Overview
In the realm of medtech trials, best practices for data management are paramount, focusing on the assurance of information quality, the strategic leverage of technology, the promotion of collaboration, and the investment in training for data handling professionals. This article underscores the critical nature of implementing standardized processes, utilizing advanced systems such as Electronic Data Capture (EDC), and fostering open communication among stakeholders. These essential strategies not only enhance the integrity and reliability of clinical data but also lead to improved study outcomes and regulatory compliance. By adopting these practices, organizations can navigate the complexities of clinical research with confidence and authority.
Introduction
In the rapidly evolving landscape of Medtech trials, the significance of effective data management cannot be overstated. As organizations strive to enhance the accuracy and integrity of clinical data, they encounter a myriad of challenges, including:
- Regulatory compliance
- Data volume
- Complexity
With the stakes higher than ever, prioritizing robust data management strategies is essential for ensuring successful trial outcomes and advancing innovative medical devices. This article delves into the critical role of data management in Medtech trials, exploring:
- Best practices
- Technological advancements
- The importance of collaboration among stakeholders
These elements are all aimed at navigating the complexities of clinical research in today's dynamic environment.
The Critical Role of Data Management in Medtech Trials
Information oversight acts as a crucial foundation in Medtech studies, significantly influencing the precision, dependability, and integrity of clinical information. Efficient data management in medtech trials ensures that the information gathered during experiments is meticulously arranged, verified, and prepared for examination. This meticulous approach is essential not only for regulatory compliance but also pivotal for the overall success of the study.
As we look to 2025, the emphasis on robust information handling strategies for data management in medtech trials is more pronounced than ever, reflecting the evolving landscape of clinical trials.
Establishing organized information handling procedures is vital for data management in medtech trials. This enables Medtech firms, such as bioaccess®, to enhance their decision-making capabilities, which in turn leads to improved patient outcomes and a faster time-to-market for innovative medical devices. For instance, a clearly outlined transition plan when migrating to a new Electronic Data Capture (EDC) system can minimize disruptions to ongoing studies, ensuring that information integrity is preserved throughout the process. The case study titled "Establishing a Transition Plan" illustrates how careful planning—including data management in medtech trials, outlining steps, training needs, and risk mitigation strategies—can facilitate a smooth transition and enhance the overall effectiveness of information handling.
Moreover, incorporating industry benchmarks like the Study Data Tabulation Model Implementation Guide (SDTMIG) and Clinical Data Acquisition Standards Harmonization (CDASH) is essential for effective data management in medtech trials, ensuring high-quality information oversight. These standards provide a framework that enhances the consistency and quality of healthcare information, ultimately leading to increased success rates in studies.
The Clinical Information Coordination (CIC) team at bioaccess® plays a vital role in this process, comprising various roles including Information Manager, Database Programmer, Medical Coder, and Quality Control Associate. Each role carries specific responsibilities that ensure effective oversight.
As Chris Rush, a biomedical engineer with 13 years of experience in the medical device field, emphasizes, prioritizing effective data management in medtech trials is crucial for navigating the complexities of research studies. With over 20 years of experience in the Medtech industry, bioaccess® is well-equipped to manage various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). As the Medtech industry continues to expand, the significance of data management in medtech trials cannot be overstated.
It is essential for ensuring that studies not only meet regulatory standards but also achieve their desired outcomes. By prioritizing effective information handling strategies, bioaccess® can navigate the complexities of clinical studies in Latin America with greater confidence and success.
Challenges in Data Management for Medtech Trials
Medtech studies often encounter significant challenges in information handling, particularly regarding data management in medtech trials, which includes issues related to volume, complexity, and adherence to regulatory compliance. The sheer volume of information generated can overwhelm traditional management systems, leading to problems such as inconsistent entry and a lack of standardization. These challenges frequently result in errors that can delay proceedings and jeopardize the effectiveness of data management in medtech trials.
Moreover, the combination of various information sources adds another layer of complexity. As experimental studies increasingly rely on diverse platforms and technologies, effective data management in medtech trials becomes essential for unifying information. A recent examination revealed that 33% of research professionals view AI-driven information discrepancy detection as a critical feature for analytics platforms, highlighting the necessity for advanced solutions to enhance information quality.
bioaccess®, with over 15 years of expertise in the Medtech sector, provides comprehensive clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
- Feedback on study documents
These capabilities streamline testing workflows through intelligent insights and ensure that information integrity is preserved throughout the process.
