Overview
The article emphasizes best practices for ensuring data quality in Medtech trials, underscoring the critical role of data integrity in producing reliable and safe outcomes. It highlights the importance of:
- Standardized data collection methods
- Regular staff training
- Adoption of technology, such as Electronic Information Capture systems
These elements collectively enhance the accuracy and reliability of clinical data, which are essential for regulatory compliance and successful trial outcomes.
Introduction
In the rapidly evolving landscape of medical technology, the integrity of data collected during clinical trials stands as a critical pillar for ensuring patient safety and regulatory compliance.
As Medtech companies navigate the complexities of clinical research, the quality of data becomes paramount, influencing not only the reliability of study outcomes but also the trust placed in new medical devices by healthcare professionals and patients alike.
With a significant percentage of data quality oversight resting on the shoulders of chief investigators, the importance of leadership and robust data management practices cannot be overstated.
As organizations strive for excellence, embracing technology and collaborative strategies emerges as essential for overcoming the challenges of data quality, ultimately paving the way for successful innovations in healthcare.
The Critical Role of Data Quality in Medtech Trials
Data quality in medtech trials is paramount, as it directly influences the reliability of study outcomes and the safety of participating patients. Ensuring high-quality information is essential for maintaining data quality in medtech trials, leading to valid results that regulatory bodies, healthcare professionals, and patients can trust. Notably, a significant 65% of personnel responsible for reviewing quality reports are chief investigators, underscoring the critical role of leadership in maintaining integrity.
At bioaccess, our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This includes review and feedback on study documents to comply with country requirements and reporting on serious and non-serious adverse events. These capabilities are meticulously designed to enhance information management efficiency and integrity, ensuring compliance with regulatory requirements. Poor data quality in medtech trials can lead to misleading conclusions, potentially resulting in unsafe medical devices entering the market.
A case study highlighting typical pitfalls in healthcare information management reveals that reliance on paper forms and spreadsheets often leads to integrity issues and regulatory non-compliance. By adopting electronic information capture solutions, Medtech companies can significantly enhance data quality in medtech trials, as well as management efficiency, integrity, and compliance with regulatory requirements.
The integrity of medical information transcends mere regulatory compliance; it is essential for ensuring data quality in medtech trials, which demonstrates the safety and effectiveness of medical devices. The selection of information collection tools plays a pivotal role in maintaining this integrity. As Lauren Houston from the University of Wollongong aptly states, "It is essential to guarantee the scientific integrity of research studies to assess information management practices, ensure the precision of results, and minimize mistakes."
As we advance through 2025, the significance of robust information standards for achieving data quality in medtech trials cannot be overstated for any Medtech firm striving for success in research and subsequent commercialization. Best practices for information management should be disseminated widely in the published literature to ensure that all stakeholders are equipped with the necessary knowledge to uphold these standards, thereby reinforcing the regulatory implications of maintaining high integrity.
Challenges to Data Quality in Medtech Clinical Trials
Guaranteeing data quality in medtech trials presents significant challenges that can undermine the validity of research results. A prevalent issue is human error during information entry, which negatively impacts data quality in these trials. Participants have indicated a pressing need for additional education and training on information monitoring procedures, underscoring the significance of meticulous attention to detail.
In addition to human error, data quality in medtech trials is frequently compromised by issues such as inconsistent formats, missing records, and duplicate entries. These challenges are exacerbated by the complex regulatory landscape that Medtech firms must navigate, often leading to compliance difficulties that further jeopardize data integrity. As highlighted in recent discussions among research professionals, there is an urgent need for tailored standard operating procedures (SOPs) that align with the specific contexts of various studies, particularly within academic settings.
Moreover, the lack of clear instructions for overseeing information integrity has been identified as a major barrier. A recent case analysis titled "Challenges in Information Assurance" revealed that numerous medical studies suffer from non-standardized information checks, resulting in a high rate of publication retractions due to inaccurate data. This underscores the necessity for ongoing research to establish a 'gold standard methodology' for ensuring data quality in medtech trials.
To address these challenges, a proactive approach to information management is essential. Conducting regular audits and employing standardized information collection methods can significantly enhance the accuracy and thoroughness of health records. Involving statisticians early in the study design process is crucial; as Scott R. Evans, Ph.D., notes, their participation can help prevent potential issues and develop effective strategies for addressing research questions.
