Overview
Best practices for CRO collaboration in Latin America emphasize the necessity of transparent communication, the cultivation of mutual trust, and the strategic use of local expertise to adeptly navigate the regulatory and cultural challenges inherent in clinical research. This article underscores that effective partnerships—manifested through regular strategy meetings and the implementation of advanced project management tools—are pivotal in enhancing trial outcomes and operational efficiency. Consequently, these practices position Latin America as a compelling hub for clinical studies, inviting stakeholders to consider the potential benefits of such collaboration.
Introduction
In the swiftly evolving landscape of clinical research, Contract Research Organizations (CROs) are pivotal in bridging the divide between pharmaceutical companies and regulatory mandates. This is especially evident in Latin America, where CROs are not merely simplifying the intricacies of clinical trials; they are also bolstering the region's standing as a credible hub for innovative research.
As they adeptly navigate diverse regulatory frameworks and cultural subtleties, CROs are transforming the operational paradigm of clinical studies, delivering customized solutions that enhance efficiency and outcomes. This article examines the multifaceted roles of CROs, investigates best practices for collaboration with sponsors, and underscores the emerging trends that are shaping the future of clinical research in Latin America.
By leveraging local expertise and innovative methodologies, the prospects for successful clinical trials in this region have never been more promising.
Understanding the Role of Contract Research Organizations (CROs) in Clinical Research
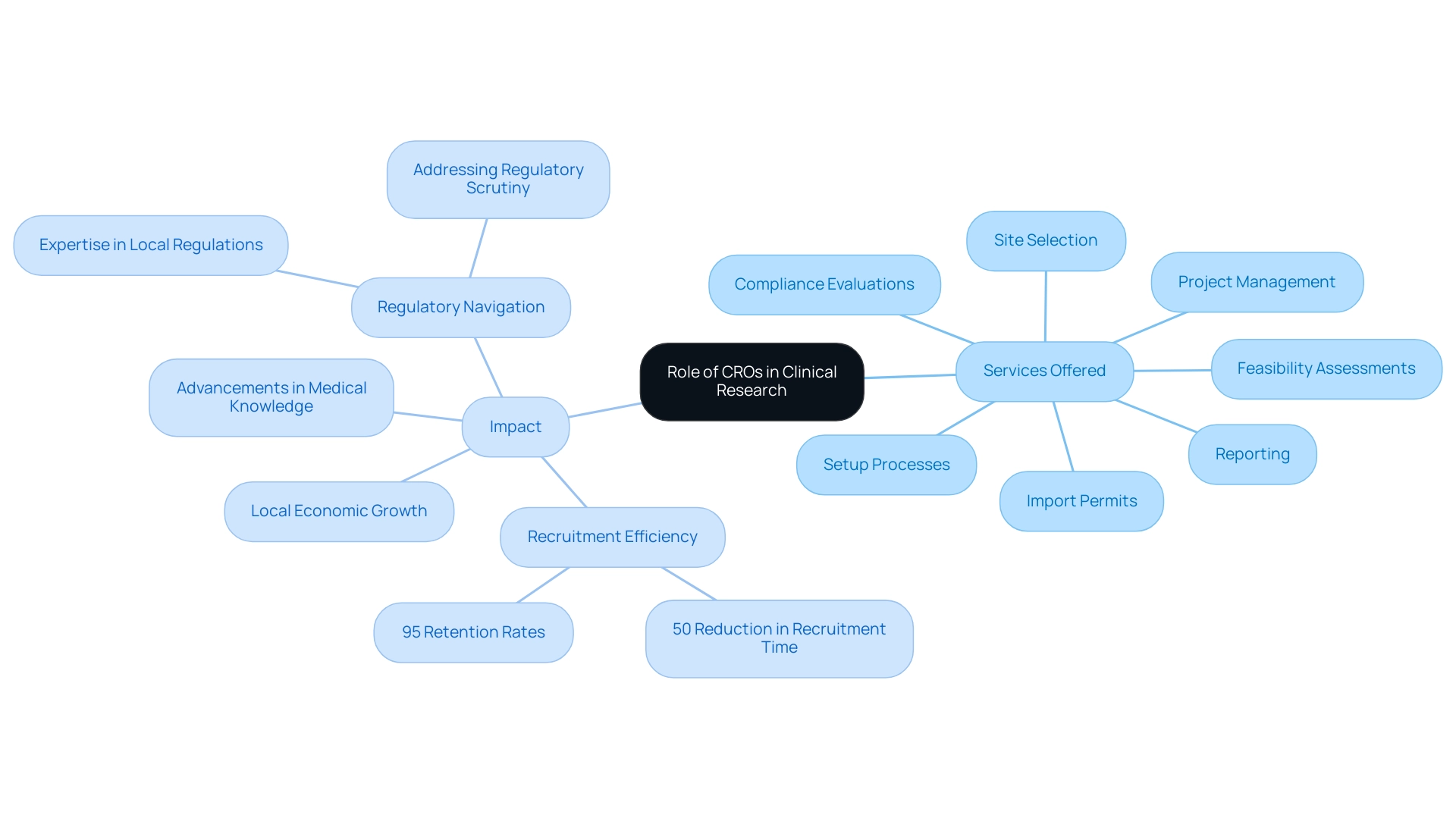
Contract Research Organizations (CROs) play a crucial role as collaborators within the clinical study ecosystem, delivering outsourced services that encompass the design, management, and execution of studies. In Latin America, the collaboration of CROs has become a pivotal element in aligning pharmaceutical companies with regulatory demands. These organizations provide invaluable expertise in navigating local regulations, adeptly recruiting participants from diverse populations, and managing the intricate logistics involved in clinical trials.
