Overview
Best practices for clinical trial materials focus on ensuring compliance with regulatory standards and enhancing operational efficiency throughout the research process. The article emphasizes the importance of meticulous planning, effective documentation, and systematic management, which are crucial for navigating regulatory requirements and ultimately improving trial outcomes, as evidenced by increased success rates in recent studies.
Introduction
In the intricate world of clinical trials, the significance of well-organized materials cannot be overstated. These essential documents and tools, ranging from study protocols to informed consent forms, are the backbone of effective research execution, ensuring compliance with stringent regulatory standards.
As the landscape of clinical trials evolves, the demand for meticulous planning and management grows, particularly with recent statistics indicating a rise in Phase III success rates. This article delves into the multifaceted role of clinical trial materials, exploring their impact on research outcomes, the challenges of regulatory navigation, and the ethical imperatives surrounding informed consent.
By highlighting best practices and innovative strategies, it aims to illuminate how organizations can enhance participant engagement and streamline trial operations, ultimately contributing to more effective and trustworthy clinical research.
Understanding Clinical Trial Materials: A Foundation for Compliance
Clinical study resources play a vital role in the efficient implementation of research, encompassing a variety of essential documents and tools. These clinical trial materials include:
- Research protocols
- Informed consent forms
- Case report forms
- Participant recruitment documents
All of which must comply with specific regulatory standards. Maintaining adherence to these standards is vital as it ensures compliance and facilitates the seamless operation of the study.
Thorough healthcare research management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
Enhance this process. Significantly, the evaluation and input on research documents are crucial to fulfill national standards, guaranteeing that all resources are compliant. For example, a well-organized research protocol acts as a thorough roadmap, outlining the project's objectives, design, and methodology, which greatly influences the quality of results.
Recent statistics indicate that Phase III success rates have increased to 66%, exceeding the 56% pre-pandemic average, highlighting the significance of effective trial resources in achieving positive outcomes. The influence of Medtech clinical research on local economies—such as job creation, economic growth, healthcare enhancement, and international collaboration—cannot be overlooked in this context. As noted by Arnaud Levain Chavanon, a Swiss quality assurance consulting firm with a deep understanding of both local and international regulations, ObelysQ is ideally positioned to help pharmaceutical and biotech companies navigate these changes.
