Overview
Best practices for device trial compliance strategies necessitate a multifaceted approach that prioritizes participant safety, data integrity, and adherence to regulatory standards. Effective communication, continuous training, and technological advancements are paramount in fostering a culture of compliance. This, in turn, significantly enhances the success of clinical research within the medical device sector. By addressing these key elements, stakeholders can navigate the complexities of the Medtech landscape more effectively, ensuring that trials are conducted with the utmost integrity and efficacy.
Introduction
In the intricate realm of clinical research, compliance emerges as a pivotal element that guarantees the integrity and success of medical device trials. As the landscape evolves—driven by regulatory changes and technological advancements—the emphasis on adherence to ethical guidelines and protocols is more critical than ever.
With a surge in participant enrollment and a heightened focus on patient experience, organizations are re-evaluating their compliance strategies to foster trust and enhance trial outcomes. This article delves into the multifaceted dimensions of compliance, exploring the challenges faced, the role of technology in streamlining processes, and the vital importance of training and continuous improvement in navigating the complexities of clinical trials.
As the industry progresses, understanding and implementing effective compliance measures will be essential for the successful advancement of medical devices and the overall integrity of clinical research.
Understanding Compliance in Medical Device Trials
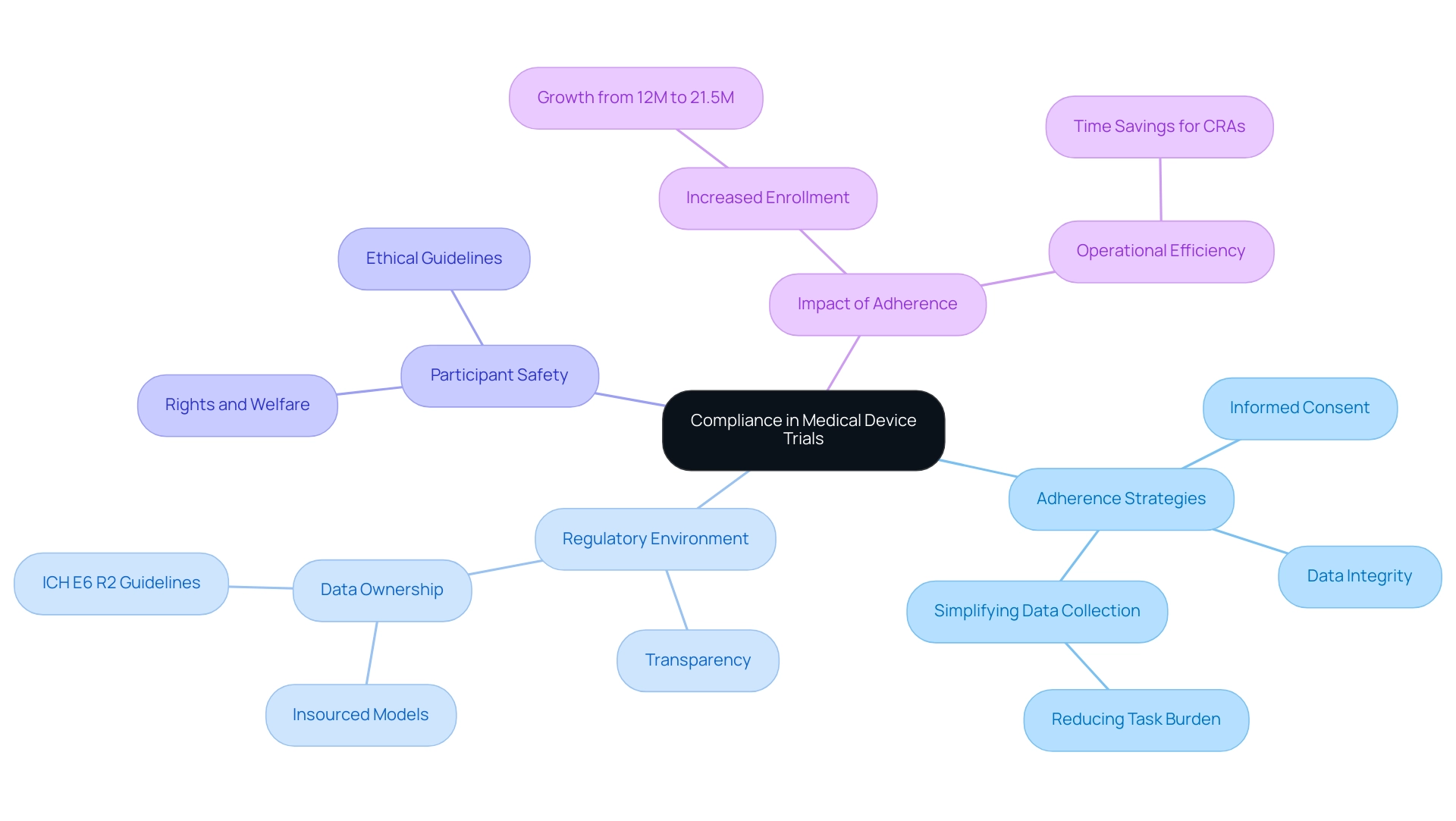
Adherence in medical device studies is a cornerstone of successful clinical research, relying heavily on effective device trial compliance strategies that encompass standards, protocols, and ethical guidelines. This multifaceted approach includes critical elements such as informed consent, data integrity, and participant safety. The importance of adherence extends beyond simple regulatory observance; it is vital for protecting the rights and welfare of participants, thereby promoting trust among stakeholders, including regulatory agencies and the participants themselves.
