Introduction
Argentina has rapidly emerged as a central hub for medtech clinical trials in Latin America, distinguished by its comprehensive healthcare infrastructure and regulatory framework. With a diverse patient population and a commitment to enhancing medical technology, the country presents an attractive landscape for clinical research organizations (CROs) and medtech companies alike.
The collaboration between local entities and international partners, alongside favorable regulatory changes, has paved the way for accelerated innovation and streamlined processes. As the demand for clinical trials grows, understanding the intricacies of patient recruitment, regulatory compliance, and best practices becomes essential for researchers aiming to capitalize on Argentina's burgeoning potential in the medtech sector.
This article delves into the key factors driving Argentina's position as a leader in medtech clinical trials, exploring the role of CROs, effective patient recruitment strategies, and future trends shaping the industry.
Argentina: The Emerging Hub for Medtech Clinical Trials in Latin America
Argentina has quickly positioned itself as a crucial center for Medtech CRO Services Argentina within the realm of medtech studies in Latin America. Characterized by a robust healthcare infrastructure encompassing public, social security, and private sectors, the country is overseen by the Ministry of Health, ensuring regulation and quality across these domains. This distinctive structure not only enhances the variety of patient groups available for studies but also supports medical activities, especially in private hospitals where a larger patient pool is accessible compared to other Latin American countries.
The ongoing economic growth and rising healthcare expenditures are driving forces behind the enhanced operational capacity of Medtech CRO Services Argentina in the country. Moreover, the partnership between Greenlight Guru and bioaccess™ illustrates the dedication to speeding up medtech advancements and studies, especially emphasized by PAVmed's successful initial in-human research in Colombia. Favorable regulatory changes enacted on June 5, 2017, aimed at streamlining timelines and enhancing the efficiency of the research oversight environment, further solidify the country's position as an appealing landscape for researchers.
As stated, 'Revenues are allocated to the nation where the funds are utilized,' emphasizing the financial advantages of carrying out research studies in that region. This is especially pertinent for U.S. Medtech firms encountering regulatory challenges and financial constraints, as utilizing Medtech CRO Services Argentina offers a more streamlined process and cost-effective solutions. Furthermore, the dedication to enhancing medical technology and improving patient results through groundbreaking research, exemplified by bioaccess®'s cost-effective and high-quality Medtech CRO Services Argentina, strengthens the nation's leadership in the region and establishes it as an ideal location for top-notch studies.