The importance of meticulous planning and organization in these materials cannot be overstated, as it enhances compliance and streamlines processes. This is especially significant considering recent trends where only 15% of healthcare organizations conduct research, thereby increasing pressure on active sites to meet trial timelines. A case study on Research Site Readiness illustrates the challenges faced by research locations, highlighting the necessity of effective site enablement strategies to optimize research sites, improve participant recruitment, and ultimately enhance overall study efficiency.
Furthermore, tools such as Trial 360 can streamline research management and boost credibility and trust in studies, further aiding in improved patient outcomes.
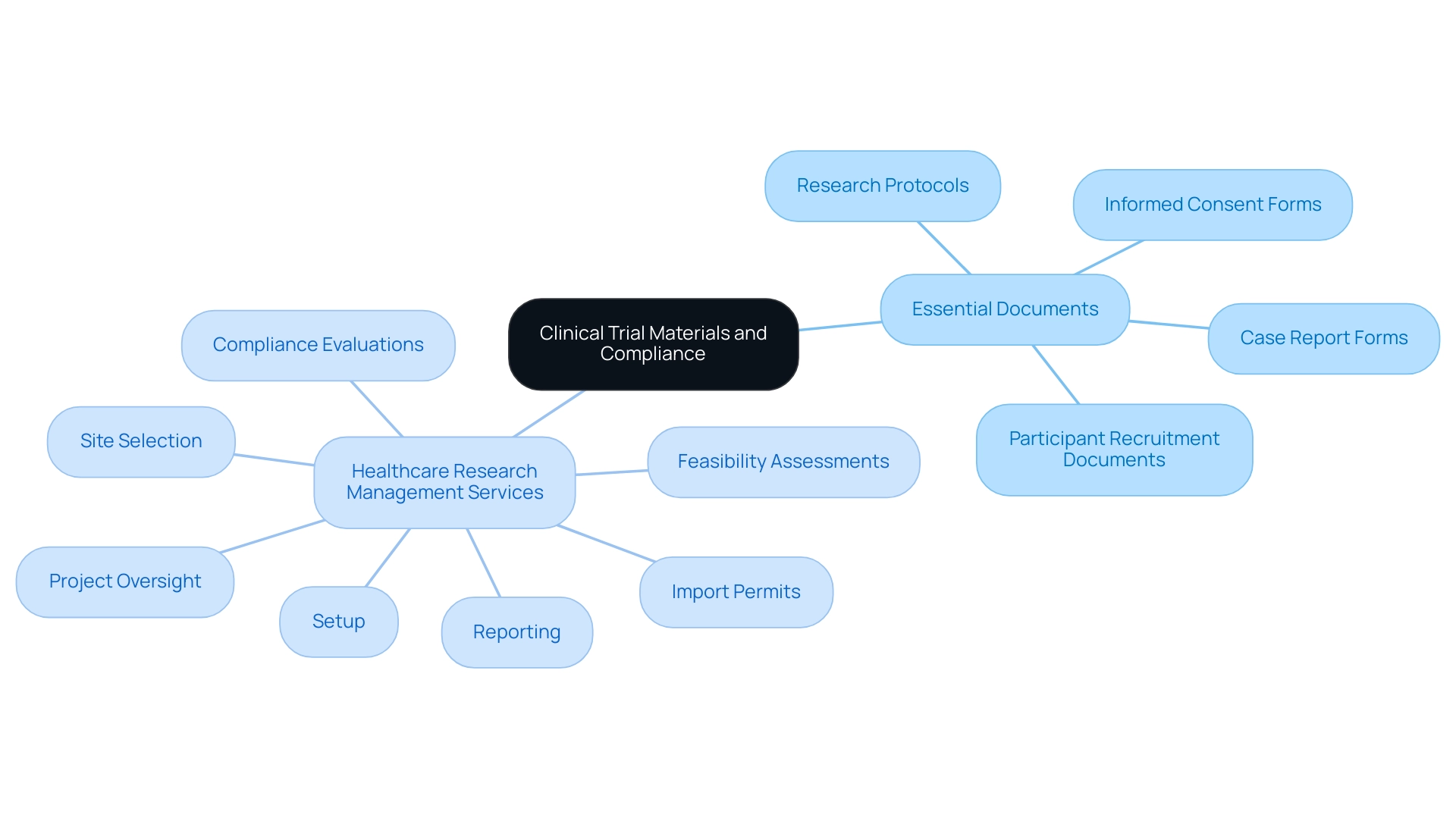
Navigating Regulatory Requirements for Clinical Trial Materials
Navigating the regulatory environment of research studies is crucial for ensuring compliance and the success of research initiatives. Our extensive management services for research include:
- Feasibility and selection of research locations
- Review and feedback on project documents to ensure compliance with country requirements
- Setup and approval through ethics committees and health ministries
- Management of import permits and nationalization of investigational devices
Additionally, we provide thorough reporting on research status, inventory management, and tracking serious and non-serious adverse events.
Regulatory bodies, notably the FDA (Food and Drug Administration) and the EMA (European Medicines Agency), impose stringent guidelines that dictate the content, structure, and approval processes for clinical trial materials. For example, informed consent documents, which are part of clinical trial materials, must not only communicate potential risks and benefits clearly but also comply with both agencies' standards. It is crucial to note that the FDA typically mandates two controlled trials or one trial with confirmatory evidence for drug approval, while the EMA has its own distinct requirements.
As Michael Banks, the global head of Regulatory Affairs for Sandoz, aptly states, 'There is an opportunity to fix this by encouraging regulators such as the FDA and the EMA to agree on an aligned regulatory approach to the development of complex generics.' This highlights the ongoing need for harmonization in Regulatory practices. Furthermore, Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, adds valuable insights into navigating these complexities.