The services offered by CROs include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup processes
- Import permits
- Project management
- Reporting
A notable partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier hub for medical studies, supported by the assistance of Colombia's Minister of Health. This collaboration underscores the significance of CROs in fostering local economic growth through clinical studies, with GlobalCare Clinical Trials reporting over a 50% reduction in recruitment time and achieving 95% retention rates, thus demonstrating the effectiveness of these organizations.
This collaboration seeks to enhance Barranquilla's reputation by streamlining processes and improving outcomes. However, the U.S. FDA has observed that inconsistent data management practices among CROs have led to delays and increased regulatory scrutiny in several high-profile drug studies. As reported on June 27, 2024, CROs significantly contribute to accelerating the drug development process by leveraging their expertise and innovative strategies.
Understanding the operational framework and capabilities of CROs enables sponsors to effectively harness their strengths, ultimately leading to more successful trial outcomes. This evolution in CRO services is further illustrated by the case study titled 'Transforming Perceptions of Latin America in Clinical Research,' which highlights how CRO collaboration in Latin America, once questioned for its ability to meet U.S. and European standards, is now recognized for its cost and time efficiencies, alongside a wealth of experienced investigators. This shift reflects a broader trend, as CRO collaboration in Latin America is increasingly regarded as a preferred location for clinical research.
Building Effective Sponsor-CRO Relationships: Key Strategies
Establishing clear communication channels and fostering mutual trust from the onset is crucial for cultivating effective relationships, particularly in the realm of CRO collaboration in Latin America. Regular strategy meetings dedicated to discussing project milestones, challenges, and expectations significantly enhance alignment. For instance, a multinational sponsor that implemented routine strategy sessions with its CRO experienced a remarkable 30% reduction in delays, highlighting the direct impact of effective communication on clinical trial timelines.
