Overview
Enhancing patient diversity in Mexican clinical trials is of paramount importance. Best practices to achieve this include:
- Engaging local communities
- Employing culturally competent research teams
- Implementing flexible recruitment strategies
These approaches are essential for overcoming barriers and ensuring that clinical studies accurately reflect the diverse demographics of Mexico. Ultimately, this leads to more relevant and effective healthcare outcomes, reinforcing the need for collaboration in clinical research.
Introduction
In the realm of clinical research, the inclusion of diverse patient populations transcends ethical considerations; it is a pivotal factor that directly influences the efficacy and safety of medical treatments.
As the global landscape of clinical trials evolves, the necessity of mirroring the rich tapestry of demographics—encompassing various races, ethnicities, genders, and socioeconomic backgrounds—has become increasingly evident.
This is especially pertinent in Mexico, a nation characterized by its cultural diversity yet frequently underrepresented in clinical studies.
By embracing patient diversity, researchers can significantly enhance the relevance of their findings and promote equitable healthcare outcomes.
Nonetheless, considerable challenges remain, ranging from socioeconomic barriers to historical mistrust, which demand innovative strategies and robust community engagement to ensure that clinical trials are inclusive and representative of the populations they aim to serve.
Understanding Patient Diversity in Clinical Trials
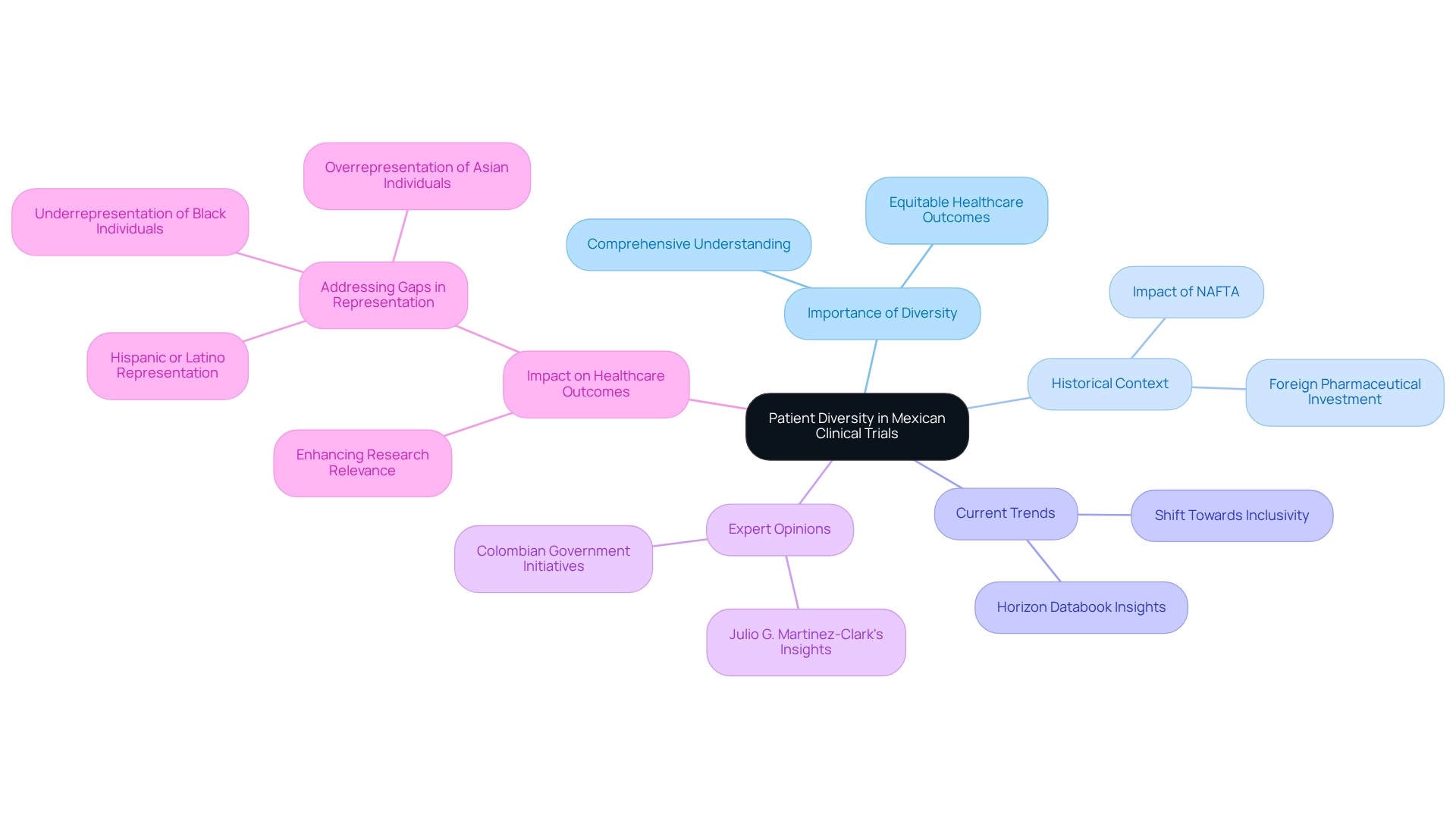
The concept of patient diversity in Mexican clinical trials is crucial, encompassing participants from a wide array of backgrounds, including various races, ethnicities, genders, ages, and socioeconomic statuses. This diversity is essential for a comprehensive understanding of how different populations respond to medical treatments and interventions. In Mexico, a nation characterized by its rich cultural and ethnic tapestry, ensuring that research studies reflect patient diversity in clinical trials is increasingly vital.