By prioritizing information quality, Medtech firms such as bioaccess can ensure that their clinical studies yield reliable outcomes, ultimately contributing to the advancement of medical technology and positively impacting local economies through job creation, economic growth, and healthcare enhancement.
Regulatory Compliance: A Foundation for Data Integrity
Ensuring regulatory compliance is paramount for maintaining data quality in medtech trials. Adhering to guidelines established by regulatory agencies such as the FDA and EMA not only safeguards patient safety but also fortifies the reliability of the data collected. Compliance necessitates the application of Good Clinical Practice (GCP) standards, which provide a structured framework for recording, managing, and storing information.
By fostering a culture of compliance, organizations can significantly mitigate the risk of discrepancies, ensuring that their findings are both reliable and actionable.
The comprehensive services offered by bioaccess include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are instrumental in navigating the complexities of regulatory compliance. They are specifically designed to tackle the challenges faced by medical device startups in clinical trials, including regulatory hurdles, competition, recruitment issues, and financial constraints.
The impact of GCP standards on information integrity is profound. Research indicates that organizations that rigorously apply these standards experience a marked enhancement in information reliability. For instance, a recent analysis revealed that only a small fraction of studies employed advanced statistical methods to account for compliance, highlighting a gap in methodological rigor that could influence the interpretation of results.
This underscores the necessity for heightened awareness and the adoption of appropriate statistical techniques, such as Complier Average Causal Effect (CACE) analysis, which was utilized in 56% of the reviewed studies. As Páll Jóhannesson, co-founder of Greenlight Guru Clinical, aptly stated, "It is better to be safe than sorry, and to require this documentation up-front instead of only relying on the vendor’s statements."
Moreover, the integration of robust information management practices is essential for maintaining compliance. Tools like Stata facilitate seamless interoperability with various file formats, thereby enhancing the efficiency of import and export processes. This capability is crucial for ensuring that information integrity is upheld throughout the evaluation process, directly supporting adherence to regulatory standards.
In conclusion, the commitment to regulatory compliance and adherence to GCP standards not only preserves the integrity of research data but also enhances data quality in medtech trials. As the landscape of medical research evolves, organizations must prioritize these practices to ensure the reliability of their findings and the safety of participants. The case study titled "Statistical Methods Used to Account for Compliance" further illustrates the gaps in methodological rigor, reinforcing the need for improved statistical methods in compliance analysis.
Reach out to discover how bioaccess can support your research studies.
Leveraging Technology for Enhanced Data Management
Technology is revolutionizing information management in Medtech trials, significantly enhancing data quality and the reliability of clinical information. At the forefront of this transformation are Electronic Information Capture (EIC) systems, which enable real-time information entry and validation, minimizing human error. With approximately 8 billion mobile phone subscriptions worldwide, the availability of these technologies has never been greater, streamlining information collection processes.
Moreover, the incorporation of advanced analytics and machine learning algorithms plays a vital role in identifying anomalies and trends. This proactive approach not only facilitates timely issue resolution but also enhances the overall integrity of the information collected. As the landscape of research studies evolves, the focus on patient-centricity and real-world evidence is expected to further shape data management practices, ensuring they remain essential in advancing medical knowledge and improving patient outcomes.
For instance, case studies have demonstrated that organizations leveraging EDC systems experience significant improvements in data quality and study efficiency. The partnership between bioaccess® and Caribbean Health Group to establish Barranquilla as a prominent research location in Latin America illustrates this trend, supported by Colombia's Minister of Health. Additionally, GlobalCare Clinical Studies' collaboration with bioaccess® has achieved over a 50% reduction in recruitment duration and 95% retention rates, underscoring the effectiveness of comprehensive research management services.
These systems not only assist in meeting regulatory requirements but also enhance data quality in Medtech trials by fostering a collaborative atmosphere among stakeholders, ultimately leading to more successful study outcomes. As Bloss and associates noted, reworking the existing human research protection system is crucial for improving information management practices. Approaching 2025, the role of technology in information management for Medtech studies will continue to expand, providing innovative solutions to tackle the challenges of clinical research.
bioaccess® plays a pivotal role in connecting innovative Medtech firms in Latin America, offering essential support to enhance medical devices through effective information management strategies. This includes regulatory approval, patient recruitment, and timely information delivery. bioaccess® specializes in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, ensuring a customized approach tailored to the unique requirements of each study.