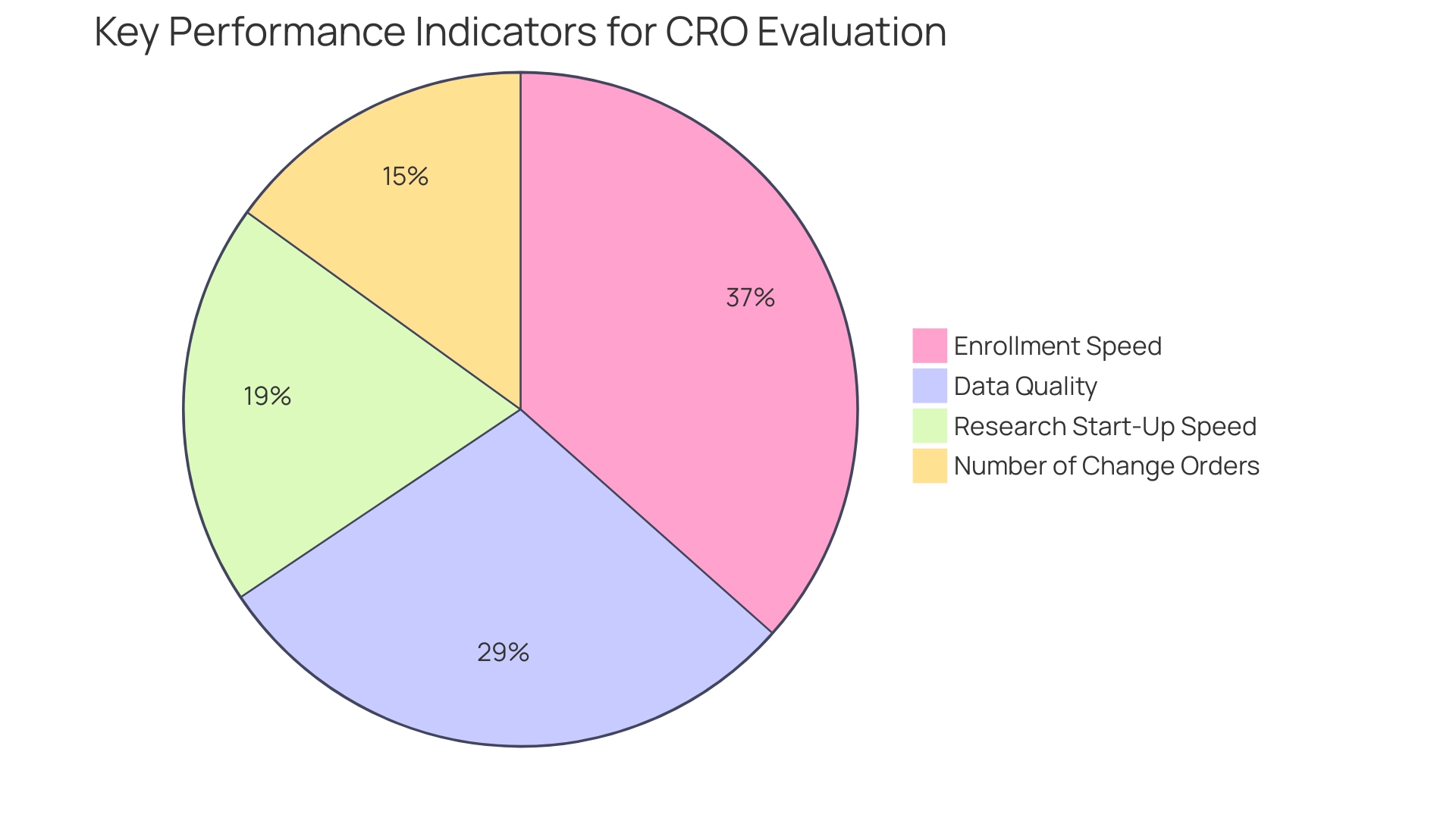
Furthermore, sponsors frequently emphasize key indicators such as:
- Enrollment speed (34 percent)
- Data quality (27 percent)
- Research start-up speed (18 percent)
- The number of change orders (14 percent)
when assessing CRO performance. Involving organizations like bioaccess®, with their 20+ years of experience in Medtech and expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research, early in the protocol design phase proves beneficial; their insights can shape feasible research designs, ensuring both parties are aligned on objectives and methodologies.