Moreover, off-patent therapies represent around 80% of medicines used worldwide, underscoring the importance of effective regulatory compliance in the pharmaceutical landscape. The Patients’ and Consumers’ Working Party (PCWP) by the EMA serves as a case study in this regard, providing a platform for exchanging information between the agency and patients, thereby facilitating patient engagement in the regulatory process. Such initiatives promote the active participation of patients in the drug evaluation process, enhancing the relevance of regulatory decisions.
Furthermore, a comparison between EMA's policy on medicinal data publication and the Clinical Trials Regulation reveals significant differences in the range of medicinal products included and the publication channels, which can have profound implications for studies. Implementing regular training sessions for research staff on these evolving regulations is essential. Such training not only fosters adherence to compliance but also significantly reduces the risk of delays and enhances the credibility of research outcomes.
By actively engaging with the latest regulatory updates for 2024, stakeholders can ensure that all research materials meet the required standards, ultimately contributing to more effective and dependable medical studies.
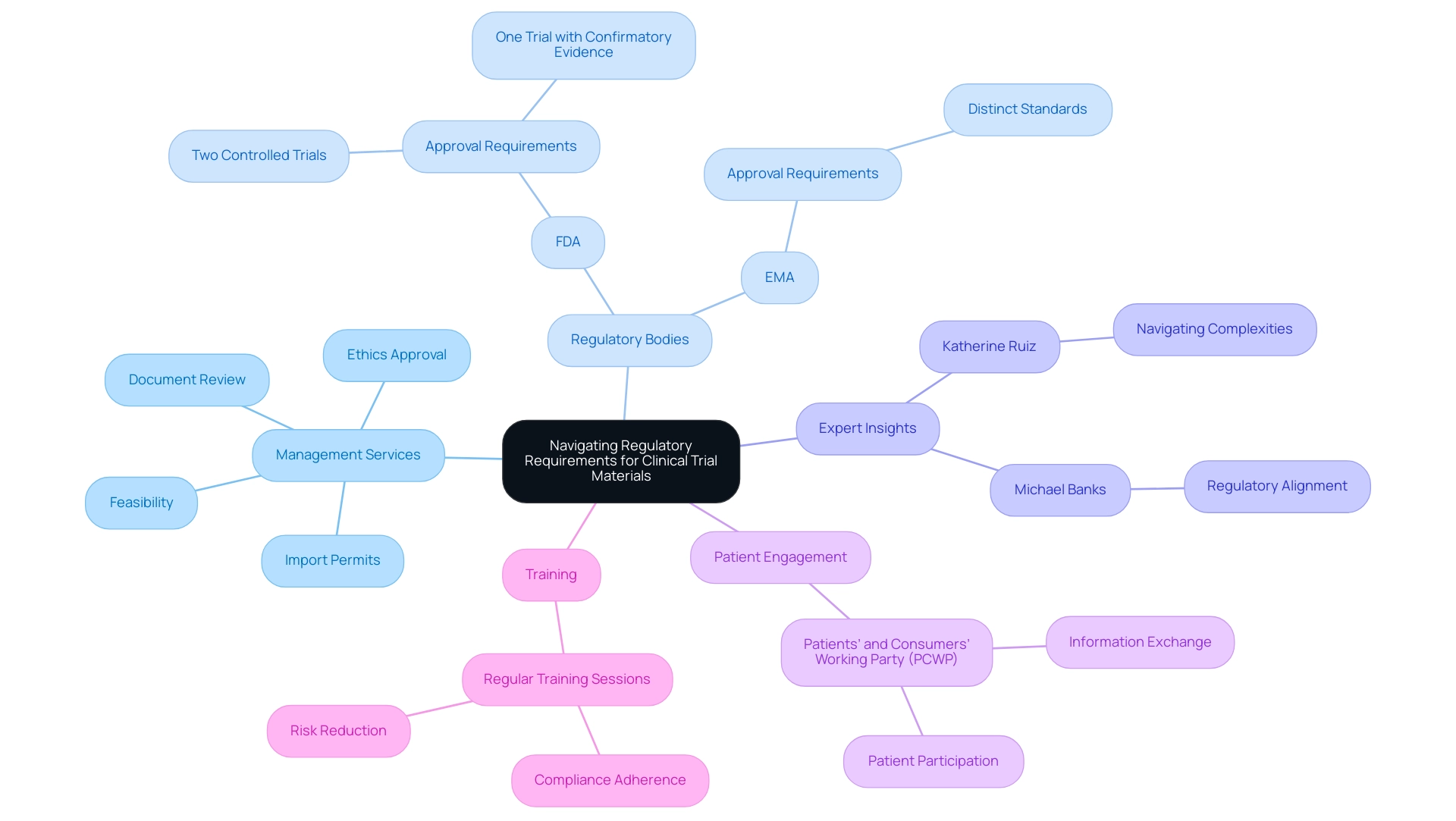
The Role of Informed Consent in Clinical Trials
Informed consent transcends a mere procedural formality; it is a fundamental ethical obligation that guarantees participants are comprehensively informed about the study's objectives, methodologies, potential risks, and anticipated benefits. Research staff have reported challenges such as time pressures and the complexity of information presented during the consent process, which can hinder participants' understanding. A well-crafted informed consent form must be accessible, utilizing straightforward language to avoid the bewilderment that can arise from complex jargon.