Involving individuals from diverse communities allows researchers to evaluate the safety and effectiveness of new medical technologies with greater precision. The importance of patient diversity is underscored by recent findings revealing significant gaps in racial representation within medical studies globally. A study involving 8,015 participants from 47 nations found that 85.6% were White, highlighting the underrepresentation of Black individuals and the overrepresentation of Asian individuals in certain studies. Additionally, Hispanic or Latino participants in the U.S. were also underrepresented relative to their population percentage.
These disparities emphasize the urgent need for innovative approaches in study design to foster inclusivity, thereby enhancing the relevance of research findings. Historically, the North American Free Trade Agreement (NAFTA), enacted in 1993, significantly bolstered Mexico's economy and attracted foreign pharmaceutical investment, laying the groundwork for the current testing landscape. Today, this environment is evolving, with a growing acknowledgment of the importance of patient diversity in Mexican clinical trials. The Horizon Databook forecasts significant trends in the Mexican medical study market from 2018 to 2030, indicating a shift towards more inclusive methodologies and underscoring the importance of patient diversity in these trials.
As bioaccess® leads advancements in Medtech medical studies across Latin America, it prioritizes innovation and regulatory excellence, ensuring that medical evaluations reflect patient diversity in Mexican clinical trials while adhering to compliance standards. Our comprehensive management services for studies include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
All designed to foster an inclusive research environment while generating jobs and stimulating economic growth.
Expert opinions further bolster this perspective. Julio G. Martinez-Clark, a prominent figure in the field, notes that the Colombian government is actively pursuing research studies as part of its strategy to cultivate a knowledge-based economy by 2031. This proactive approach serves as a model for other Latin American nations, including Mexico, to enhance their research frameworks.
Ultimately, patient diversity in Mexican clinical trials not only enriches the research process but also contributes to more equitable healthcare outcomes. By ensuring that medical studies accurately reflect the population, researchers can enhance the significance of their findings, paving the way for advancements in medical technology that benefit all segments of society.