In 2025, the regulatory environment in medical device studies is increasingly shaped by the demand for transparency and data ownership. Recent trends highlight that sponsors are shifting towards insourced models for data management, driven by the ICH E6 R2 guidelines. This transition not only enhances operational efficiency but also improves the quality of results delivered to patients.
A case study exemplifying this shift demonstrates how increased data ownership leads to improved adherence outcomes, ultimately benefiting both sponsors and participants. Current statistics reveal a notable increase in adherence rates, with the individual market growing from 12 million enrolled participants in 2021 to 21.5 million in 2024. This surge underscores the growing focus on patient experience in study design, as data leaders prioritize simplifying data gathering processes to lessen the burden on participants. Expert opinions indicate that removing unnecessary tasks during visits can significantly enhance adherence; for instance, eliminating a single 20-minute task across 130,000 visits can save 43,000 hours of work, enabling Clinical Research Associates (CRAs) to concentrate on what truly matters.
At bioaccess, our extensive trial management services encompass feasibility studies, site selection, adherence reviews, trial setup, import permits, project management, and reporting. These services are designed to address the challenges faced by medical device startups, such as regulatory hurdles, competition, recruitment issues, and financial constraints. The significance of adherence in medical studies cannot be overstated.
It serves as the foundation for developing effective device trial compliance strategies throughout the testing process. By grasping and applying essential regulatory components, including the assessment and input on research documents to meet national standards, as well as the significance of reporting on study progress and adverse occurrences, researchers can navigate the intricacies of medical studies more efficiently, ensuring not only adherence to regulations but also the ethical treatment of participants. As the field progresses, the development of medical devices will increasingly depend on effective device trial compliance strategies to ensure adherence and the overall success of research programs.
Key Factors Influencing Compliance in Clinical Trials
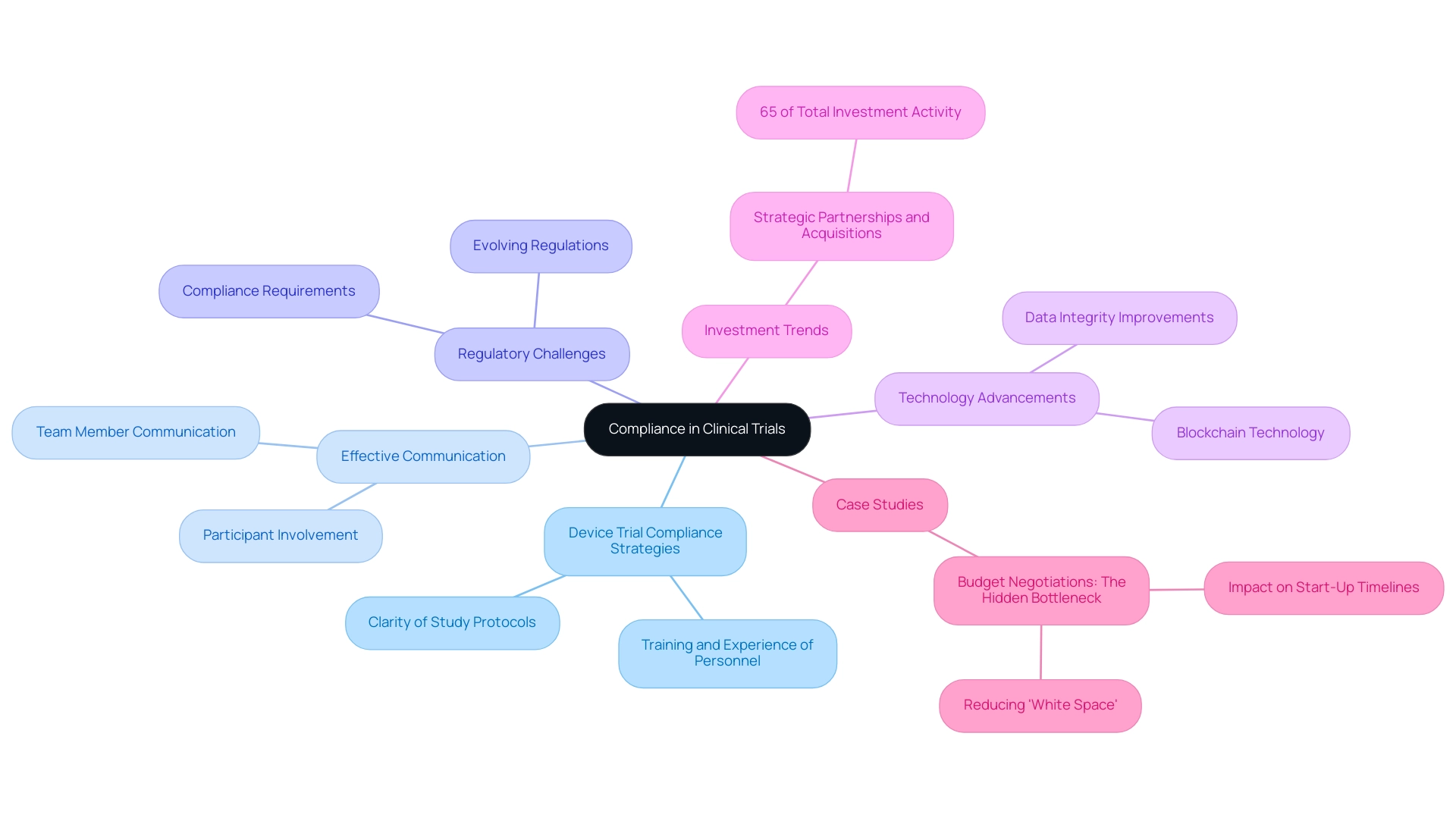
