medical devices and in vitro diagnostics in Colombia, underscore the importance of Corrective and Preventive Action (CAPA) in research, highlighting that maintaining data integrity requires stringent access controls, regular backups, and thorough training on electronic systems.
The implementation of blockchain technology can further enhance data integrity and traceability in research, offering a promising avenue for addressing compliance challenges. Moreover, the FDA's 21 CFR Part 11 regulation establishes standards for electronic records and signatures, emphasizing the need for system validation and detailed audit trails to maintain data integrity. To mitigate these challenges, it is crucial for researchers to establish robust communication channels with professionals in clinical trial regulatory affairs, such as those at INVIMA, the Colombia National Food and Drug Surveillance Institute, and remain vigilant about updates in the compliance environment.
This proactive approach not only enhances compliance but also fosters adaptability in an increasingly complex oversight landscape.
Future Trends and Directions in Regulatory Affairs
The landscape of compliance matters in clinical research is poised for significant change, driven by comprehensive clinical trial regulatory affairs and management services that include:
- Viability assessments
- Site selection
- Adherence reviews
- Trial preparation
- Import permits
- Project oversight
- Reporting
Our service capabilities encompass the review and feedback on study documents to ensure compliance with country requirements, as well as detailed reporting on study status, inventory, and serious and non-serious adverse events. Leaders in this field, such as Ana Criado, Director of Affairs and CEO of Mahu Pharma, alongside Katherine Ruiz, an expert in compliance for medical devices and in vitro diagnostics in Colombia, exemplify the expertise guiding these advancements.
The shift towards electronic submissions is becoming more pronounced, with organizations increasingly leveraging artificial intelligence to improve compliance decision-making processes. This evolution not only streamlines submissions but also enhances the accuracy and efficiency of compliance interactions. For example, Walgreens signed 15 agreements for research recruitment in Q3 of 2023, up from 8 agreements in Q2, emphasizing the rising trend in research recruitment and its effects on compliance matters.
Moreover, a crucial trend is the focus on patient-centric methods, which are anticipated to greatly influence the design of clinical trials and the framework surrounding patient involvement. These approaches necessitate a more profound integration of patient perspectives, ultimately influencing compliance requirements. As articulated in a recent quote, 'It’s the balance that we need to strike between technology and human interaction,' underscoring the importance of maintaining human connections amidst increasing technological reliance.
Additionally, researchers should remain vigilant regarding ongoing global governance harmonization efforts, which aim to create a cohesive framework that simplifies processes across various regions. A case study on trust-building in medical research illustrates how education and transparency can enhance patient involvement and compliance with guidelines. By staying informed on these developments, researchers can strategically navigate the changing landscape of clinical trial regulatory affairs, thereby enhancing the efficiency and effectiveness of their clinical trials.

Conclusion
Regulatory affairs play a crucial role in the management of clinical trials, ensuring that research involving human subjects adheres to stringent guidelines and legal requirements. Professionals in this field are tasked with navigating the complexities of regulatory submissions, maintaining compliance with evolving regulations, and safeguarding participant rights. The processes outlined, from feasibility studies to the importance of informed consent, underscore the meticulous nature of trial management and the necessity for skilled regulatory professionals.
The challenges faced in regulatory affairs, particularly in diverse jurisdictions like Latin America, highlight the need for innovative solutions and robust communication channels. As the landscape continues to evolve, the adoption of technology, such as electronic data capture and artificial intelligence, is poised to enhance the efficiency and accuracy of regulatory interactions. Moreover, the emphasis on patient-centric approaches and global regulatory harmonization reflects an ongoing shift towards creating a more cohesive and responsive regulatory environment.
In conclusion, a comprehensive understanding of regulatory affairs is essential for the successful execution of clinical trials. By prioritizing compliance and embracing emerging trends, researchers can navigate the regulatory landscape effectively, ultimately contributing to the integrity of clinical research and the safety of participants. As the field continues to advance, staying informed and adaptable will be key to overcoming the challenges and leveraging the opportunities that lie ahead in regulatory affairs.
Frequently Asked Questions
What are the key elements of regulatory matters in clinical trial regulatory affairs?
Key elements include policies, guidelines, and legal requirements that oversee investigations involving human participants, such as FDA requirements, ICH guidelines, and Good Clinical Practice (GCP) standards.
What services are offered in clinical trial regulatory affairs?
Services include comprehensive feasibility studies, meticulous selection of research sites, in-depth compliance reviews, feedback on study documents, setup, import permits, project management, and detailed reporting processes.
Why is compliance with regulations important in clinical trials?
Compliance is crucial for safeguarding the safety, rights, and well-being of participants and for upholding the integrity of the data collected. Non-compliance can lead to delays, fines, and jeopardized patient safety.
What is a Certificate of Analysis (CoA) and why is it important?
A CoA records the identity, purity, and strength of investigational products used in research trials and is essential for compliance with regulations.
What is the registration requirement for ethics committees (ECs) involved in clinical investigations?
All ethics committees must register electronically with the HHS Office for Human Research Protections (OHRP), and this registration is effective for three years upon approval.
What role do specialists play in clinical trial regulatory affairs?
Specialists prepare and submit compliance documents, oversee trial setup, maintain communication with oversight agencies, implement informed consent procedures, and train research staff on compliance protocols.
How have submission success rates in clinical trial regulatory affairs changed recently?
Submission success rates have improved by 15% over the past five years, indicating the effectiveness of professionals in navigating the approval processes.
What is the significance of informed consent in clinical trials?
Informed consent ensures that participants understand their rights and the nature of the studies they are involved in, which is a fundamental ethical requirement in clinical research.
What are the potential consequences of failing to comply with clinical trial regulations?
Consequences can include delays in research, monetary fines, and compromised patient safety, highlighting the importance of thorough compliance management.
Who are some experts in clinical trial regulatory affairs mentioned in the article?
Ana Criado, Director of Compliance at Mahu Pharma, and Katherine Ruiz, who focuses on compliance for medical devices and in vitro diagnostics in Colombia, are noted experts in the field.