Challenges to Achieving Patient Diversity in Mexico
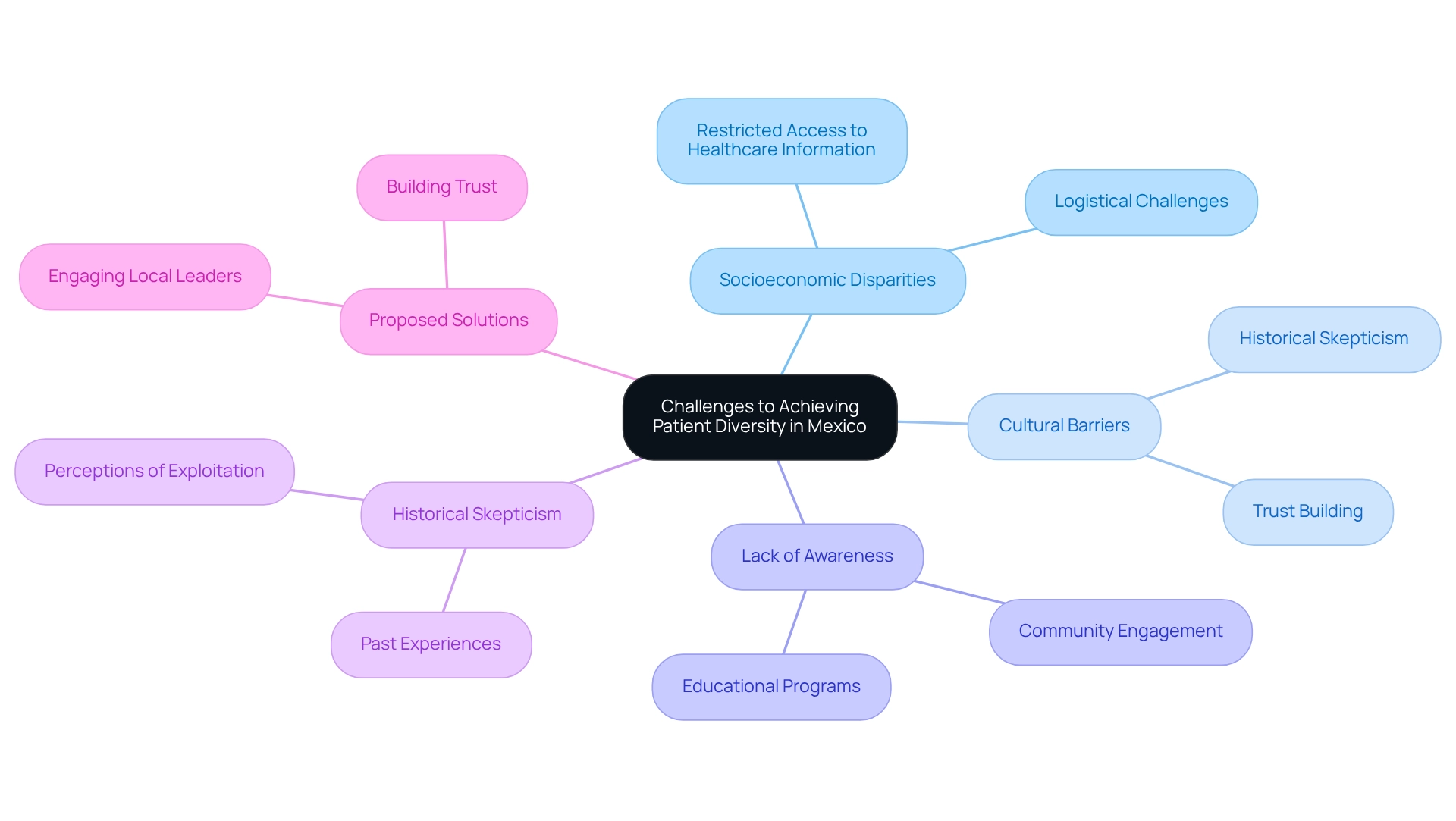
Significant challenges in achieving patient diversity in Mexican clinical trials stem from socioeconomic disparities, cultural barriers, and a pervasive lack of awareness about clinical trials among underrepresented populations. Many prospective participants encounter restricted access to healthcare information, compounded by logistical challenges that deter their involvement in study initiatives. Furthermore, historical skepticism towards medical studies within certain groups can severely impede recruitment efforts, as individuals may hesitate to participate due to past experiences or perceptions of exploitation.
To effectively tackle these challenges, a multifaceted approach is crucial. This strategy must take into account the unique cultural and social dynamics of the Mexican population, acknowledging the importance of building trust and encouraging open communication. Engaging local leaders and community groups can facilitate connections between researchers and potential participants, ensuring that studies are designed with the community's needs and concerns in mind.
Moreover, educational programs aimed at increasing awareness of the benefits and safety of medical studies can empower individuals to participate, ultimately enhancing diversity in research.