Adherence in medical studies is significantly influenced by device trial compliance strategies, which are shaped by factors such as the clarity of study protocols, the training and experience of research personnel, and the degree of participant involvement. A pivotal element in fostering adherence is effective communication among team members and participants, ensuring that all parties fully comprehend their roles and responsibilities. In 2023, strategic partnerships and acquisitions among pharmaceutical companies, CROs, and technology firms accounted for 65% of total investment activity in the research market, underscoring the industry's commitment to enhancing communication and collaboration to expedite drug development.
bioaccess™ offers a comprehensive suite of clinical study management services, which includes:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
These services are tailored to tackle the challenges faced by medical device startups, such as regulatory hurdles, competition, recruitment issues, and financial constraints. Furthermore, the implementation of blockchain technology has emerged as a notable advancement, facilitating secure and transparent data sharing among trial stakeholders, thereby improving data integrity and trial efficiency.
The complexity of regulatory requirements can also pose significant challenges. Staying informed about evolving regulations is crucial for maintaining standards. Regular training sessions and updates provided by bioaccess™ not only enhance staff knowledge but also bolster device trial compliance strategies, ultimately leading to more successful outcomes.
For instance, a case study titled "Budget Negotiations: The Hidden Bottleneck" illustrates that inefficient budget negotiations can prolong timelines due to unproductive waiting periods. By minimizing 'white space' in negotiations through effective communication and planning, organizations can substantially shorten start-up timelines and enhance overall trial efficiency. As noted by a Senior Director within a global biopharma’s clinical sciences and study management group, "Eliminating one 20-minute task per visit across 130,000 visits avoids 43,000 hours of work. CRAs can focus on what matters."
Moreover, the significance of training on adherence cannot be overstated. Research indicates that well-trained personnel are more adept at navigating intricate protocols and regulatory environments, which directly correlates with the effectiveness of device trial compliance strategies. In 2025, data reveal that the remaining research studies classified as 'Other' constituted 16% of the total conducted, emphasizing the need for targeted strategies to enhance adherence across various study types.
Expert opinions highlight that device trial compliance strategies necessitate clarity in protocols; when protocols are well-defined, compliance improves, yielding more reliable and valid results.