The flexibility that bioaccess® offers is essential in navigating the complexities of medical device trials. As Anthony Encarnacao, Vice President of Global Partnerships, states, 'When everyone collaborates, partnerships thrive, and success becomes a shared achievement.' Such collaborative practices, including tailored methodologies and processes employed by bioaccess®, not only streamline operations but also pave the way for successful outcomes, enabling vetted CRO collaboration in Latin America to leverage technology effectively to meet study goals.
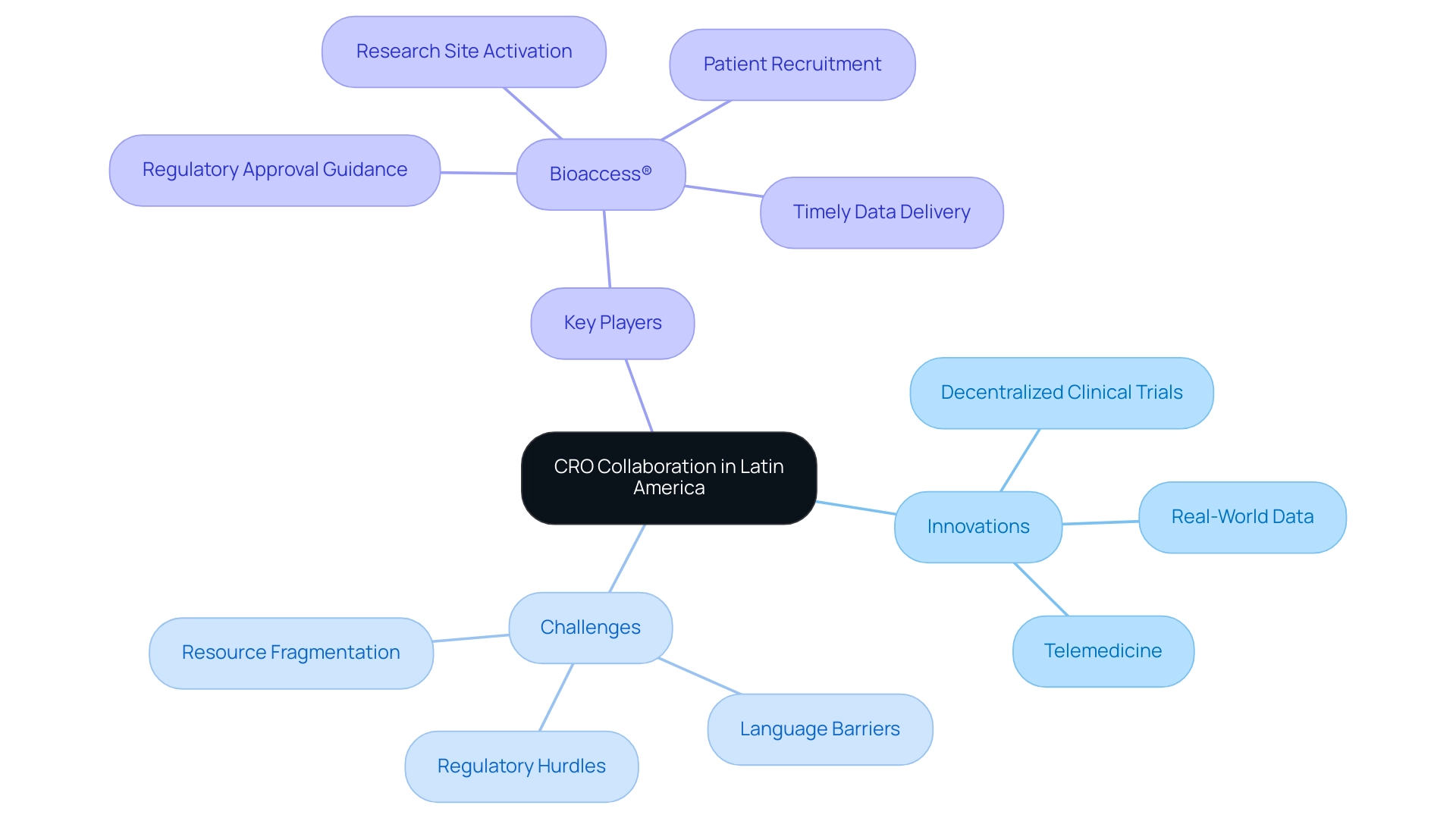
Navigating Challenges in Latin American Clinical Research: Regulatory and Cultural Considerations