Engaging participants in meaningful discussions before they endorse the consent form is vital, as it not only cultivates trust but also bolsters participant safety and reinforces ethical compliance. Lydia O'Sullivan aptly notes, 'The authors declare that they have no competing interests,' emphasizing the importance of transparency in research. Recent insights from research staff indicate that a shorter, more straightforward Participant Information Leaflet (PIL) could significantly enhance the informed consent process.
With only 39.4% of participants demonstrating a clear understanding of randomization, it is evident that simplification is necessary. Moreover, the informed consent process regarding clinical trial materials is closely linked to supply chain management in research studies, as ensuring that participants are well-informed can lead to better engagement and retention, ultimately affecting the efficiency of operations. Routine assessments of informed consent documents are crucial to guarantee that they comply with current regulations and best practices, promoting a more knowledgeable and reliable research environment.
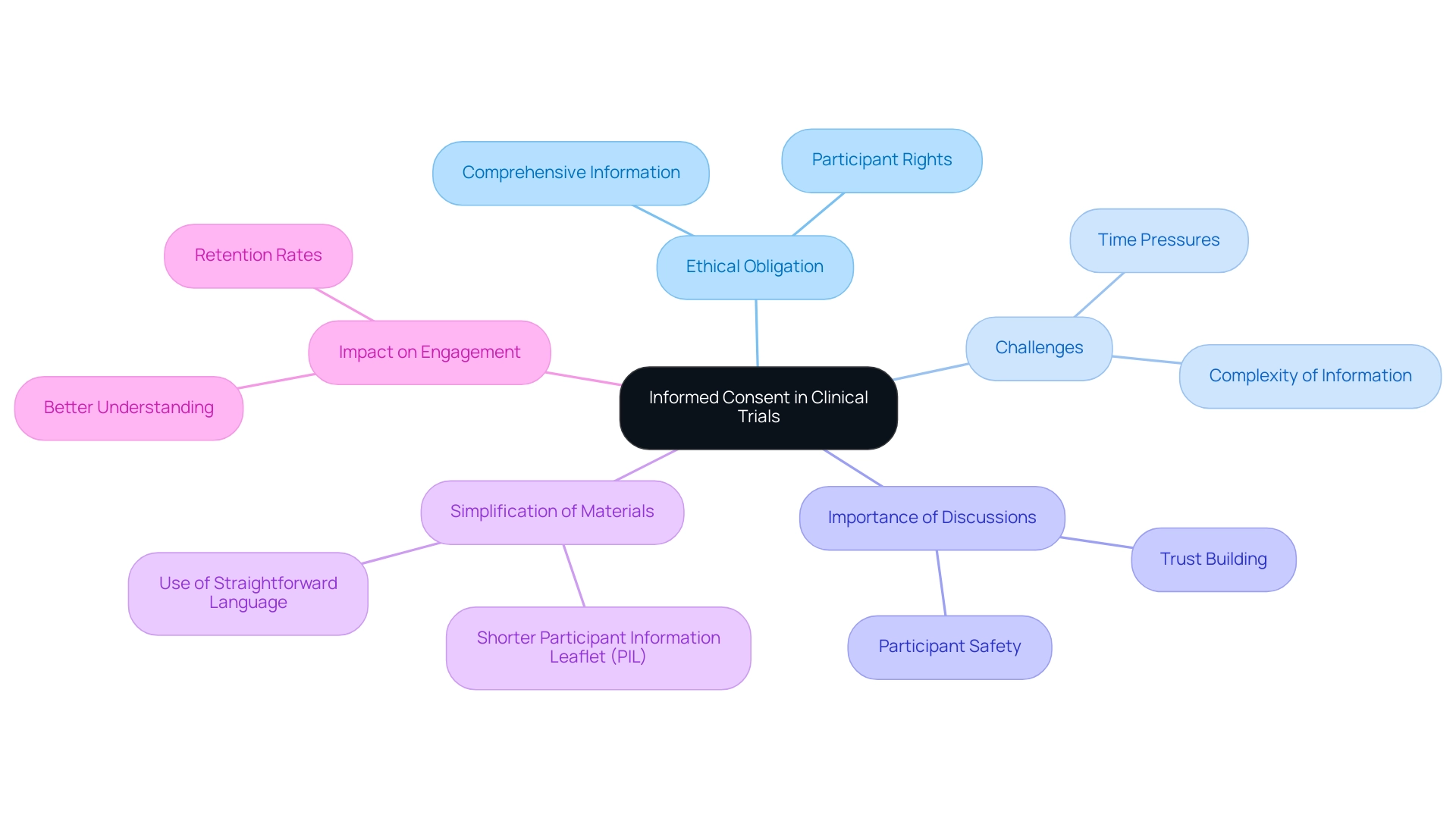
Enhancing Readability: Making Clinical Trial Materials Accessible to Participants
To promote participant comprehension and involvement in studies, it is crucial that study materials are created with clarity and brevity. Recent findings indicate that clinical trial consent forms are, on average, written at a late high school or undergraduate level, representing a significant gap in education for many potential participants. This highlights the critical need for improvement.
Utilizing techniques such as:
- Bullet points
- Short sentences
- Accessible terminology
can significantly enhance readability, making complex information more digestible. Additionally, the integration of visual aids, such as charts and infographics, plays a pivotal role in effectively conveying intricate data and concepts. Evidence suggests that the use of visual aids not only aids comprehension but also fosters greater participant retention.