Navigating Regulatory Requirements for Clinical Trials
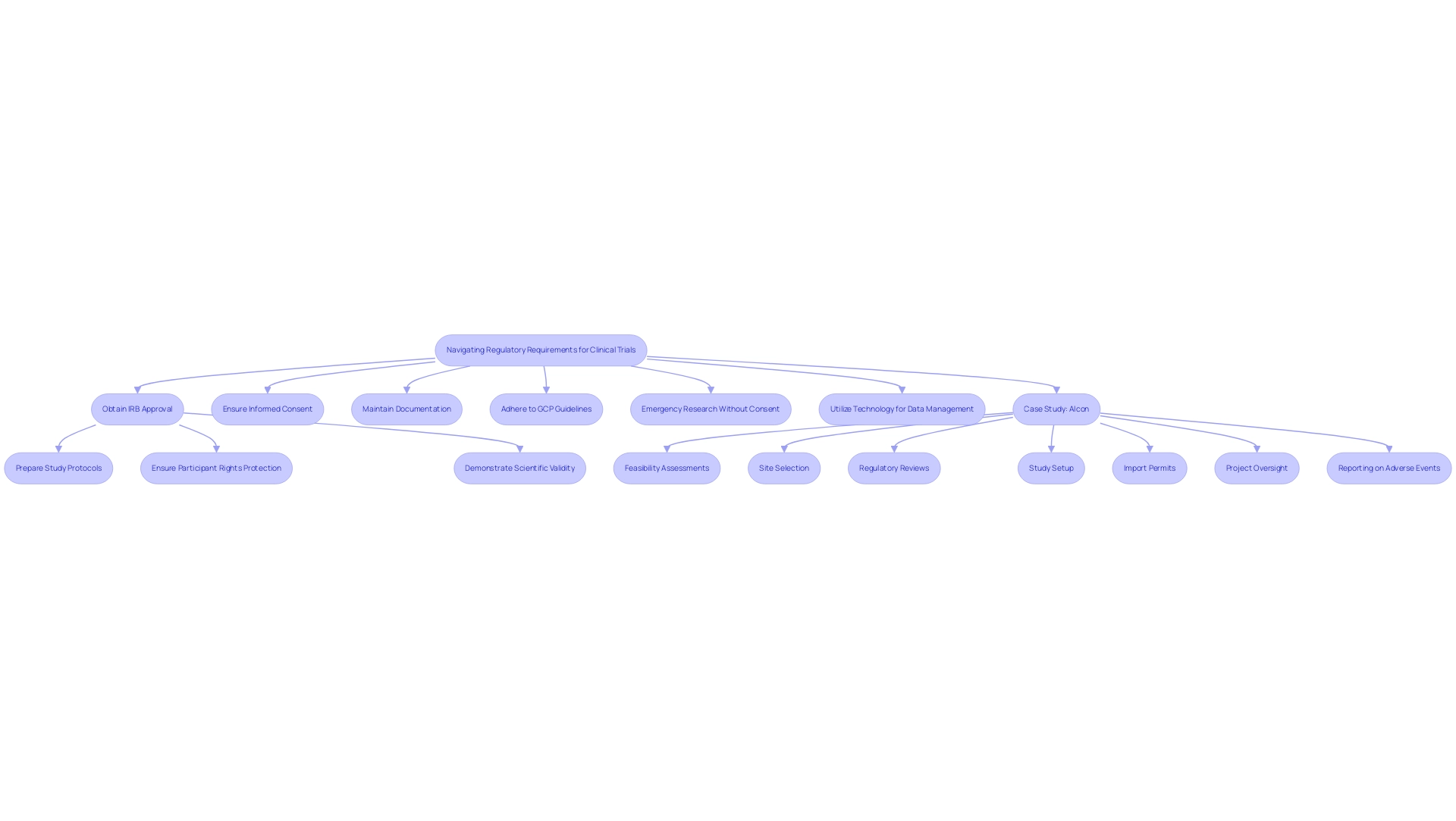
Navigating the regulatory landscape for clinical studies necessitates a comprehensive understanding of the requirements established by regulatory bodies such as the FDA and EMA. Essential steps in this process include:
- Obtaining Institutional Review Board (IRB) approval
- Ensuring informed consent from participants
- Maintaining meticulous documentation throughout the study
Adherence to Good Clinical Practice (GCP) guidelines is crucial, as these standards uphold the ethical and scientific quality of studies.
In 2025, the FDA and EMA updated their regulations to streamline the approval process, reflecting a growing emphasis on patient safety and data integrity. For instance, sponsors can now ship investigational products as soon as 30 days after the FDA receives the Investigational New Drug (IND) application, provided they meet specific criteria. This alteration seeks to accelerate the initiation of research studies while guaranteeing adherence to safety standards, thus improving patient safety.
Effective navigation of compliance requirements is further demonstrated by case studies like the one conducted by Alcon, which aimed to enhance site experience in clinical studies through device trial compliance strategies. By simplifying data management and improving data entry processes, Alcon reduced the time burden on sites, ultimately leading to improved patient care and more efficient data collection. This situation underscores the practical effects of adhering to regulations in enhancing the overall examination experience.