The evolving regulatory landscape further complicates matters, as Medtech companies must continuously adapt their data management strategies in trials to meet new compliance requirements. This can be resource-intensive, diverting focus from core research activities. bioaccess® excels in navigating these complexities, offering support in trial setup, ethics committee approvals, nationalization of investigational devices, and reporting on study status and adverse events.
A case study on common pitfalls in healthcare information handling revealed that many Medtech companies still rely on outdated methods, such as paper and Excel for information collection, exacerbating these challenges. By avoiding such traps and leveraging bioaccess's expertise, organizations can enhance project oversight, ensure information integrity, and maintain compliance with regulatory standards.
Expert insights underscore the importance of adopting innovative data management strategies in medtech trials to effectively navigate these complexities. As Chris, Solutions Engineer at Greenlight Guru, states, 'When you decide to collaborate with Greenlight Guru Clinical, you’re not only obtaining the top toolkit for MedTech research collection, you’re receiving committed customer support from MedTech experts who comprehend the distinct characteristics of medical device studies.' As the Medtech industry continues to evolve, staying informed about the latest trends and technologies in information handling will be crucial for success in clinical studies.
Navigating Regulatory Compliance in Data Management
Navigating regulatory compliance is crucial for effective information handling in Medtech trials. Adherence to standards established by regulatory bodies such as the FDA and EMA is imperative, as these dictate the protocols for data collection, storage, and reporting. A meticulously structured data management plan in Medtech trials is vital; it should encompass regular audits, rigorous data validation processes, and comprehensive documentation to uphold compliance standards.
Statistics reveal that over 80% of clinical study protocols submitted to ethics committees undergo more than three amendments within a decade, underscoring the dynamic nature of regulatory requirements.
Integrating technology solutions can significantly enhance compliance tracking, thereby streamlining processes and mitigating the risk of non-compliance. For instance, a systematic review on statistical methods for managing noncompliance in randomized studies involving medical devices emphasizes the necessity of employing appropriate statistical techniques to ensure accurate treatment effect estimates. This highlights the importance of addressing noncompliance to maintain the integrity of results.
Moreover, staying updated with the latest FDA and EMA guidelines is imperative for 2025 and beyond. The FDA has recently issued guidance on notifying the agency about manufacturing interruptions, stressing the need for transparency and accountability in clinical trials. By developing a robust information organization strategy that aligns with these regulatory standards, companies can enhance their operational efficiency and facilitate the successful advancement of medical devices through effective data management in Medtech trials.
bioaccess® is committed to ensuring information security and client trust through its grievance and information protection procedures. If you have any queries or concerns regarding the processing of your information, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"). We will address your concerns in accordance with applicable law, ensuring compliance and transparency.
Additionally, bioaccess® provides a pre-validated platform that includes templates and SOPs to aid compliance with stringent regulations, simplifying adherence for Medtech companies. As noted by bioaccess®, "This practice is still very good to follow before you gather any information," which underscores the significance of following best practices in information handling. By leveraging bioaccess®'s expertise and tailored approach, companies can navigate the complexities of regulatory compliance more effectively.
![]()
Leveraging Technology for Effective Data Management
Technology plays a crucial role in transforming data management within medtech trial practices. The integration of Electronic Data Capture (EDC) systems, Clinical Trial Management Systems (CTMS), and advanced analytics platforms significantly enhances data management by streamlining collection processes, improving integrity, and enabling real-time monitoring. By automating information entry and validation, medtech companies can minimize human error, thereby elevating the overall quality of the information collected.
The adoption of cloud-based solutions further revolutionizes data management in medtech trials by providing secure storage and facilitating easy access for all stakeholders involved. This fosters collaboration and enhances transparency throughout the trial process. As the industry evolves, the emphasis on robust medical information gathering becomes increasingly vital, particularly in light of the growing trend towards value-based purchasing in healthcare.
As highlighted in a recent case analysis, producers are now required to furnish significant medical information to validate their device selections, underscoring the necessity for effective information handling strategies. Vivienne van der Walle, Founder and Medical Director, aptly noted, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." This perspective underscores the critical need for effective information handling methods that prioritize patient care.
In 2025, the technological landscape in data management for medtech trials continues to advance, with EDC systems emerging as a cornerstone for efficient clinical information management. These systems facilitate data management by incorporating built-in compliance features, ensuring adherence to regulatory standards while optimizing the information collection process. Notably, solutions such as Greenlight Guru Clinical's eClinical platform provide templates and standard operating procedures (SOPs) that assist with compliance and enhance information collection.