As mentioned in research, higher reading complexity correlates with increased dropout rates, reinforcing the necessity of improving readability. To further refine these materials, conducting readability assessments with target participant demographics can yield valuable insights, guiding researchers in crafting documents that truly resonate with their audience. The case study titled "Implications of Literacy Barriers in Clinical Trials" emphasizes the importance of addressing literacy barriers in clinical trial materials to improve participant comprehension and retention.
By prioritizing accessibility, researchers can empower participants to make informed choices about their involvement, ultimately enriching the ethical conduct of studies and enhancing inclusivity. Future research should also explore the long-term effectiveness of AI-simplified consent forms to further advance this field.
Best Practices for Managing and Distributing Clinical Trial Materials
Effectively handling and distributing clinical trial materials requires a systematic method that emphasizes adherence and operational effectiveness. Studies indicate that distribution errors occur in around 20% of clinical trial materials, highlighting the essential requirement for frequent audits to detect discrepancies and compliance problems swiftly. Our comprehensive services encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Nationalization of investigational devices
- Project management
These services are essential in overcoming the regulatory hurdles that medical device startups often face.
The adoption of centralized systems for monitoring resources has proven invaluable in mitigating loss and ensuring that all team members access the most current documentation. Moreover, setting clear guidelines for managing sensitive information, including patient data, is essential for preserving confidentiality and complying with regulatory standards, especially the necessity for informed consent from both parents in research involving nonviable neonates. A culture of responsibility and openness within research groups not only improves the handling of study resources but also leads to better research results.