Bioaccess offers comprehensive management services for studies that can significantly assist in overcoming these obstacles. Our capabilities include:
- Feasibility studies
- Site selection
- Compliance reviews
- Test setup
- Import permits
- Project management
- Reporting
By ensuring that research trials are well-managed and compliant with local regulations, we can attract more studies to Mexico, reflecting the diverse demographics of the population.
For example, our feasibility studies have successfully pinpointed key sites that are accessible to underrepresented groups, while our compliance reviews guarantee that all ethical considerations are addressed, fostering trust within communities.
Statistics reveal that despite Mexico's robust medical infrastructure and a population of over 323,000 speakers of indigenous languages, the country currently occupies a minor position in global medical studies. This underscores the urgent need for strategies that not only attract more research studies but also ensure that these investigations embody patient diversity in Mexican clinical trials. The case study titled "Clinical Investigation in Mexico: An Overview" explores the current state of medical studies in Mexico, highlighting both the opportunities and challenges faced in attracting more medical experiments.
By surmounting cultural barriers and addressing socioeconomic inequalities, the potential for increased patient diversity in Mexican clinical trials can be realized, resulting in more inclusive and representative findings. Additionally, as Julio G. Martinez-Clark noted, the government of Colombia is actively working to attract more research studies, emphasizing the necessity for similar initiatives in Mexico. Furthermore, the varying levels of commitment to medical research throughout Latin America, as evidenced by the 79.2% approval rate among oncologists in Argentina for research continuity strategies, illustrate the broader context of challenges faced in Mexico.
Building Trust with Diverse Patient Populations
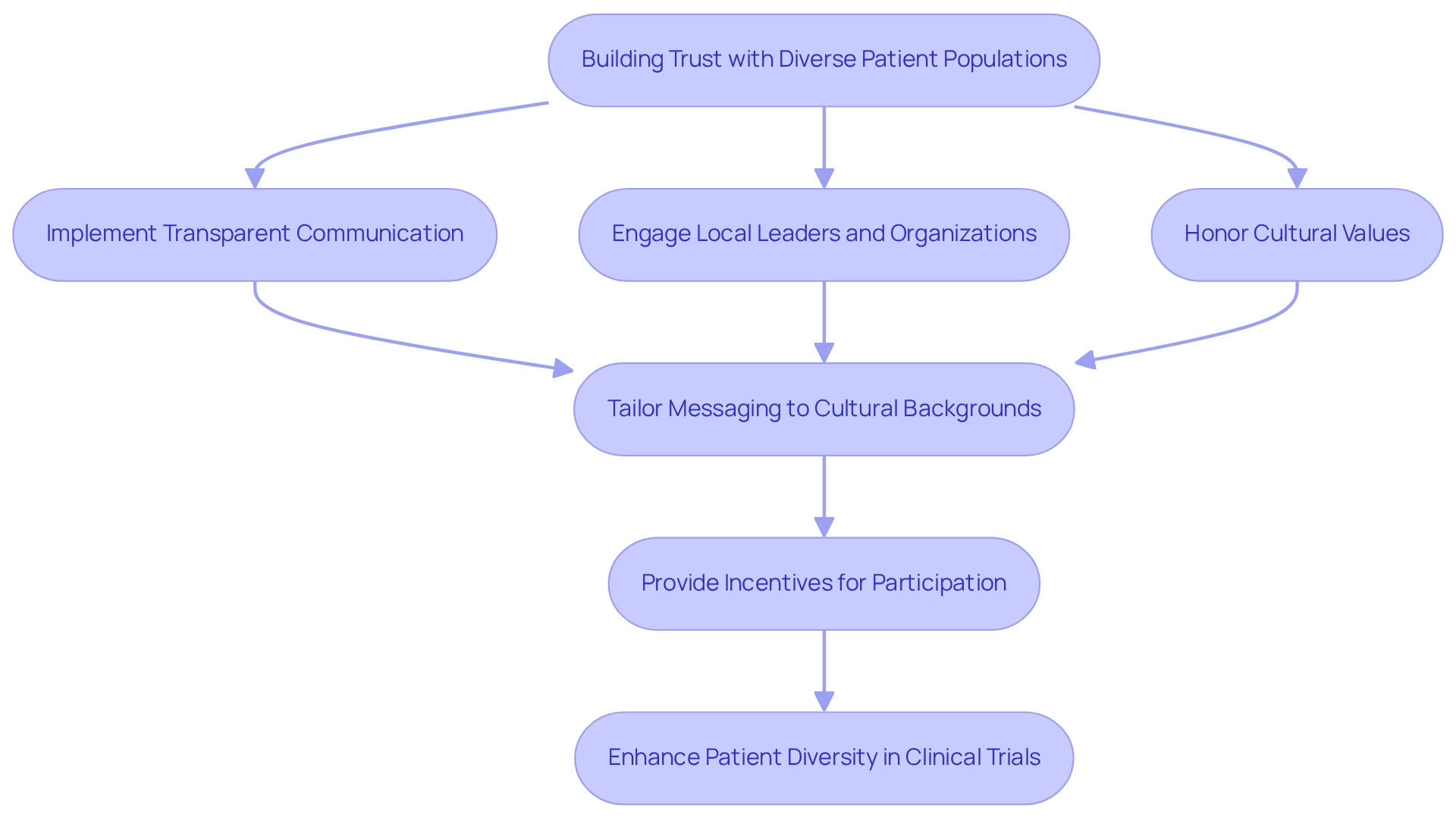