The impact of these technologies is profound, as they not only improve the efficiency of studies but also bolster market access and sales strategies through effective data management in medtech trials, enhancing data quality and reliability. With over 20 years of experience in the medtech field, bioaccess® is well-positioned to leverage these technological advancements to enable successful studies, particularly in overseeing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up assessments. Furthermore, bioaccess® offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance reviews, and project management, specifically tailored for the Latin American market, ensuring accelerated and effective study execution.
Ensuring Data Quality and Integrity in Trials
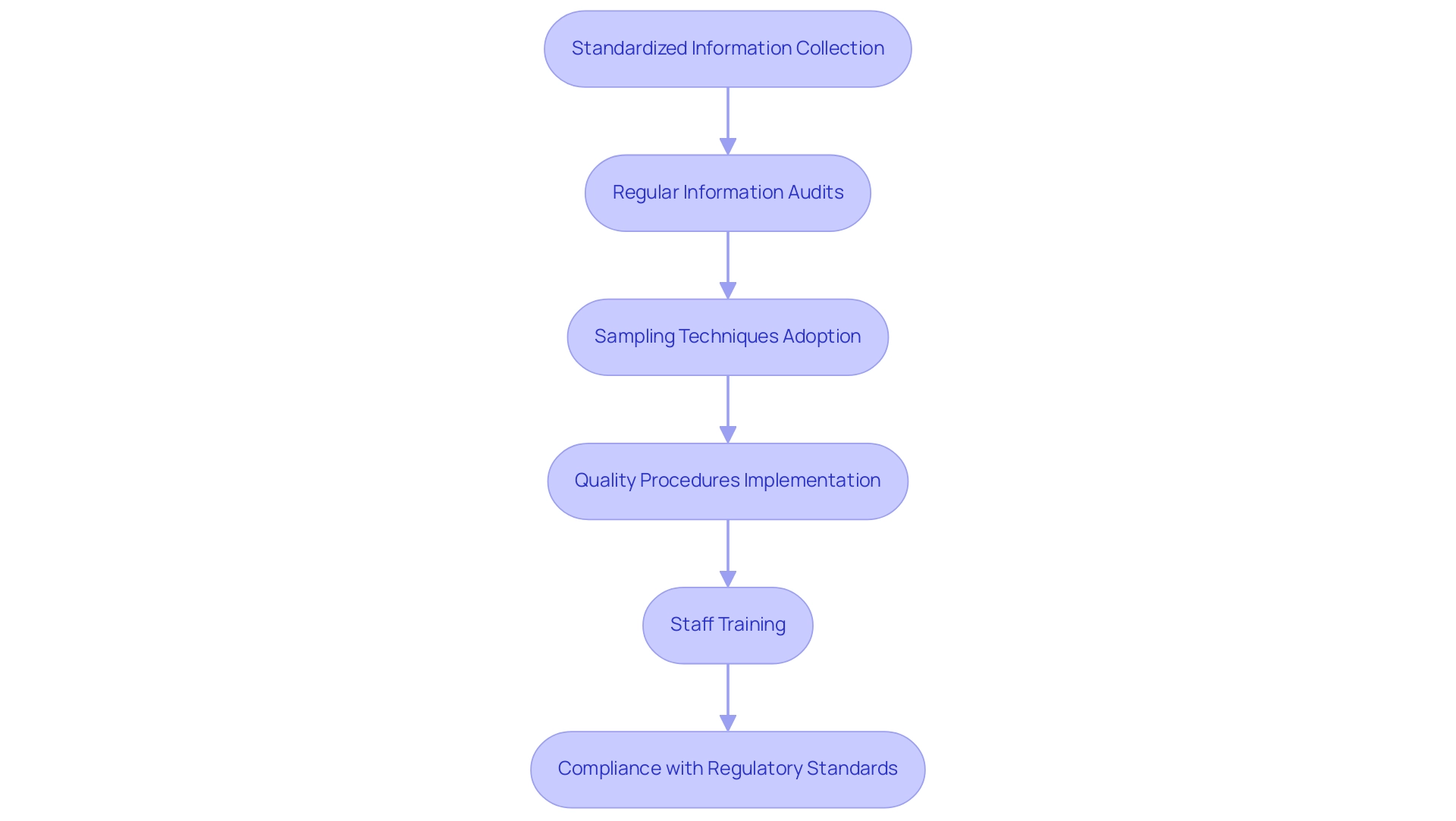
Data management in medtech trials is critical for ensuring information quality and integrity, directly impacting the reliability of trial outcomes. Implementing standardized information collection methods serves as a foundational practice that streamlines processes and reduces variability in data management. Regular information audits, overseen by the Safety Monitoring Board, play a vital role in identifying safety risks and ensuring compliance with regulatory standards.