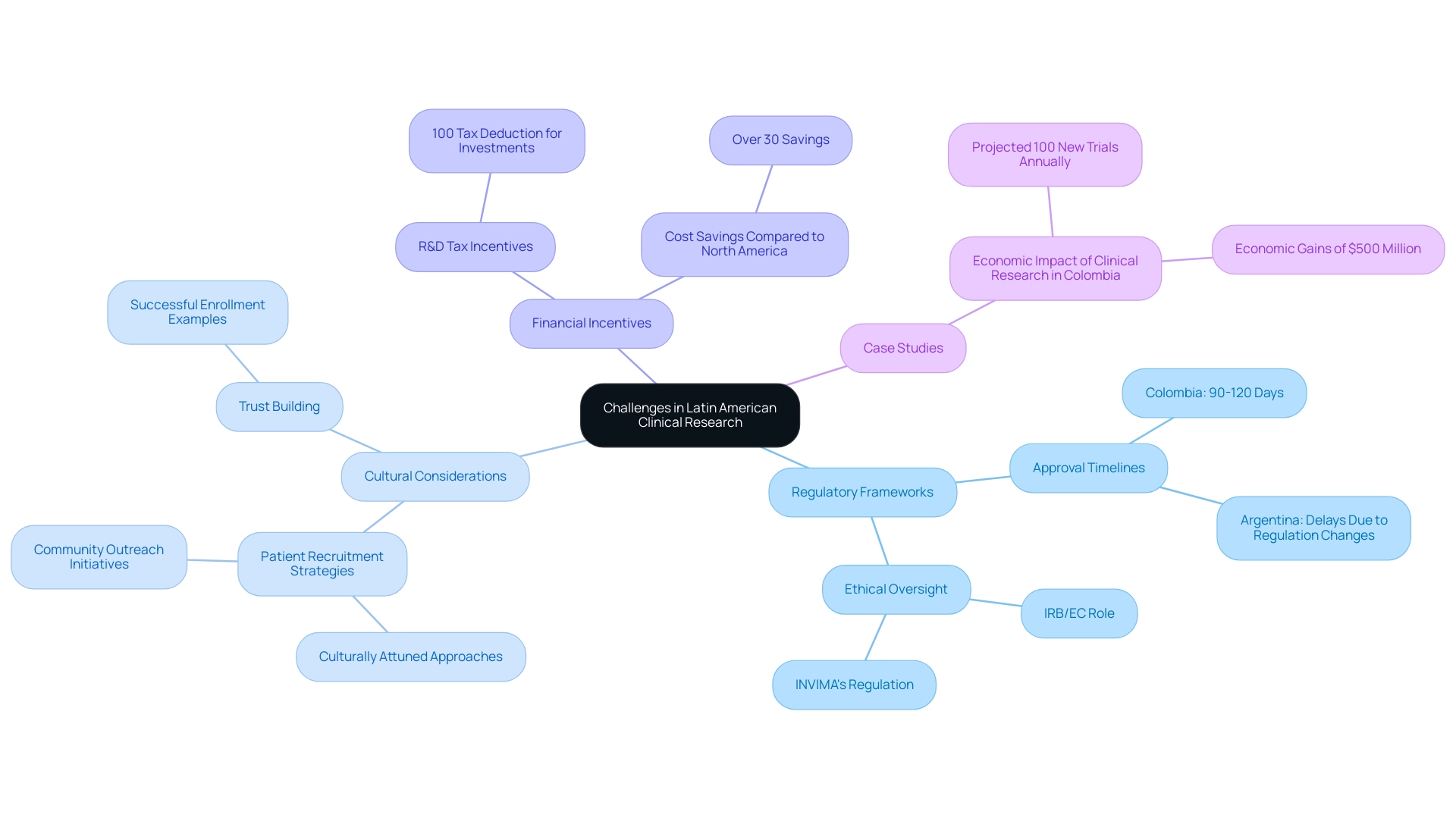
Navigating medical research in Latin America presents a multitude of challenges, particularly due to its diverse regulatory frameworks and cultural intricacies. A recent examination of 297 studies underscores the urgent need for a more inclusive research approach that focuses on overlooked diseases, highlighting the importance of adapting to local contexts. In Colombia, the process for obtaining clinical research approval is notably efficient, with a total IRB/EC and INVIMA evaluation taking only 90-120 days, positioning it as a desirable location for first-in-human (FIH) investigations.