To effectively enhance involvement from diverse patient groups in research studies, sponsors must prioritize trust-building to achieve patient diversity in Mexican clinical trials. This necessitates the implementation of transparent communication strategies and culturally sensitive outreach efforts. Involving local leaders and organizations is essential, as these collaborations can connect researchers with potential participants.
By demonstrating a commitment to ethical standards and honoring cultural values, researchers can cultivate a sense of trust that motivates various groups to engage in research studies. Furthermore, the impact of community involvement on participation statistics in research cannot be overstated. For instance, the Food and Drug Omnibus Reform Act (FDORA Act) mandates sponsors to submit diversity action plans and enrollment goals, underscoring the importance of patient diversity in Mexican clinical trials through early engagement with diverse groups during study design.
This approach not only fosters trust but also aligns with the increasing emphasis on patient diversity in Mexican clinical trials, as highlighted by the FDA's revised guidance on Diversity Action Plans, which stresses the significance of health equity and inclusivity in medical investigations.
Effective communication strategies are paramount in this context. Researchers should tailor their messaging to resonate with various cultural backgrounds, ensuring that potential participants feel understood and valued. Additionally, providing appropriate incentives for participation, as emphasized by Singh, can further encourage recruitment from underrepresented groups.
By promoting a welcoming atmosphere and actively engaging communities in the research process, study sponsors can significantly enhance patient diversity in Mexican clinical trials and representation in their investigations.
At bioaccess, our comprehensive management services for studies encompass feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting. These services not only simplify the research process but also bolster local economies through job creation and healthcare advancement. By adhering to national standards and emphasizing diversity, equity, and inclusion (DEI) in research studies, we can improve the effectiveness of our investigations and promote global cooperation, ultimately resulting in better health outcomes for everyone.