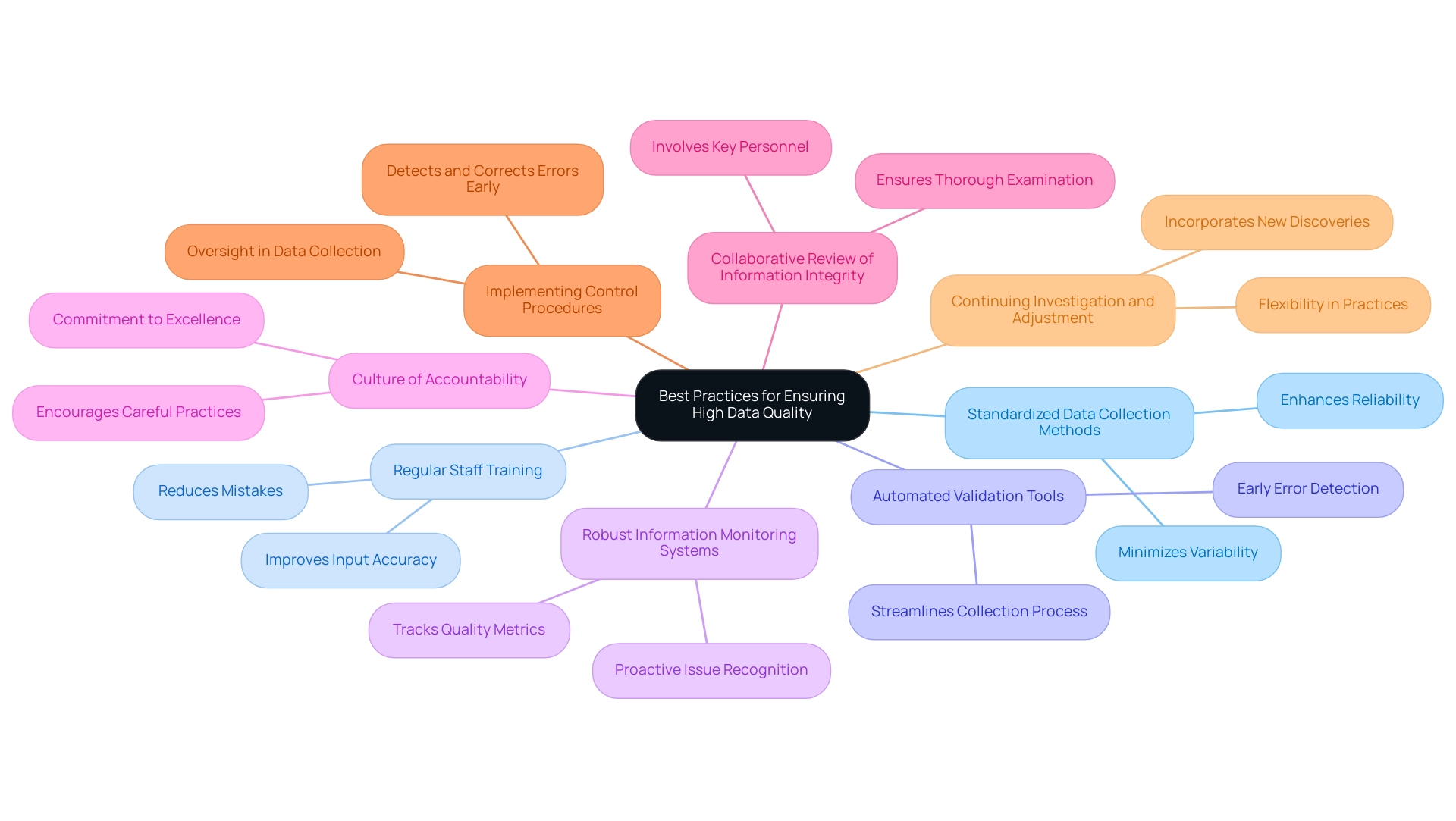
Best Practices for Ensuring High Data Quality
To achieve superior data quality in Medtech trials, organizations must adopt a series of best practices that are essential for success.