Recent surveys reveal that:
- 35% of sites have adopted at least one sampling technique for monitoring, underscoring the importance of oversight in maintaining integrity.
- 95% of survey respondents reported implementing procedures to uphold quality, highlighting the industry's commitment to high standards.
- 100% of locations conducted staff training related to quality, emphasizing the significance of training in fostering a culture of accountability.
At bioaccess, our comprehensive clinical trial oversight services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project coordination
- Reporting
We also offer review and feedback on study documents to ensure compliance with country requirements and secure necessary approvals from ethics committees and health ministries. Employing robust information validation methods is essential for identifying mistakes early in the handling process.
Establishing clear protocols for data management in medtech trials mitigates inconsistencies and enhances the overall integrity of the information collected. Educating personnel on optimal data management methods is equally crucial; it promotes a culture of responsibility and emphasizes the importance of information integrity throughout the project lifecycle. As Fatima Zaidi, CEO and Founder, states, "In today’s rapidly evolving business landscape, technology plays an essential role in ensuring data quality and integrity."
Case studies underscore the significance of adhering to regulatory guidelines, such as Good Clinical Practice (GCP), which ensure that studies are conducted with the utmost integrity. By following these standards, organizations can protect participant rights and guarantee that study outcomes are scientifically sound and trustworthy. The case study titled 'Compliance and Best Practices in Clinical Research' highlights the importance of compliance and the role of regular training for experts in maintaining high standards of quality and integrity throughout the project lifecycle.
As the Medtech landscape continues to evolve, effective data management in medtech trials will remain a cornerstone of successful clinical research, ensuring information quality and integrity.
Best Practices for Data Collection in Medtech Trials
Effective data management in medtech trials is paramount, necessitating optimal methods for information gathering to ensure success. The adoption of electronic data capture (EDC) systems significantly streamlines data management, minimizing the risks associated with manual entry errors. Research indicates that data management through EDC systems can enhance accuracy by up to 30%, establishing them as a vital component in modern clinical trials.
As Páll Jóhannesson, Managing Director of Greenlight Guru Clinical, states, "Ever since our inception, we have been focusing on assisting MedTech companies bring safer medical devices to market," highlighting the critical role of data management in medtech trials.
Employing standardized case report forms (CRFs) is another essential strategy, guaranteeing consistency in information collection across multiple sites. This standardization facilitates easier comparison of information and aids in regulatory compliance, which is paramount for effective data management in medtech trials. Bioaccess provides comprehensive clinical trial oversight services, including:
- Feasibility studies that assess site viability
- Site selection to identify optimal research locations
- Compliance reviews to ensure adherence to regulations
- Trial setup for efficient initiation
- Import permits for investigational devices
- Project coordination for streamlined operations
- Reporting to track study progress and outcomes
These services collectively reinforce the significance of compliance in data management.
Effective participant engagement is equally crucial. Clear communication regarding study expectations and comprehensive training on information reporting can significantly enhance the quality of the information collected. A study highlighted that trials with robust participant training programs experienced a 25% increase in quality metrics.
Moreover, regular assessments of collection processes enable timely adjustments, further enhancing both efficiency and precision. The success of a clinical study hinges on effective data management in medtech trials, as underscored in the case study titled 'Final Thoughts on Clinical Information Management,' which emphasizes the necessity of choosing the right tools and partners for clinical information collection. Furthermore, with the oncology sector serving as a central focus in research due to the rising occurrence of cancer, data management in medtech trials becomes even more essential in addressing the need for innovative therapies.
By concentrating on these optimal methods and utilizing the extensive services provided by bioaccess, including thorough reporting that offers insights into study status and adverse events, Medtech companies can ensure that their studies yield dependable and actionable outcomes. This ultimately promotes the progress of innovative medical devices and aids in local economic development and global cooperation.
Enhancing Collaboration for Better Data Management
Improving cooperation among stakeholders is crucial for optimizing data management in medtech trials. By creating clear communication pathways among researchers, information managers, and regulatory bodies, we promote an environment conducive to sharing essential information and insights. Regular meetings and updates are vital for aligning goals and expectations, ensuring that all parties remain synchronized throughout the trial process.