The IRB/EC provides essential ethical oversight, while INVIMA, as a Level 4 health authority, regulates the safety and efficacy of medical devices, thereby enhancing the overall quality of the research environment. Furthermore, Colombia offers significant cost savings of over 30% compared to trials conducted in North America or Western Europe, alongside rigorous healthcare quality standards recognized by the World Health Organization and other international bodies. However, regulatory approval timelines can vary significantly among nations, impacting both project initiation and data collection.
For instance, a recent investigation in Argentina encountered considerable delays due to unexpected changes in local regulations, illustrating the unpredictable nature of the regulatory landscape. To adeptly navigate these complexities, sponsors are advised to collaborate with local Contract Research Organizations (CROs) in Latin America that possess in-depth knowledge of the regional regulatory environment and provide extensive management services, including feasibility studies, compliance reviews, and project management. Additionally, Colombia offers substantial R&D tax and financial incentives, such as a 100% tax deduction for investments in science and technology, making it financially advantageous for companies to conduct experiments there.
As Anil Kumar P., a Research Manager in Healthcare, articulates, 'I am committed to staying at the forefront of industry innovations, ensuring that my work consistently exceeds client expectations.' This partnership can facilitate smoother project execution. Moreover, culturally attuned patient recruitment strategies are crucial for enhancing participant engagement and retention.
A notable example is a successful experiment in Colombia, where community outreach initiatives fostered trust among participants, ultimately leading to improved enrollment outcomes. Furthermore, Colombia's government is actively promoting medical research as part of its strategy to evolve into a knowledge economy by 2031, with experts projecting over 100 new studies each year, generating nearly $500 million in economic benefits annually. Such culturally sensitive methods are essential for overcoming obstacles and ensuring the successful implementation of studies across the region.