-
Standardized Data Collection Methods are paramount. Establishing uniform protocols minimizes variability and ensures consistency across information sets. This approach not only enhances reliability but also facilitates easier comparison and analysis of results.
-
Regular Staff Training is equally critical. Ongoing training on information entry protocols is essential. Studies indicate that effective training significantly enhances input accuracy, thereby minimizing mistakes that could jeopardize study integrity.
-
The implementation of Automated Validation Tools cannot be overlooked. Utilizing automated systems for validation allows for early detection of errors, streamlining the collection process and ensuring that inaccuracies are promptly addressed.
-
Moreover, Robust Information Monitoring Systems must be established. Comprehensive monitoring systems enable organizations to track key quality metrics throughout the trial. This proactive method aids in recognizing potential issues before they escalate, ensuring that information remains reliable and valid.
-
Fostering a Culture of Accountability is vital. When team members acknowledge the significance of information integrity, it leads to more careful practices and a commitment to excellence. Each individual must understand their responsibilities in upholding superior information standards.
-
Collaborative Review of Information Integrity is crucial as well. Individuals accountable for examining information integrity reports, including chief investigators, auditors, information managers, and sponsors, play a pivotal role in maintaining integrity. Their joint efforts ensure that all elements of information integrity are thoroughly addressed.
-
The importance of Implementing Control Procedures is illustrated by the case study titled "Introducing Control Into the Methods of Information Gathering." This highlights the role of oversight in ensuring that information collected during research meets established standards. Efficient control strategies can detect and correct mistakes early in the information gathering process, ensuring that the information remains trustworthy and accurate throughout the study.
-
Finally, Continuing Investigation and Adjustment is essential. The necessity for ongoing examination into standard methods for information monitoring in healthcare studies underscores the continuing importance of this subject. Organizations must remain flexible and receptive to incorporating new discoveries into their information management practices.