Navigating the Regulatory Landscape and Infrastructure for Medtech in Argentina
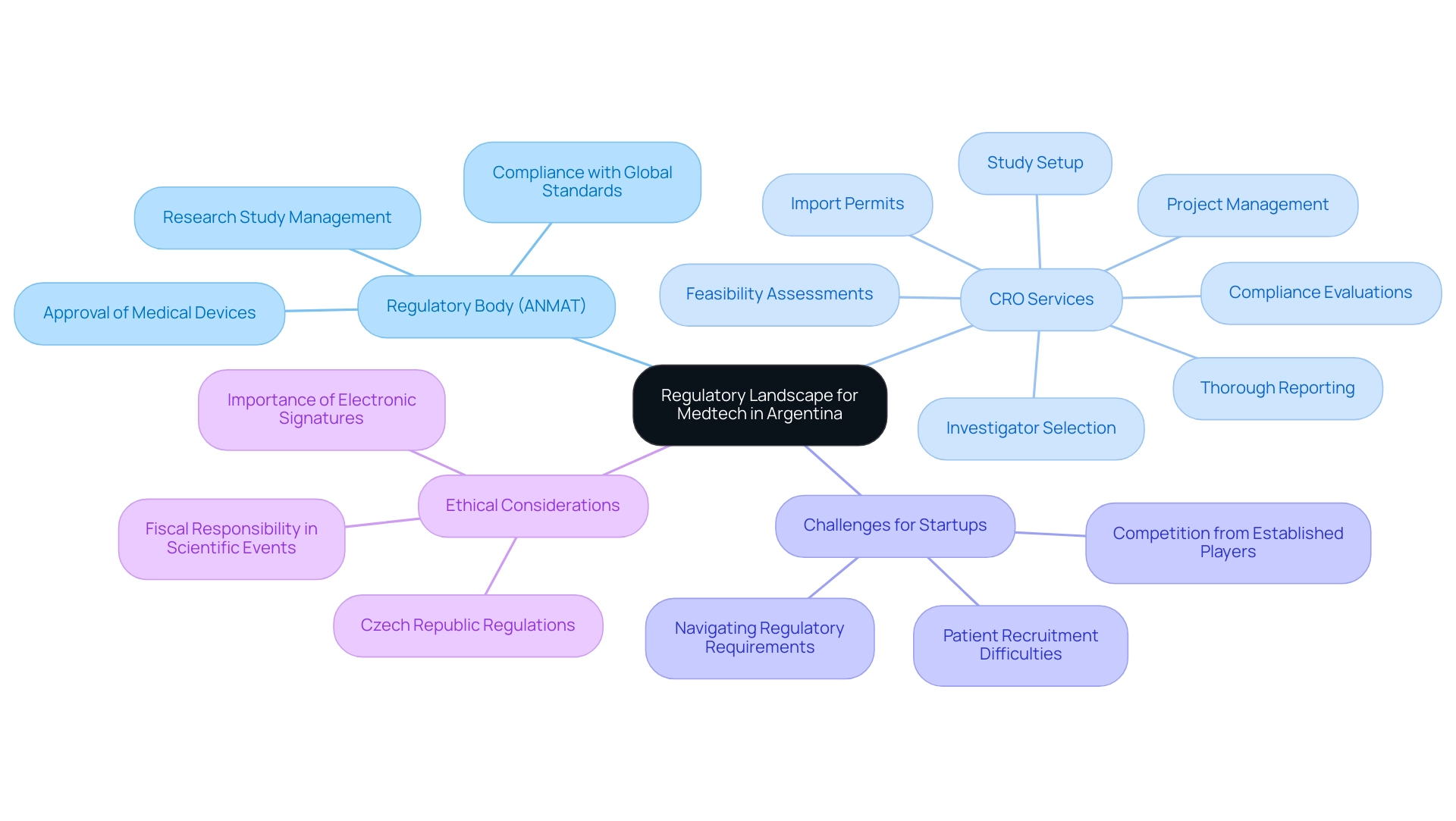
In Argentina, the regulatory framework for medtech is mainly overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT), which manages the approval of medical devices and research studies. Grasping the specific regulatory requirements for submitting research protocols, obtaining ethical approvals, and complying with local statutes is crucial for researchers. Existing guidelines not only conform to global standards but also highlight ethical factors in medical studies.
Additionally, collaborating with experienced Medtech CRO Services Argentina like bioaccess® can significantly ease the navigation of these regulatory processes. With more than 20 years of experience in overseeing research studies, bioaccess® provides Medtech CRO Services Argentina, including customized services such as:
- Feasibility assessments
- Investigator selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Thorough reporting
This partnership ensures adherence to both national and global standards while optimizing study timelines and resource utilization.
The country also gains from a strong hospital framework, with various institutions actively involved in research studies, offering a solid basis for research initiatives. However, medical device startups often face challenges such as:
- Navigating convoluted regulatory requirements
- Competition from established players
- Difficulties in patient recruitment due to limited financial resources
As articulated by the Argentine Chamber of Medical Specialties (CAEME), 'travel expenses must be reasonable and limited to the days on which the scientific or professional event is planned to be held,' reflecting the need for fiscal responsibility in scientific endeavors.
Moreover, the importance of electronic signatures in regulatory documentation, emphasized by their reference five times, highlights their role in ensuring compliance and authenticity in research processes. The Czech Republic's regulations on advertising and ethical codes binding pharmaceutical industry associations further illustrate the necessity of adhering to ethical practices within the industry.
Understanding the Role of Clinical Research Organizations (CROs) in Argentina
Clinical Research Organizations (CROs) in the region, such as bioaccess®, play a pivotal role in facilitating medtech studies by providing Medtech CRO Services Argentina, which include a comprehensive suite of essential services. Their offerings consist of:
- Feasibility studies
- Meticulous site selection
- Compliance reviews
- Setup processes that ensure adherence to local regulations
- Import permits
- Project management
- Robust reporting
The assessment procedure for medical studies in the country usually requires approximately 70 working days, emphasizing the essential demand for effective CRO services.