Best Practices for Enhancing CRO-Sponsor Collaboration
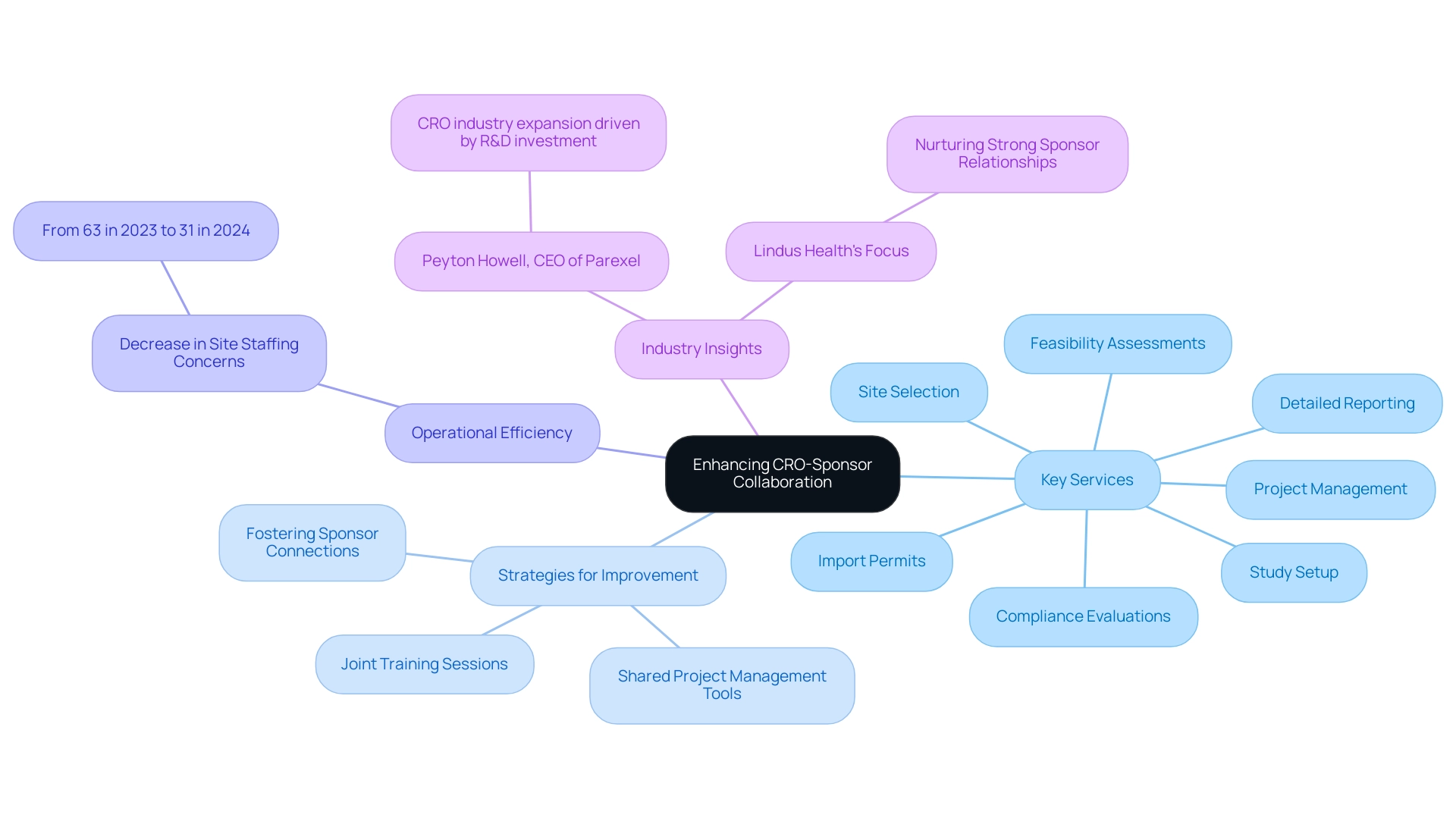
To foster effective collaboration between CROs and sponsors, it is essential to establish a culture rooted in transparency and open communication. Our extensive research study management services are crucial for navigating the complexities of research studies in Latin America. These services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Detailed reporting
All of which support CRO collaboration in the region. Recent statistics indicate a promising trend: site staffing concerns have decreased from 63% in 2023 to just 31% in 2024, reflecting improved operational efficiency.
Implementing a shared project management tool can significantly streamline information exchange, ensuring that all stakeholders remain informed about progress and developments. For instance, a recent partnership between a leading pharmaceutical company and a CRO employed a centralized platform that facilitated real-time updates. This approach led to quicker decision-making and effective issue resolution. Moreover, fostering a collaborative environment through joint training sessions can align both teams on best practices and mutual expectations, enhancing transparency and contributing to more successful outcomes.
Fostering robust sponsor connections is also essential, as highlighted by Lindus Health, to guarantee the success of research studies. Additionally, focusing on participant experience and investing in staff development, as recommended in recent reports, are vital steps for research sites. This approach not only aligns with insights from industry leaders like Peyton Howell, CEO of Parexel, who notes that CRO collaboration in Latin America is poised for significant expansion driven by increased R&D investment, but also emphasizes the necessity of utilizing available tools and embracing AI to improve drug development timelines.
By incorporating these thorough services, such as detailed compliance evaluations that guarantee adherence to local regulations, and an efficient process for securing import permits for investigational devices, we can enhance CRO-sponsor partnerships and promote global health advancement in the evolving landscape of research studies.