By adhering to these best practices, organizations can significantly enhance data quality in Medtech trials, ultimately leading to more dependable outcomes and advancing the development of innovative medical technologies. As Matthew P Smeltzer observed, the integrity of information is paramount in clinical research, underscoring the importance of these practices.
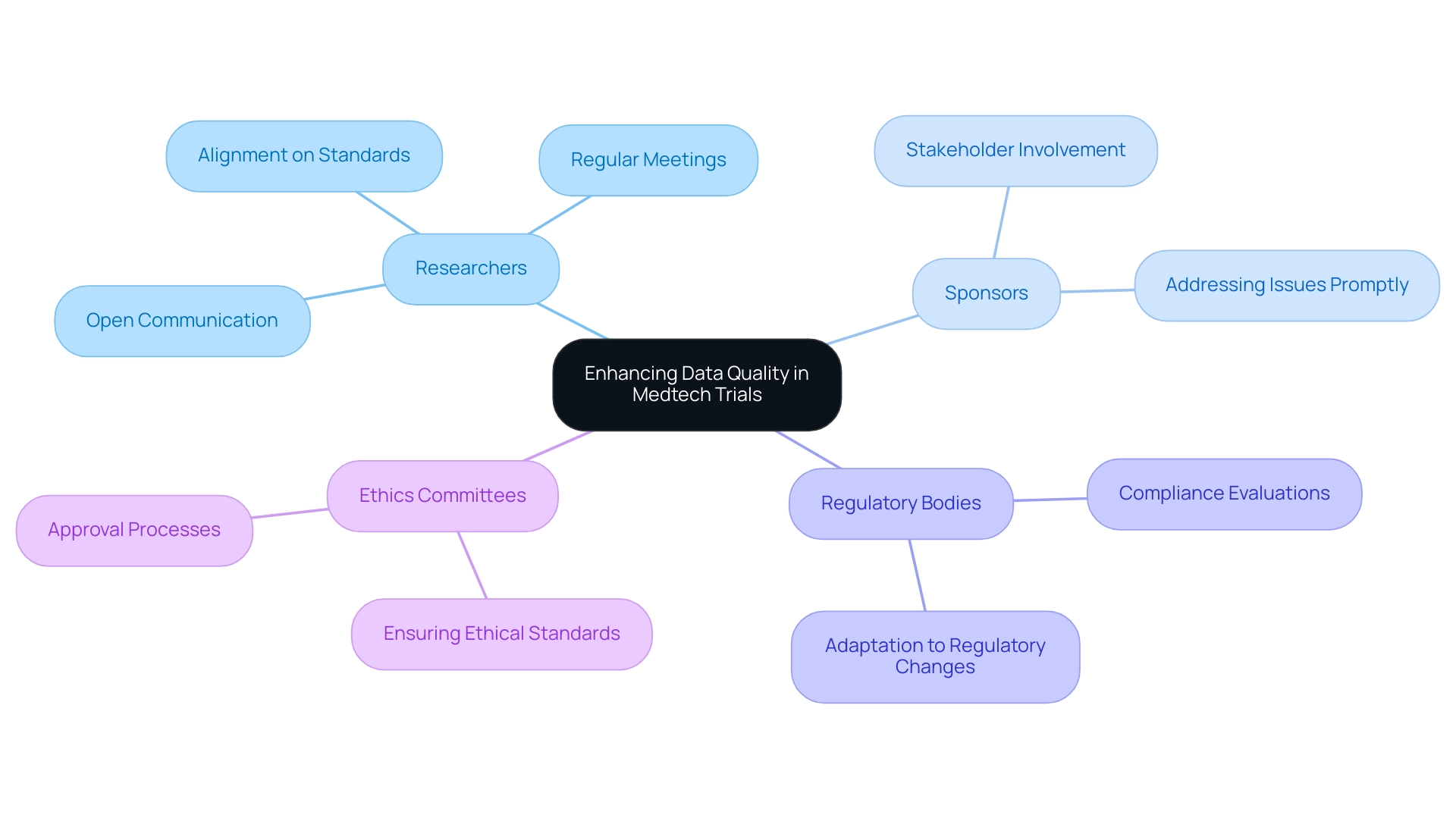
Collaboration Among Stakeholders: Enhancing Data Quality
Cooperation among stakeholders is essential for ensuring data quality in medtech trials. Involving all parties—including researchers, sponsors, regulatory bodies, and ethics committees—ensures alignment on expectations and standards for information quality. Regular meetings and open communication channels facilitate the exchange of insights and best practices, which are crucial for effective data management.
For instance, the partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, aims to establish Barranquilla as a leading location for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative illustrates how systematic control measures can significantly reduce errors, thereby enhancing the reliability and validity of clinical study outcomes.
Furthermore, statistics indicate that organizations with robust stakeholder involvement experience a marked improvement in information standards. Companies that prioritize collaboration are more likely to achieve successful results, as they can swiftly address potential issues and adapt to evolving regulatory requirements. This is particularly relevant in a landscape where data quality in medtech trials is critical; unreliable information can severely impact trial outcomes and the health of subjects involved.
As Miro Kazakoff, a senior lecturer, aptly noted, "In a world of increasing information, the companies with more information-literate individuals are the ones that are going to succeed." This underscores the significance of fostering a culture of communication and teamwork, which not only enhances information integrity but also stimulates innovation in research.
Moreover, the extensive services provided by bioaccess™, including feasibility studies, site selection, compliance evaluations, experiment setup, import permits, project management, and reporting, highlight the importance of ethical standards in ensuring information integrity. By cultivating a cooperative atmosphere, organizations can significantly enhance data quality in medtech trials, leading to improved results and ultimately promoting the advancement of medical technologies. Notably, platforms with strong stakeholder involvement, such as those boasting over 200K followers, demonstrate the potential for elevated information standards and successful outcomes.