As pointed out by Julio G. Martinez-Clark, CEO of bioaccess®, 'The demand for medical device research in the nation is backed by the substantial healthcare spending, the occurrence of chronic illnesses, a regulatory framework that requires the demonstration of device safety and effectiveness, and the availability of highly skilled healthcare professionals.' Collaborating with a local CRO like bioaccess®, which offers Medtech CRO Services Argentina, grants researchers invaluable insights into the Argentine market, leverages established relationships with healthcare providers, and provides expert guidance in navigating the complex regulatory landscape, including the challenges posed by varying local regulations. This partnership not only speeds up the trial process but also significantly improves the quality and dependability of results.
Additionally, with regulatory modifications enacted on June 5, 2017, intended to streamline timelines and enhance efficiency, Medtech CRO Services Argentina has become increasingly essential in the changing environment of trials. They employ top-tier integrated services and technology solutions for safety, regulatory compliance, quality assurance, and medical information, reinforcing their position as essential allies in the process of medical investigation. Furthermore, the healthcare system of the nation, which encompasses public, social security, and private sectors, offers a varied array of services and a greater patient base for research studies in comparison to other Latin American countries, thus further promoting research endeavors in private hospitals.
Patient Recruitment Strategies for Successful Trials
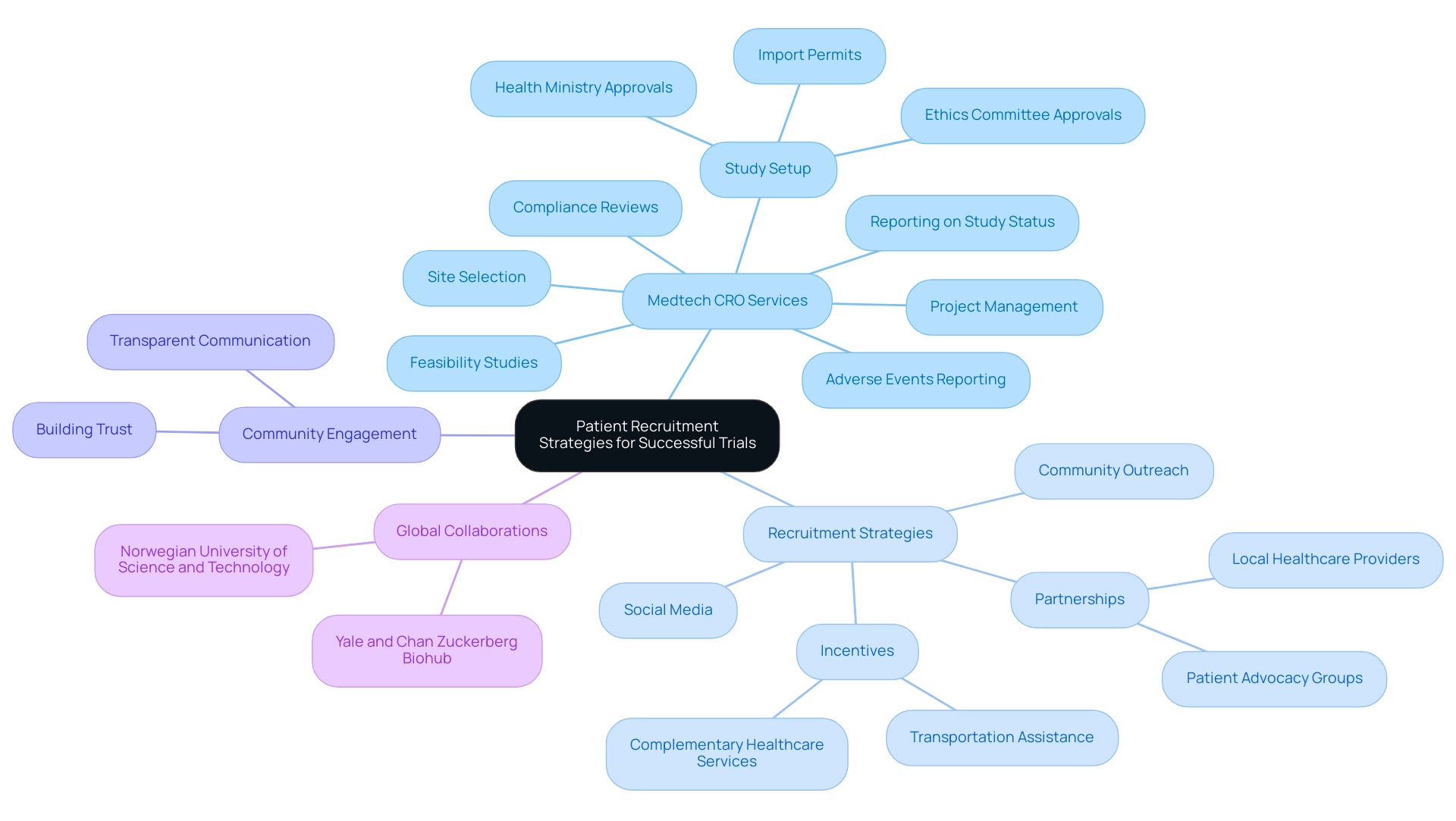
Recruiting participants for research studies in Argentina can be effectively managed through Medtech CRO Services Argentina, requiring a nuanced approach that aligns with the cultural and social dynamics of the local population. With Yale New Haven Hospital's capacity of 1,541 licensed beds, it's clear that effective strategies are essential to overcome recruitment challenges. Our thorough Medtech CRO Services Argentina include research management services that encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup—which involves obtaining necessary approvals from ethics committees and health ministries
- Import permits
- Project management
- Reporting on study status and adverse events
This ensures that all aspects of the research are meticulously handled.