Future Trends in CRO Collaboration: Innovations and Opportunities in Latin America
The landscape of CRO collaboration in Latin America is undergoing a significant transformation, primarily driven by technological advancements and an increasing focus on patient-centric methodologies. Innovations such as decentralized clinical trials (DCTs) and the integration of real-world data are gaining momentum, allowing for more adaptable and efficient study designs. A notable example is a recent trial conducted in Mexico that effectively employed telemedicine to monitor participants remotely.
This approach not only reduced the necessity for frequent site visits but also greatly enhanced patient engagement and compliance. As Natalie Gershman, CEO and medical director of Geny Research, asserts, 'This relationship significantly helps to increase subject enrollment and retention rates, and improves patient compliance.' However, Medtech companies in Latin America face considerable challenges, including regulatory hurdles, language barriers, and resource fragmentation, which can impede effective CRO collaboration in the region.
With Research Grid securing USD 6.5 million in November 2024, there is evident financial support for these advancements. Yet, addressing these challenges is paramount for success. As CROs embrace innovative technologies, it is essential for sponsors to remain agile, adapting their research strategies to incorporate these advancements. Bioaccess® emerges as a pivotal entity in this landscape, offering expert guidance on regulatory approval, research site activation, patient recruitment, and timely data delivery.
Their commitment to supporting Medtech startups ensures a seamless transition into the Colombian market, effectively addressing the regulatory complexities that impact CRO collaboration in Latin America. Furthermore, bioaccess® provides cost-effective solutions that bridge the gap between innovation and execution, positioning them advantageously within the dynamic and evolving clinical trial landscape in the region.
Conclusion
In conclusion, the future of clinical research in Latin America is indeed promising, with Contract Research Organizations (CROs) playing a transformative role. Their adeptness at navigating complex regulations, coupled with a steadfast commitment to innovative practices, positions them as invaluable partners for sponsors. As the industry continues to advance, the collaboration between CROs and sponsors will be pivotal in achieving significant medical advancements in the region. Embracing the evolving landscape of clinical research, CROs are set to drive successful trials, enhancing patient engagement and optimizing trial designs through local expertise and adaptability.
Frequently Asked Questions
What is the role of Contract Research Organizations (CROs) in clinical studies?
CROs play a crucial role as collaborators within the clinical study ecosystem, providing outsourced services that include the design, management, and execution of studies.
How do CROs assist pharmaceutical companies in Latin America?
CROs help pharmaceutical companies align with regulatory demands by navigating local regulations, recruiting participants from diverse populations, and managing the logistics of clinical trials.
What services do CROs offer?
CROs offer a variety of services including feasibility assessments, site selection, compliance evaluations, setup processes, import permits, project management, and reporting.
Can you provide an example of a successful CRO partnership in Latin America?
A notable partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a hub for medical studies, supported by Colombia's Minister of Health, demonstrating the significance of CROs in local economic growth.
What impact have CROs had on recruitment and retention rates in clinical trials?
GlobalCare Clinical Trials reported over a 50% reduction in recruitment time and achieved 95% retention rates, showcasing the effectiveness of CROs.
What challenges have CROs faced according to the U.S. FDA?
The U.S. FDA has noted that inconsistent data management practices among CROs have led to delays and increased regulatory scrutiny in several high-profile drug studies.
How do CROs contribute to the drug development process?
CROs significantly accelerate the drug development process by leveraging their expertise and innovative strategies.
What is the importance of establishing communication channels with CROs?
Clear communication channels and mutual trust are crucial for effective relationships, with regular strategy meetings enhancing alignment and reducing delays in clinical trials.
What key performance indicators do sponsors focus on when assessing CRO performance?
Sponsors emphasize indicators such as enrollment speed (34%), data quality (27%), research start-up speed (18%), and the number of change orders (14%).
How does early involvement of organizations like bioaccess® benefit clinical research?
Involving organizations like bioaccess® early in the protocol design phase helps shape feasible research designs and ensures alignment on objectives and methodologies.
What is the significance of collaboration in CRO partnerships?
Collaborative practices, including tailored methodologies, streamline operations and pave the way for successful outcomes, enabling effective use of technology to meet study goals.