Moreover, understanding the nuances of IRB approval processes is vital for researchers. Key steps include:
- Preparing comprehensive study protocols
- Ensuring that all participant rights are protected
- Demonstrating the scientific validity of the research
Statistics indicate that trials adhering to these protocols experience higher rates of IRB approval, which is essential for upholding FDA and EMA regulations.
Furthermore, it is crucial to acknowledge that emergency research may proceed without consent in life-threatening situations, highlighting the flexibility that exists in critical scenarios. Additionally, the NIH adheres to the Privacy Act to safeguard records accessed by personal identifiers, emphasizing the significance of upholding participant confidentiality and meeting legal obligations.
By prioritizing device trial compliance strategies, researchers can enhance the credibility of their studies and foster smoother interactions with regulatory authorities, ultimately contributing to the successful advancement of medical devices in the research environment. Bioaccess provides extensive research study management services, including:
- Feasibility assessments
- Site selection
- Regulatory reviews
- Study setup
- Import permits
- Project oversight
- Reporting on serious and non-serious adverse events
These services are crucial for navigating these complexities. As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We did a small AI initiative to see if we can generate meaningful test data for setting up and validating our systems. It turns out we can." This innovative approach emphasizes the potential for utilizing technology to enhance clinical study processes.
Challenges in Ensuring Compliance in Device Trials
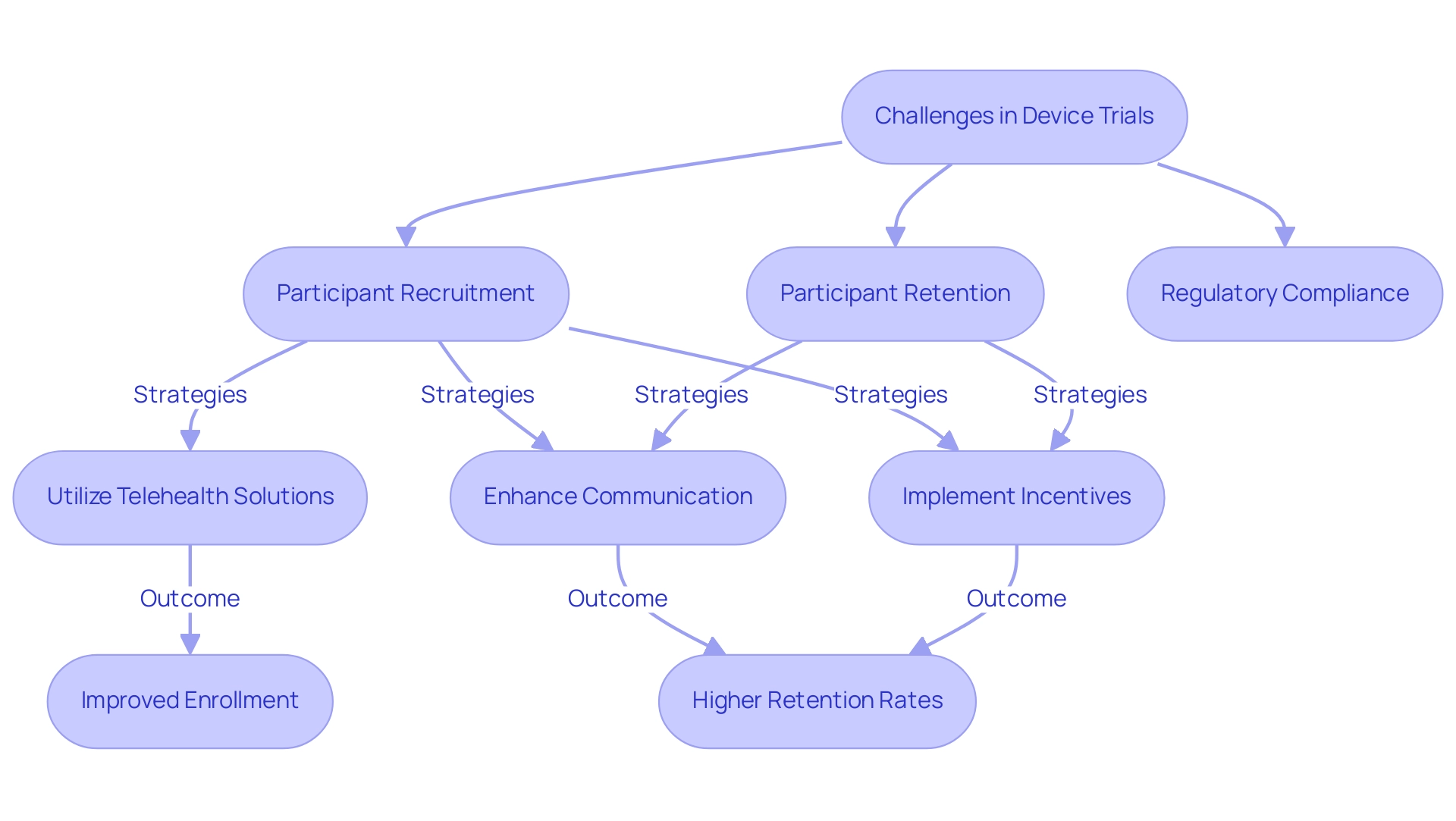
Ensuring compliance in medical device studies is paramount, necessitating effective device trial compliance strategies to navigate a landscape fraught with challenges, particularly in participant recruitment, retention, and adherence to regulatory requirements. Recent statistics reveal that many studies encounter significant hurdles, with enrollment rates frequently falling short of expectations—compromising the integrity of study results. As we approach 2025, dropout rates in medical device studies remain a pressing concern; disengagement can lead to incomplete data and skewed outcomes.