Key strategies for recruitment include:
- Harnessing social media platforms to increase visibility
- Forming partnerships with local healthcare providers to access established trust networks
- Implementing community outreach programs that foster engagement
Collaborating with patient advocacy groups enhances these efforts further by raising awareness and building trust regarding the study's objectives. As Robert A. Lisak emphasizes, training compassionate, patient-centered professionals is crucial in fostering these relationships.
Offering incentives for participation, such as transportation assistance or complementary healthcare services, can significantly motivate potential participants. Moreover, transparent communication about the benefits and safety measures of the trial is vital in encouraging enrollment and ensuring a diverse patient population. This versatile approach not only tackles practical hiring challenges but also corresponds with recent trends in patient engagement, highlighting the significance of relationship-building and community involvement in studies.
Furthermore, our alliances, including Yale's partnership with the Chan Zuckerberg Biohub New York and the Norwegian University of Science and Technology, demonstrate the importance of global cooperation in promoting medical research, which could aid comparable projects in the region and enhance local economic development and healthcare advancements.
Best Practices for Managing Clinical Trials in Argentina
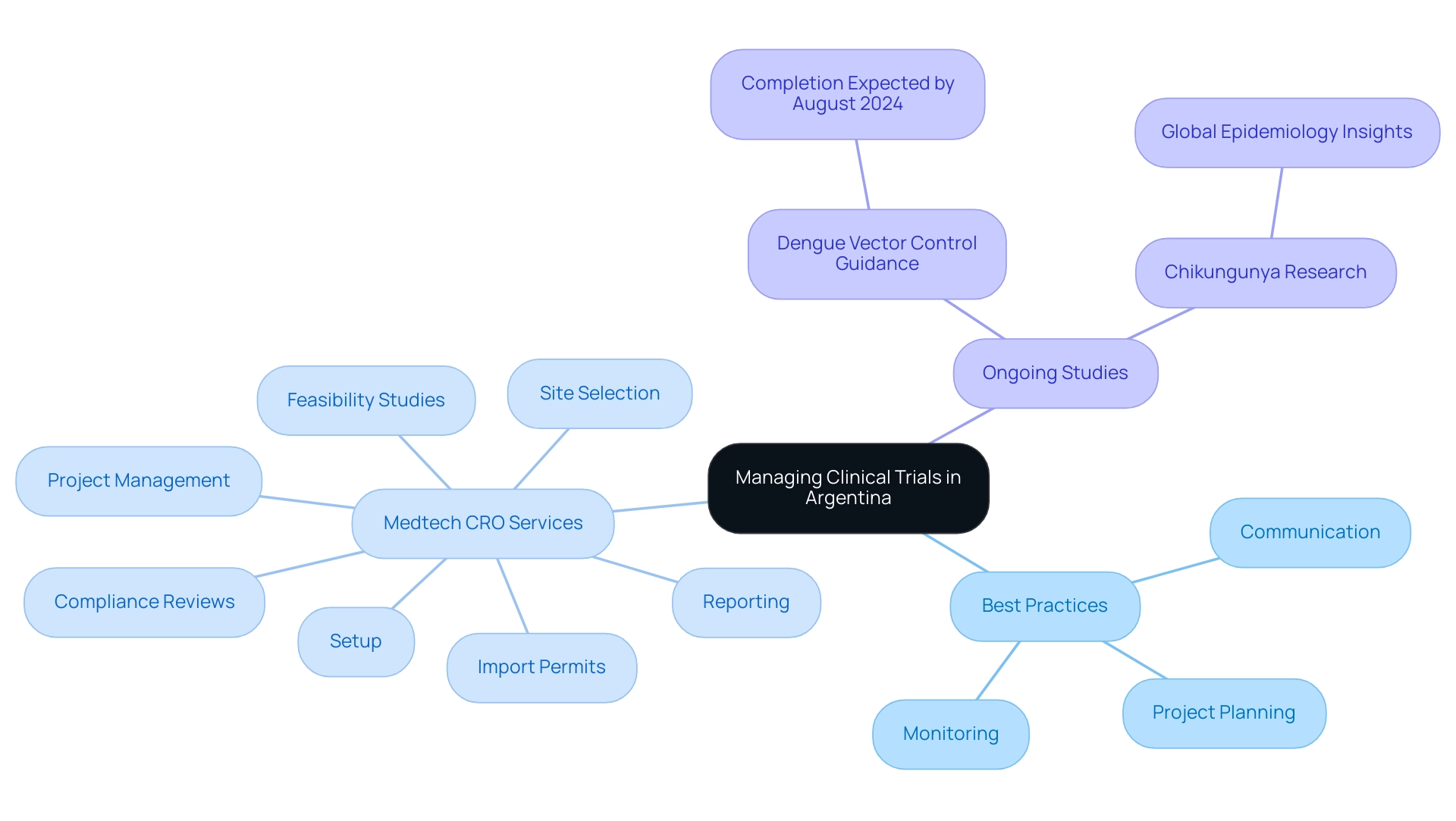
To effectively manage research studies in Argentina, researchers should adopt a comprehensive approach that incorporates Medtech CRO Services Argentina, along with best practices such as:
- Meticulous project planning
- Consistent communication with stakeholders
- Ongoing monitoring of study progress
Notably, bioaccess® provides a suite of comprehensive clinical study management services, including Medtech CRO Services Argentina such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
All tailored to ensure successful outcomes in medical device evaluations. This includes detailed attention to setup and monitoring processes, ensuring adherence to ethical guidelines and local regulations.