For example, the European Commission's recent public consultation on phthalates in medical devices demonstrates the significance of regulatory factors and best practices in handling research materials. As noted by EFPIA, the overarching aim is to reclaim Europe’s position as a leader in health research and development, ensuring that European patients receive priority access to innovative treatments and technologies. This strategic alignment with best practices in clinical trial materials supply chain management, including the management of import permits and nationalization processes, is essential for fostering advancements in the field.
Conclusion
The intricate landscape of clinical trials is underscored by the pivotal role of well-organized materials, which serve as the backbone for compliance and successful research execution. From study protocols to informed consent forms, these documents not only ensure adherence to regulatory standards but also significantly impact research outcomes. As evidenced by the rising Phase III success rates, the meticulous planning and management of clinical trial materials are essential for achieving positive results and fostering trust in the research process.
Navigating the complex regulatory requirements is paramount for maintaining compliance and enhancing the credibility of clinical trials. The emphasis on harmonization among regulatory bodies, alongside the implementation of best practices, facilitates smoother operations and contributes to more effective research outcomes. Moreover, the ethical obligation of informed consent highlights the importance of participant engagement and understanding, which are critical for the integrity of clinical trials.
Ultimately, the commitment to enhancing the accessibility and readability of clinical trial materials empowers participants to make informed decisions about their involvement. By prioritizing clarity and transparency, researchers can improve participant comprehension and retention, fostering a more ethical and inclusive environment for clinical research. In a rapidly evolving field, embracing innovative strategies and best practices will not only streamline trial operations but also contribute to the advancement of medical science, benefiting both patients and the broader healthcare landscape.
Frequently Asked Questions
What are the essential documents included in clinical study resources?
Essential documents include research protocols, informed consent forms, case report forms, and participant recruitment documents.
Why is compliance with regulatory standards important in clinical studies?
Compliance with regulatory standards is vital as it ensures the seamless operation of the study and guarantees that all resources are compliant with national standards.
What services enhance healthcare research management?
Healthcare research management services include feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
How does a well-organized research protocol impact a study?
A well-organized research protocol acts as a comprehensive roadmap, outlining the project's objectives, design, and methodology, which greatly influences the quality of results.
What recent statistics highlight the effectiveness of clinical trial resources?
Recent statistics show that Phase III success rates have increased to 66%, exceeding the pre-pandemic average of 56%, indicating the significance of effective trial resources in achieving positive outcomes.
What role does Medtech clinical research play in local economies?
Medtech clinical research contributes to job creation, economic growth, healthcare enhancement, and international collaboration.
What challenges do research sites face in conducting studies?
Only 15% of healthcare organizations conduct research, increasing pressure on active sites to meet trial timelines, which highlights the need for effective site enablement strategies.
How can tools like Trial 360 assist in research management?
Tools like Trial 360 can streamline research management, enhance credibility and trust in studies, and ultimately improve patient outcomes.
What are the key aspects of navigating the regulatory environment for research studies?
Key aspects include feasibility and selection of research locations, compliance reviews of project documents, setup and approval through ethics committees, and management of import permits.
What are the regulatory requirements for informed consent documents?
Informed consent documents must clearly communicate potential risks and benefits while complying with standards set by regulatory bodies like the FDA and EMA.
How do the FDA and EMA differ in their drug approval requirements?
The FDA typically mandates two controlled trials or one trial with confirmatory evidence for drug approval, while the EMA has its own distinct requirements.
Why is patient engagement important in the regulatory process?
Patient engagement enhances the relevance of regulatory decisions and facilitates communication between patients and regulatory agencies.
What is the significance of regular training for research staff?
Regular training fosters adherence to compliance, reduces the risk of delays, and enhances the credibility of research outcomes.
How can stakeholders ensure that research materials meet required standards?
By actively engaging with the latest regulatory updates and implementing training sessions, stakeholders can ensure compliance and improve the effectiveness of medical studies.