With over 15 years of experience in the Medtech sector, bioaccess® possesses an intimate understanding of these challenges.
To enhance compliance, researchers must implement effective device trial compliance strategies that not only attract participants but also sustain their engagement throughout the study process. Logistical obstacles—including the location of research studies, travel expenses, and time commitments—disproportionately affect individuals from lower-income backgrounds, complicating recruitment efforts. A study on diversity in medical research recruitment highlights that minorities and individuals of color are historically under-represented, underscoring the urgent need for innovative approaches to broaden participation.
This study identifies logistical issues as a primary concern for under-represented groups, indicating that addressing these barriers is essential.
Utilizing technology to reduce site visits has emerged as a promising strategy to improve enrollment among diverse populations. By leveraging telehealth solutions and remote monitoring, researchers can alleviate some logistical burdens that deter potential participants. This method not only enhances accessibility but also fosters a more inclusive environment for medical studies.
Moreover, maintaining participant engagement is crucial for the successful implementation of device trial compliance strategies. As Gueddar observes, "As medical studies expand in scale and location, across various nations, numerous languages, and currencies, sponsors and sites will aim to enhance payment processes that can manage this increasing complexity." Successful retention strategies encompass regular communication, personalized follow-ups, and incentives that resonate with participants' needs.
In partnership with Caribbean Health Group, bioaccess™ is committed to establishing Barranquilla as a premier location for medical studies in Latin America, with support from Colombia's Minister of Health. This collaboration aims to enhance ambulatory services for research studies, achieving over a 50% reduction in recruitment time and 95% retention rates. As the landscape of medical research continues to evolve, effectively addressing these challenges through robust device trial compliance strategies will be essential for ensuring the success and integrity of medical device studies.
Effective Strategies for Patient Recruitment and Retention
To enhance patient recruitment and retention in research studies, investigators must implement focused outreach techniques that effectively involve potential participants. Utilizing social media platforms has proven to be a powerful tool, with studies indicating that campaigns on these platforms can significantly enhance recruitment rates. Community engagement initiatives, such as informational sessions and collaborations with local healthcare providers, further raise awareness about the study and its benefits, fostering trust and interest among potential participants.
In the context of Barranquilla, Colombia, bioaccess™ and Caribbean Health Group's collaboration aims to position the city as a leading destination for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances the visibility of clinical studies but also contributes to local economic growth through job creation and improved healthcare services.
Providing clear, comprehensive information regarding the study's objectives, procedures, and potential benefits is essential in alleviating concerns and encouraging participation. Regular communication with participants throughout the study not only keeps them informed but also cultivates a sense of involvement and commitment, which is crucial for improving retention rates and implementing device trial compliance strategies. Consistent monitoring and assessment of recruitment objectives are essential for research success, as they enable investigators to modify strategies as required.
A notable case study involves GlobalCare Clinical Trials' partnership with bioaccess™, which achieved over a 50% reduction in recruitment time and 95% retention rates. This collaboration underscores the importance of adaptability and proactive communication in achieving recruitment goals, demonstrating how their efforts led to improved outcomes.
In 2025, expert opinions emphasize that continuous monitoring of recruitment progress and the ability to pivot tactics swiftly are vital for meeting enrollment targets. Ursula Türcke, Senior Director of Clinical Operations at FGK Clinical Research GmbH, emphasized the importance of effective recruitment strategies, stating, "AstraZeneca's 'no cure – no pay' policy highlights the critical nature of successful patient enrollment in research studies." By incorporating device trial compliance strategies, researchers can significantly improve patient recruitment and retention, ultimately leading to more successful studies.