Currently, a systematic review for updated dengue vector control guidance is in progress and is expected to be completed by August 2024, highlighting the need for timely project management in relation to ongoing study efforts. Establishing clear timelines and milestones is essential for maintaining project alignment and ensuring timely deliverables. The implementation of advanced project management tools can enhance collaboration among team members, facilitating efficient task completion and information sharing.
As Bettis AA points out, comprehending the worldwide epidemiology of chikungunya is essential for informed decision-making in research studies. Furthermore, it is critical to uphold rigorous documentation standards and adhere to ethical guidelines to maintain the integrity of the research process. The recent release of guidelines for the diagnosis and treatment of Dengue, Chikungunya, and Zika by the Pan American Health Organization highlights the significance of these diseases in management of research.
Educating team members on local regulations and cultural nuances is equally essential, as it fosters a collaborative atmosphere and greatly aids in the success of trials. This comprehensive approach not only improves operational efficiency but also aligns with the latest trends in project management within the healthcare sector, particularly with Medtech CRO Services Argentina, while simultaneously promoting job creation and economic growth in local communities.
Future Trends in Medtech Clinical Research in Argentina
The country is set to become a major participant in the Medtech medical study field, supported by various expected trends in Medtech CRO Services Argentina. A notable increase in investment in digital health technologies, including telemedicine and remote monitoring solutions, is expected to significantly enhance patient engagement and streamline data collection processes. With Phase III success rates increasing to 66%, exceeding the 10-year pre-pandemic average of 56%, the current state of research highlights Argentina's potential.
The incorporation of advanced tools like artificial intelligence and machine learning will further transform research studies, resulting in enhanced data analysis and more informed decision-making. Furthermore, bioaccess® offers extensive Medtech CRO Services Argentina, which include research study management services such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
This ensures that research studies are conducted effectively. Notably, bioaccess® specializes in Early-Feasibility Studies and First-In-Human Studies, which are critical for validating innovative medical devices early in the development process.