The integration of collaborative tools and platforms significantly streamlines workflows and enhances transparency. For instance, the case study titled "Electronic External Information in Clinical Trials" illustrates the role of integrating electronic external information, such as Patient Reported Outcomes (PRO) and central laboratory results, into Electronic Information Capture (EDC) systems. This approach not only facilitates direct information entry by subjects but also underscores the importance of data management in medtech trials, effectively managing diverse sources and thereby improving integrity.
Statistics reveal that effective communication can dramatically impact clinical trial success; studies indicate that 33% of professionals consider AI-driven discrepancy detection a critical feature for clinical analytics platforms. This emphasizes the necessity for robust communication strategies among stakeholders to address discrepancies promptly and efficiently.
With more than 20 years of experience in the Medtech sector, bioaccess® recognizes the importance of collaboration in information handling. The company’s comprehensive clinical trial oversight services encompass:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This ensures a tailored approach to each trial's unique needs. As mentioned by a guest contributor from Integral, 'In today's information-driven world, the ability to share and collaborate with information is crucial for innovation.'
Expert insights indicate that nurturing a culture of cooperation can result in enhanced information handling results. By emphasizing communication and employing innovative information handling solutions, Medtech companies can improve data management in medtech trials, ultimately speeding up the journey from research to commercialization. Moreover, the partnership between bioaccess® and Caribbean Health Group seeks to establish Barranquilla as a premier location for clinical research in Latin America, backed by Colombia's Minister of Health, which highlights the wider influence of Medtech clinical studies on local economies, including job creation and healthcare enhancement.
Training and Development for Data Management Professionals
Investing in the training and development of information handling experts is essential for upholding the highest standards in Medtech studies. Continuous education that emphasizes regulatory requirements, advanced information handling tools, and industry best practices significantly enhances staff expertise. Encouraging participation in workshops, webinars, and certification programs not only fosters professional growth but also ensures that teams stay informed about the latest industry trends.
A skilled workforce adept at managing the complexities of information oversight is vital for effective data management in Medtech trials, directly correlating to improved research outcomes. Statistics indicate that 1 in 3 employees perceive their organization’s training as outdated, emphasizing the urgent need for ongoing education in this rapidly evolving field. Furthermore, companies incur an average cost of $4,100 when onboarding new hires, highlighting the financial implications of insufficient training.
By harnessing emerging technologies that enhance the personalization and relevance of training programs, organizations can further elevate the effectiveness of their educational initiatives. The case study of bioaccess® exemplifies how robust training and development can yield successful outcomes in the Medtech sector. With extensive clinical study oversight services that encompass feasibility studies, site selection, compliance reviews, input on study documents, setup, import permits, project coordination, and reporting, bioaccess® stands as a leader in Medtech clinical research in Latin America.
By prioritizing continuous learning, organizations can bolster the capabilities of their information handling teams, which is crucial for effective data management in Medtech trials. This focus ultimately leads to more favorable test outcomes and improved patient results.
Key Takeaways for Successful Data Management in Medtech Trials
Data management in medtech trials is essential for efficient information handling to attain trustworthy results and accelerate product development. Key practices that contribute to successful data management include:
-
Prioritizing Information Quality and Integrity: Establishing standardized processes and conducting regular audits are essential to ensure accuracy and compliance with regulatory requirements. This focus on quality not only enhances the credibility of trial results but also fosters trust among stakeholders. Furthermore, gathering patient-reported outcomes (Pros) is crucial for considering the patient's viewpoint in clinical studies, further improving information quality.
-
Leveraging Technology: Utilizing advanced Clinical Information Management Systems (CDMS) can streamline collection and enhance compliance. By automating information input and oversight, Medtech companies can minimize mistakes and improve the effectiveness of their studies.
-
Fostering Collaboration: Promoting open communication among all parties—including healthcare teams, information managers, and regulatory bodies—can significantly enhance transparency and facilitate smoother operational processes. This collaborative approach ensures that everyone is aligned on objectives and methodologies, ultimately leading to better decision-making.
-
Investing in Training and Development: Ongoing education for information handling professionals is essential. By providing them with the newest skills and knowledge, organizations can improve their information handling abilities, ensuring that they are ready to confront the intricacies of contemporary research studies.