Moreover, bioaccess™ provides extensive research study management services, including feasibility assessments, site selection, regulatory reviews, study setup, import permits, project management, and reporting. These services are vital for ensuring that medical studies are carried out effectively and in accordance with local regulations, further enhancing the economic influence of Medtech research in Barranquilla.
Leveraging Technology for Enhanced Compliance
Utilizing technology in clinical studies is essential for enhancing adherence through device trial compliance strategies that automate data gathering, observation, and reporting procedures. Electronic data capture (EDC) systems exemplify this advancement by streamlining data management and significantly reducing the risk of errors that often accompany manual data entry. The implementation of mobile applications further enhances participant engagement by offering features such as appointment reminders and medication adherence notifications.
Moreover, robust data management systems facilitate real-time monitoring of regulatory metrics, which support device trial compliance strategies and empower researchers to swiftly identify and address potential issues. This proactive approach not only mitigates risks but also aligns with the growing trend of integrating diverse data sources to simplify the experience for both sites and patients. As Bree Burks, Vice President of Site Strategy at Veeva, observes, "As sponsors reconsider their site engagement strategies in 2025, they will emphasize device trial compliance strategies and standardization across sponsors for all studies."
This insight underscores the significance of technology in developing future device trial compliance strategies. Alongside technological progress, thorough management services for studies, like those offered by bioaccess, are vital in securing successful results. These services encompass:
- Feasibility studies
- Site selection
- Regulatory reviews
- Setup
- Import permits
- Project management
- Reporting
All crucial for navigating the intricacies of research studies.
Furthermore, a recent case study highlights that 61% of corporate risk and regulatory professionals have identified staying updated on regulatory changes as a top strategic priority, emphasizing the importance of device trial compliance strategies in managing the dynamic nature of regulations. Additionally, McKinsey predicts that predictive analytics may lower expenses by 15-25%, demonstrating the financial advantages of embracing advanced technologies in research studies. As the landscape of medical studies transforms, adopting these technological advancements and thorough management services is crucial for enhancing the overall efficiency and effectiveness of regulatory strategies. This ultimately results in more successful study outcomes and aids local economic development through job creation and healthcare enhancement.
Training and Education: Building a Culture of Compliance
Nurturing a culture of adherence within clinical research teams is essential for implementing device trial compliance strategies and ensuring the success of clinical trials. This commitment begins with continuous training and education, where researchers actively engage in workshops and training sessions that cover device trial compliance strategies, the latest regulatory updates, best practices, and ethical considerations. In 2025, it is crucial for organizations to recognize that 34% of entities are outsourcing regulatory functions, highlighting the need for robust internal training programs to maintain control and oversight over regulatory processes.
Establishing clear device trial compliance strategies and procedures is vital, as these frameworks guide team members in their daily activities and decision-making processes. Moreover, promoting open communication regarding regulatory issues cultivates an environment where team members feel empowered to report concerns related to device trial compliance strategies without fear of repercussions. This transparency is key to strengthening a culture of adherence.
Effective initiatives in regulatory culture demonstrate that organizations emphasizing education not only strengthen their device trial compliance strategies but also enhance outcomes. For instance, the evolution of Electronic Patient-Reported Outcomes (ePRO) illustrates the shift towards patient-oriented applications that enhance engagement and retention. This evolution necessitates a comprehensive understanding of device trial compliance strategies in study management, as ePRO systems must align with regulatory standards to ensure participant data integrity.
As sponsors increasingly seek data ownership and transparency, the demand for consistent site technology and standardization across studies will continue to grow. Bree Burks, Vice President of Site Strategy, emphasizes that "as sponsors reconsider their site engagement approaches in 2025, they will focus on consistent site technology and standardization across sponsors for all studies." By investing in training and education, organizations can better equip their teams to navigate these changes, ultimately leading to more effective research studies that adhere to device trial compliance strategies.
Furthermore, utilizing AI and machine learning can make research more adaptive, agile, and cost-efficient, further supporting regulatory efforts in this evolving landscape.
At bioaccess, our extensive research study management services encompass:
- Feasibility assessments
- Site selection
- Adherence reviews
- Study setup
- Import permits
- Project management
- Reporting
This comprehensive approach ensures that all elements of research trials are meticulously handled, aligning with the highest standards of regulatory adherence and operational excellence.
Continuous Improvement in Compliance Strategies
Ongoing enhancement in regulatory strategies is essential for navigating the evolving landscape of clinical research regulations. Organizations such as bioaccess must routinely assess their adherence practices, identify areas for improvement, and implement necessary adjustments. This process can involve conducting internal audits, gathering feedback from team members, and remaining vigilant regarding industry trends and compliance updates.