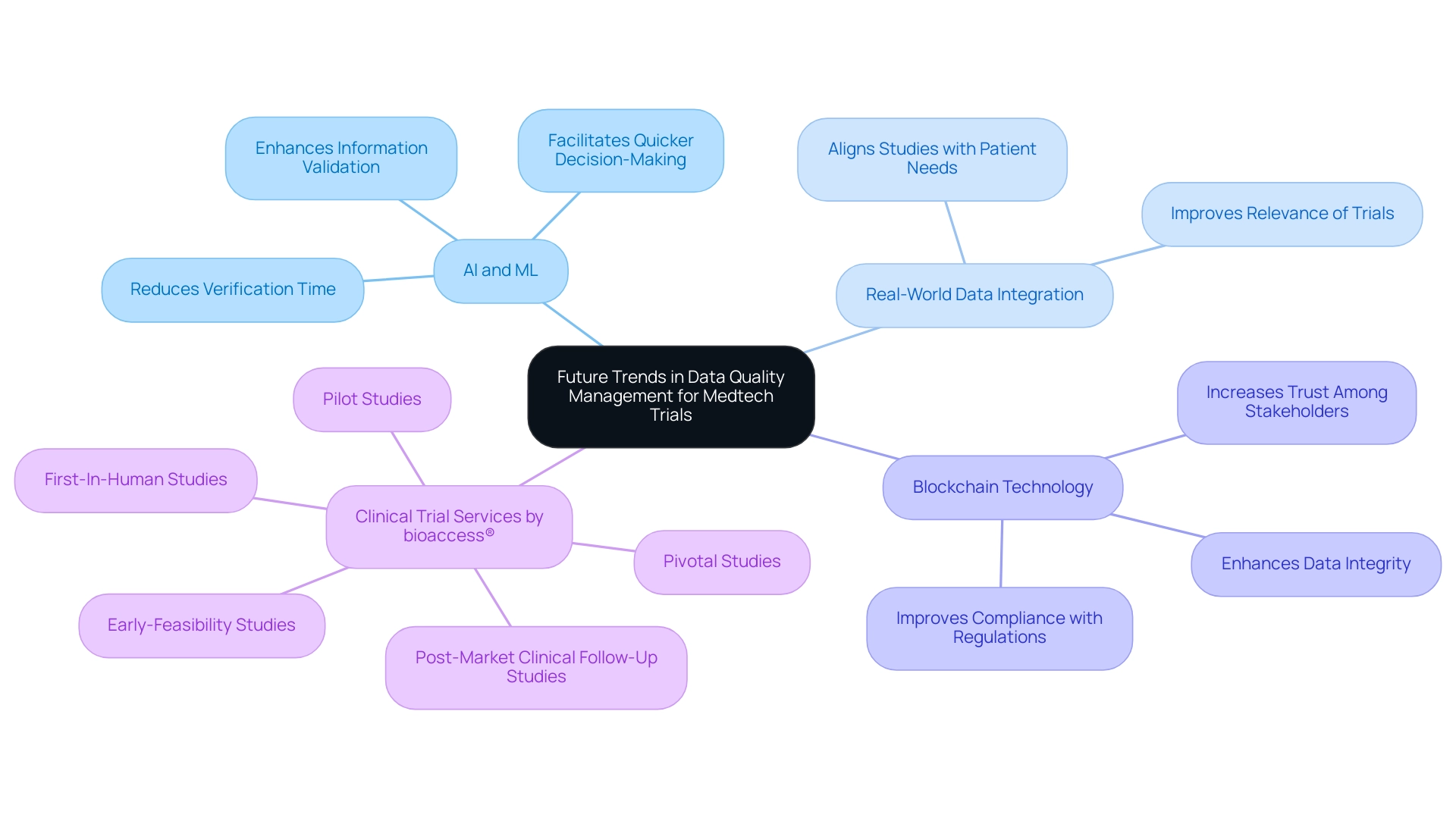
Future Trends in Data Quality Management for Medtech Trials
The landscape of data quality in Medtech trials management is on the brink of significant evolution, particularly in Latin America, where innovative medical solutions are gaining traction. A pivotal trend is the increasing reliance on artificial intelligence (AI) and machine learning (ML) to enhance and automate information validation processes. These technologies not only bolster the precision of information but also significantly reduce the time required for verification, facilitating quicker decision-making in medical studies.
With over 20 years of experience in Medtech, bioaccess® recognizes the critical importance of data quality in clinical trials, particularly under the guidance of Clinical Study Manager Dr. Sergio Alvarado. Dr. Alvarado is committed to leveraging AI for diagnosing medical conditions and improving study outcomes.
Furthermore, the integration of real-world information is becoming essential for enhancing the relevance of trials. By incorporating data that reflects actual patient experiences and outcomes, Medtech companies can ensure their studies are more aligned with the needs of healthcare providers and patients alike.