Implementing these best practices not only enhances data management in medtech trials but also leads to improved outcomes. For example, a clearly outlined transition plan when implementing a new Electronic Data Capture (EDC) system can reduce risks and ensure that research studies proceed without interruption. As highlighted in a case study titled "Establishing a Transition Plan," careful planning is essential for a smooth transition.
By concentrating on these strategies, Medtech companies can significantly influence their study success rates, ultimately speeding up the path to market for innovative medical devices. With over 20 years of experience in the Medtech sector, bioaccess® is well-positioned to guide organizations in implementing these best practices, particularly in Latin America. bioaccess® provides extensive services such as feasibility studies, site selection, compliance assessments, and project coordination, guaranteeing a customized approach to each study.
As Chris Rush, a biomedical engineer with 13 years in the medical device space, notes, "Effective data management is the backbone of successful clinical trials, ensuring that we meet both regulatory standards and patient needs.
Conclusion
Effective data management is a strategic necessity for the success of Medtech trials, significantly influencing the accuracy and integrity of clinical data. This article underscores the critical importance of robust data management strategies, including best practices, technological advancements, and collaboration among stakeholders. By prioritizing data quality through structured processes, organizations can ensure compliance with regulatory standards and enhance trial outcomes.
The integration of technologies such as Electronic Data Capture (EDC) systems and Clinical Trial Management Systems (CTMS) streamlines data collection and minimizes human error. This allows for real-time monitoring and improved data integrity, which are essential for maintaining the reliability of clinical trials. Furthermore, collaboration among researchers, data managers, and regulatory bodies fosters better communication and transparency, vital components for trial efficiency.
Investing in the training and development of data management professionals is equally vital. Ongoing education equips teams with the necessary skills to navigate the complexities of the Medtech landscape effectively, ensuring that they are well-prepared to meet emerging challenges.
In conclusion, effective data management is a strategic necessity that directly impacts clinical trial success. By implementing best practices and leveraging technological innovations, Medtech companies can ensure reliable results, improve patient outcomes, and accelerate the introduction of innovative medical devices to the market.
Frequently Asked Questions
What is the importance of information oversight in Medtech studies?
Information oversight is crucial in Medtech studies as it influences the precision, dependability, and integrity of clinical information. Efficient data management ensures that the information gathered is meticulously arranged, verified, and prepared for examination, which is essential for regulatory compliance and the overall success of the study.
How does effective data management impact Medtech trials?
Effective data management enhances decision-making capabilities for Medtech firms, leading to improved patient outcomes and a faster time-to-market for innovative medical devices. It helps maintain information integrity during transitions, such as migrating to a new Electronic Data Capture (EDC) system.
What role do industry benchmarks play in data management for Medtech trials?
Industry benchmarks like the Study Data Tabulation Model Implementation Guide (SDTMIG) and Clinical Data Acquisition Standards Harmonization (CDASH) are essential for effective data management, as they provide a framework that enhances the consistency and quality of healthcare information, ultimately increasing success rates in studies.
Who are the key members of the Clinical Information Coordination (CIC) team at bioaccess®?
The CIC team at bioaccess® includes roles such as Information Manager, Database Programmer, Medical Coder, and Quality Control Associate. Each role is responsible for ensuring effective oversight in the data management process.
What types of studies does bioaccess® manage?
bioaccess® manages various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What challenges do Medtech studies face in information handling?
Medtech studies encounter challenges such as the overwhelming volume of information, complexity from diverse data sources, and adherence to regulatory compliance. These challenges can lead to inconsistent data entry and errors that jeopardize the effectiveness of data management.
How can outdated methods affect data management in Medtech trials?
Many Medtech companies still rely on outdated methods, such as paper and Excel for information collection, which can exacerbate challenges in data management, leading to errors and compliance issues.
What strategies can enhance compliance in Medtech trials?
Developing a meticulously structured data management plan that includes regular audits, rigorous data validation, and comprehensive documentation is vital for compliance. Integrating technology solutions can also enhance compliance tracking and streamline processes.
How does bioaccess® support compliance in Medtech studies?
bioaccess® provides a pre-validated platform with templates and Standard Operating Procedures (SOPs) to aid compliance with stringent regulations. They also offer support in trial setup, ethics committee approvals, and reporting on study status and adverse events.
Why is staying updated with regulatory guidelines important for Medtech companies?
Staying updated with the latest FDA and EMA guidelines is crucial for ensuring compliance and operational efficiency. It helps companies navigate the complexities of regulatory requirements and facilitates the successful advancement of medical devices.