By cultivating a culture of continuous improvement, organizations not only fortify their regulatory efforts but also mitigate risks associated with non-compliance. This proactive approach ultimately boosts the success of medical studies, ensuring they meet both regulatory standards and stakeholder expectations.
Bioaccess offers comprehensive research study management services, including:
- Feasibility assessments
- Site selection
- Regulatory reviews
- Study setup
- Import permits
- Project oversight
- Reporting on study progress
- Managing serious and non-serious adverse events
These services are vital for ensuring that clinical trials are conducted efficiently and in accordance with local regulations, thereby enhancing the overall quality of research outcomes.
Recent statistics reveal that:
- 77% of corporate risk and governance professionals prioritize staying informed about the latest developments in environmental, social, and governance (ESG) issues, highlighting the increasing importance of adherence in the broader corporate landscape.
- 76% of consumers would withdraw their support from businesses that mistreat employees and communities, underscoring the critical role of adherence to standards in maintaining corporate reputation and stakeholder trust.
As Sharavanan notes, '91% of business leaders believe their company has a responsibility to act on ESG issues,' reinforcing the notion that adherence is not merely a regulatory requirement but a corporate obligation.
Effective internal audits have proven to significantly enhance device trial compliance strategies in medical research, as illustrated by the case study titled 'Navigating Industry Changes and Data Management Challenges,' which demonstrates how organizations are adapting their device trial compliance strategies in response to external challenges. Moreover, leveraging AI and machine learning can render clinical research more adaptive, agile, and cost-effective, providing organizations with innovative tools to refine their regulatory strategies. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, exemplifies the expertise required to navigate these complex compliance landscapes effectively.
Conclusion
Ensuring compliance in medical device trials is paramount for the integrity and success of clinical research. Compliance encompasses adherence to regulatory standards, ethical guidelines, and the safeguarding of participant rights. As the landscape evolves, driven by technological advancements and regulatory changes, organizations must prioritize effective compliance strategies that embrace transparency, data ownership, and participant engagement.
The significance of training and continuous improvement cannot be overstated. Regular education for research teams fosters a culture of compliance, empowering staff to navigate complex protocols and maintain high standards of ethical conduct. By leveraging technology, such as electronic data capture systems and mobile applications, researchers can enhance data management and participant engagement, leading to improved compliance outcomes.
Challenges remain, particularly in participant recruitment and retention, where innovative strategies and effective communication are essential. Addressing logistical barriers and utilizing technology for remote monitoring can significantly enhance enrollment among diverse populations, ensuring trials are more inclusive and representative.
Ultimately, the future of clinical research hinges on the commitment to compliance, supported by robust training, technological innovations, and a proactive approach to continuous improvement. By prioritizing these elements, organizations can not only meet regulatory requirements but also contribute to the advancement of medical devices and the overall success of clinical trials, fostering trust and integrity in the research process.
Frequently Asked Questions
What is the importance of adherence in medical device studies?
Adherence in medical device studies is crucial for successful clinical research as it protects the rights and welfare of participants, promotes trust among stakeholders, and ensures compliance with regulatory standards.
How are compliance strategies in device trials evolving?
Compliance strategies are increasingly shaped by the demand for transparency and data ownership, with sponsors shifting towards insourced models for data management, driven by ICH E6 R2 guidelines, enhancing operational efficiency and result quality.
What recent trends have been observed in participant enrollment in medical studies?
The individual market for enrolled participants has grown from 12 million in 2021 to 21.5 million in 2024, indicating a heightened focus on patient experience in study design.
How can simplifying data gathering processes improve adherence?
Removing unnecessary tasks during visits can significantly enhance adherence; for example, eliminating a single 20-minute task across 130,000 visits can save 43,000 hours of work for Clinical Research Associates (CRAs).
What services does bioaccess offer to support clinical studies?
Bioaccess provides a comprehensive suite of services including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, aimed at addressing challenges faced by medical device startups.
What role does communication play in adherence to medical studies?
Effective communication among team members and participants is pivotal in fostering adherence, ensuring that all parties understand their roles and responsibilities.
How has blockchain technology impacted clinical trials?
Blockchain technology facilitates secure and transparent data sharing among trial stakeholders, which improves data integrity and overall trial efficiency.
Why is training important for adherence in medical device studies?
Well-trained personnel are better equipped to navigate complex protocols and regulatory environments, which directly enhances the effectiveness of device trial compliance strategies.
What are the challenges posed by regulatory requirements in medical studies?
The complexity of regulatory requirements can create significant challenges, making it essential for organizations to stay informed about evolving regulations and provide regular training to staff.
What expert opinions suggest about the clarity of study protocols?
Experts indicate that well-defined protocols improve compliance, leading to more reliable and valid results in medical studies.