There is also a growing focus on patient-centered approaches, aimed at enhancing participant experiences and driving better outcomes. Furthermore, R&D funding levels and deal activity have reset in 2023 after a decline from peak levels in 2020-21, indicating a revitalized investment climate that bioaccess® is well-positioned to capitalize on with its tailored services. The impact of Medtech clinical studies on local economies is significant, creating jobs, promoting healthcare improvement, and fostering international collaboration.
Argentina's active collaboration with international study networks is likely to foster innovation and optimize trial processes, thereby positioning Medtech CRO Services Argentina as a prime choice for future studies. As noted by Murray Aitken, the overall landscape is shifting, with a total of 69 novel active substances launched globally in 2023, marking a 10% increase from the previous year and indicating a robust recovery in research and development efforts.
Conclusion
Argentina stands at the forefront of medtech clinical trials in Latin America, characterized by its robust healthcare infrastructure, favorable regulatory environment, and diverse patient populations. The effective collaboration between local and international entities, along with the pivotal role of Contract Research Organizations (CROs), has facilitated a streamlined process for conducting clinical trials. This synergy not only accelerates innovation but also enhances the quality of research outcomes, positioning Argentina as an attractive destination for medtech companies seeking to navigate complex regulatory landscapes.
Key strategies for patient recruitment and trial management have emerged as essential components of successful clinical studies in Argentina. By leveraging local insights, employing innovative patient engagement techniques, and adhering to ethical standards, researchers can effectively address recruitment challenges while ensuring diverse participant representation. Furthermore, the commitment to integrating advanced technologies and patient-centered approaches signifies a promising shift in the future of clinical research in the region.
As the demand for clinical trials continues to grow, Argentina's landscape presents unique opportunities for researchers and medtech companies. Investing in this burgeoning sector not only contributes to the advancement of medical technology but also fosters economic growth and healthcare improvement within local communities. With a focus on collaboration, innovation, and best practices, Argentina is poised to solidify its status as a leading hub for medtech clinical trials in the years to come.
Frequently Asked Questions
Why is Argentina considered a crucial center for Medtech CRO Services in Latin America?
Argentina has a robust healthcare infrastructure that includes public, social security, and private sectors, regulated by the Ministry of Health. This structure enhances the variety of patient groups for studies and supports medical activities, particularly in private hospitals with larger patient pools compared to other Latin American countries.
What factors are driving the growth of Medtech CRO Services in Argentina?
The ongoing economic growth and rising healthcare expenditures are key drivers behind the enhanced operational capacity of Medtech CRO Services in Argentina. Additionally, partnerships, such as that between Greenlight Guru and bioaccess™, aim to accelerate medtech advancements.
What role does the National Administration of Drugs, Food and Medical Technology (ANMAT) play in Argentina's medtech regulatory framework?
ANMAT oversees the approval of medical devices and research studies in Argentina. Understanding the specific regulatory requirements for submitting research protocols and obtaining ethical approvals is crucial for researchers.
What services do Medtech CROs like bioaccess® provide in Argentina?
Bioaccess® offers a comprehensive suite of services, including feasibility assessments, investigator selection, compliance evaluations, study setup, import permits, project management, and thorough reporting.
What challenges do medical device startups face in Argentina?
Startups often encounter challenges such as navigating complex regulatory requirements, competition from established players, and difficulties in patient recruitment due to limited financial resources.
How do Medtech CRO Services assist in participant recruitment for research studies?
Medtech CRO Services utilize strategies such as leveraging social media, forming partnerships with local healthcare providers, and implementing community outreach programs to effectively recruit participants.
What is the significance of electronic signatures in Argentina's regulatory processes?
Electronic signatures are emphasized in regulatory documentation to ensure compliance and authenticity in research processes, highlighting their importance in the regulatory landscape.
How do recent regulatory changes impact Medtech CRO Services in Argentina?
Favorable regulatory changes enacted on June 5, 2017, aimed at streamlining timelines and enhancing research oversight efficiency, solidifying Argentina's appeal as a location for conducting studies.
What are the benefits of collaborating with local CROs for researchers?
Collaborating with local CROs like bioaccess® provides researchers with insights into the Argentine market, established relationships with healthcare providers, and guidance in navigating complex regulatory landscapes, thereby improving trial processes and results quality.
What trends are expected to influence Medtech studies in Argentina?
An increase in investment in digital health technologies, the use of advanced tools like artificial intelligence, and a focus on patient-centered approaches are expected to enhance patient engagement and streamline data collection processes in Medtech studies.