Blockchain technology is emerging as a transformative force in information quality management. Its ability to provide secure and transparent information sharing fosters trust among stakeholders, a crucial element in clinical research. As organizations increasingly recognize the importance of information integrity, adopting blockchain can significantly enhance data quality in Medtech trials by mitigating risks associated with tampering and improving compliance with regulatory standards.
Looking ahead to 2025, the market for information monetization is projected to reach $15.5 billion, with an annual growth rate of 20%. This trend underscores the growing recognition of information as a valuable asset within the Medtech sector. A recent case study illustrates that information has transitioned from being merely a business tool to becoming a product in its own right, reflecting a shift in how organizations perceive and utilize this resource.
However, it is crucial to note that a substantial 67% of organizations currently report a lack of trust in their information for decision-making, which can impede the success of AI initiatives. This highlights the urgent need for robust governance and organizational commitment to ensure data quality in Medtech trials. As Lior Solomon, VP of Data at Drata, asserts, "Information is the lifeblood of all AI — without secure, compliant, and reliable information, enterprise AI initiatives will fail before they get off the ground."
In conclusion, the future of information management in Medtech studies will be characterized by the strategic application of AI and ML, the incorporation of real-world information, and the assurance of data quality in Medtech trials through blockchain technology. These advancements, supported by the expertise of bioaccess® and Dr. Sergio Alvarado, will not only enhance data quality and integrity in Medtech trials but also enable companies to conduct more efficient and effective research studies. Additionally, bioaccess® provides a comprehensive range of clinical trial services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
ensuring robust support for Medtech innovations.
Conclusion
The significance of data quality in Medtech clinical trials is paramount. High-quality data is critical for ensuring patient safety, regulatory compliance, and the overall reliability of study outcomes. With chief investigators tasked with data oversight, strong leadership and rigorous data management practices emerge as essential components for success. The adoption of electronic data capture technologies and advanced analytical tools significantly enhances the integrity and efficiency of data collection, minimizing human error and ensuring adherence to regulatory standards.
Furthermore, collaboration among stakeholders—including researchers, sponsors, and regulatory bodies—cultivates a culture of accountability and shared best practices, ultimately leading to enhanced data quality. As organizations navigate the complexities of clinical trials, the implementation of standardized data collection methods, ongoing training, and robust monitoring systems will be vital in preserving high data integrity.
Looking forward, the integration of artificial intelligence and blockchain technology is poised to revolutionize data management in Medtech trials. These innovations will not only streamline processes but also bolster trust and transparency among stakeholders. As the landscape of clinical research continues to evolve, prioritizing data quality remains crucial for advancing medical technologies and improving patient outcomes. Ultimately, a steadfast commitment to excellence in data management will pave the way for successful innovations in the healthcare sector, ensuring that new medical devices are both safe and effective for patients.
Frequently Asked Questions
Why is data quality important in medtech trials?
Data quality is paramount in medtech trials as it directly influences the reliability of study outcomes and the safety of participating patients. High-quality information is essential for valid results that can be trusted by regulatory bodies, healthcare professionals, and patients.
What are the key services offered by bioaccess for clinical trial management?
Bioaccess offers comprehensive clinical trial management services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What are the consequences of poor data quality in medtech trials?
Poor data quality can lead to misleading conclusions and potentially result in unsafe medical devices entering the market.
What common issues affect data quality in medtech trials?
Common issues include human error during information entry, inconsistent formats, missing records, and duplicate entries, which can undermine data integrity.
What is the significance of leadership in maintaining data quality?
A significant 65% of personnel responsible for reviewing quality reports are chief investigators, highlighting the critical role of leadership in maintaining data integrity.
How can electronic information capture solutions improve data quality in medtech trials?
By adopting electronic information capture solutions, Medtech companies can enhance data quality, management efficiency, integrity, and compliance with regulatory requirements.
What role do standard operating procedures (SOPs) play in data quality?
Tailored SOPs that align with specific study contexts are essential for addressing challenges related to data quality and ensuring compliance with regulatory standards.
What proactive measures can be taken to enhance information management in medtech trials?
Regular audits, standardized information collection methods, and involving statisticians early in the study design process can significantly improve the accuracy and thoroughness of health records.
How does ensuring data quality in medtech trials contribute to broader economic impacts?
Prioritizing information quality in clinical studies can yield reliable outcomes that contribute to advancements in medical technology, job creation, economic growth, and enhanced healthcare.