Effective Recruitment Strategies for Diverse Participants
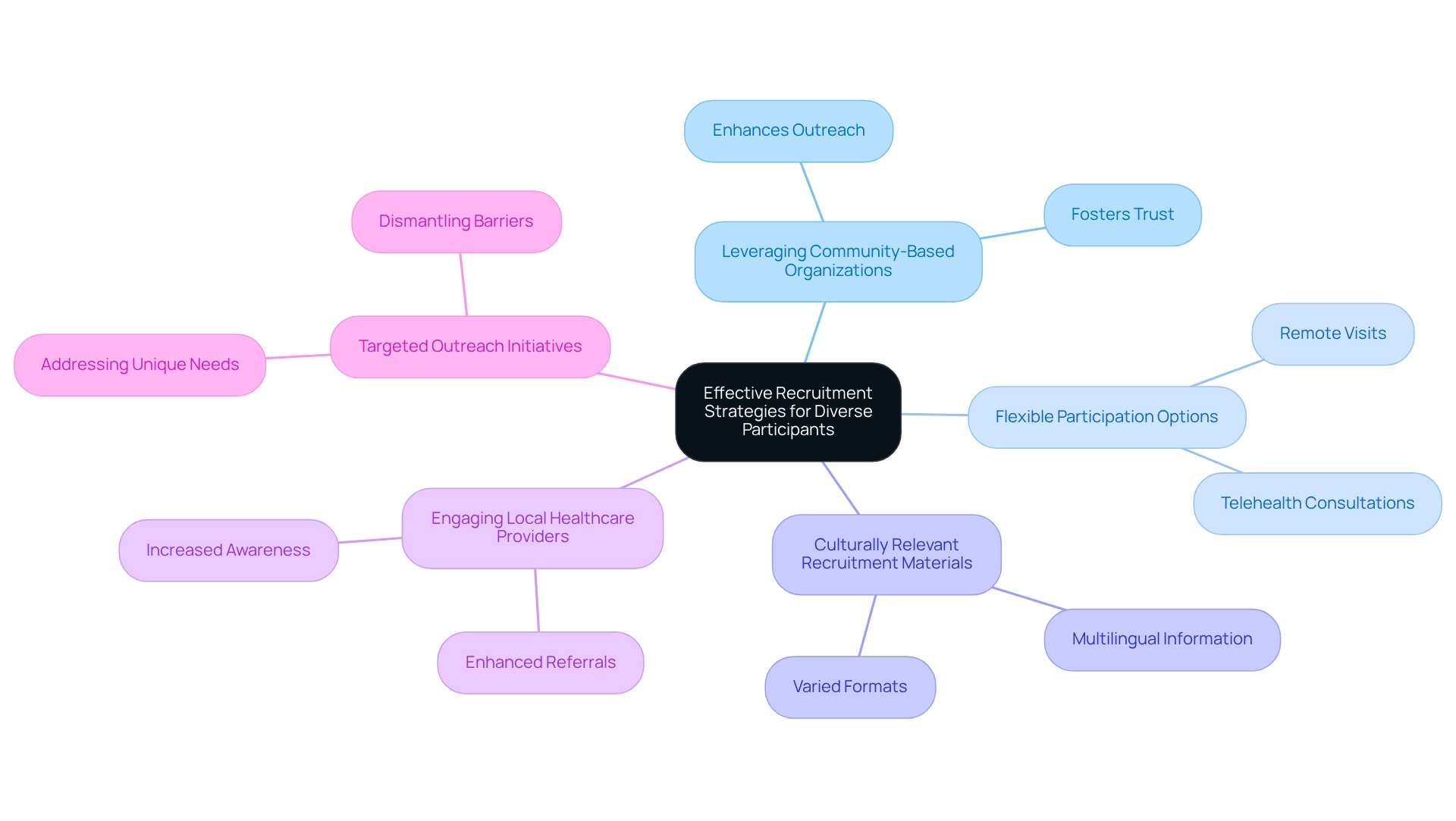
To effectively recruit diverse participants in clinical studies, clinical study teams must adopt a multifaceted approach that encompasses several key strategies:
- Leveraging Community-Based Organizations: Collaborating with local organizations significantly enhances outreach to underrepresented populations, fostering trust and encouraging participation.
- Flexible Participation Options: Providing choices such as remote visits or telehealth consultations meets the diverse needs of participants, facilitating their involvement in the process.
- Culturally Relevant Recruitment Materials: Offering information in various languages and formats ensures that diverse groups fully grasp the study's purpose and advantages.
- Engaging Local Healthcare Providers: By collaborating with local healthcare professionals, research teams can raise awareness about ongoing studies, leading to increased referrals and participation.
- Targeted Outreach Initiatives: Creating campaigns that specifically address the unique needs and concerns of various groups helps dismantle barriers to participation.
These strategies not only foster a more inclusive environment but also enhance patient diversity in Mexican clinical trials, thereby improving the overall quality of research in healthcare. Research from the University of Southern California indicates that even a 1% enhancement in the diversity of medical studies could yield significant economic benefits, projected at $40 billion for diabetes and $60 billion in total. Furthermore, case studies on Community-Based Participatory Research (CBPR) demonstrate that collaborations between academic institutions and local populations effectively close healthcare gaps, leading to improved recruitment strategies and health outcomes for racial and ethnic minorities. This emphasizes the critical importance of patient diversity in Mexican clinical trials.
By investing in local connections and education, research teams can achieve long-lasting advantages in participation and health equity.
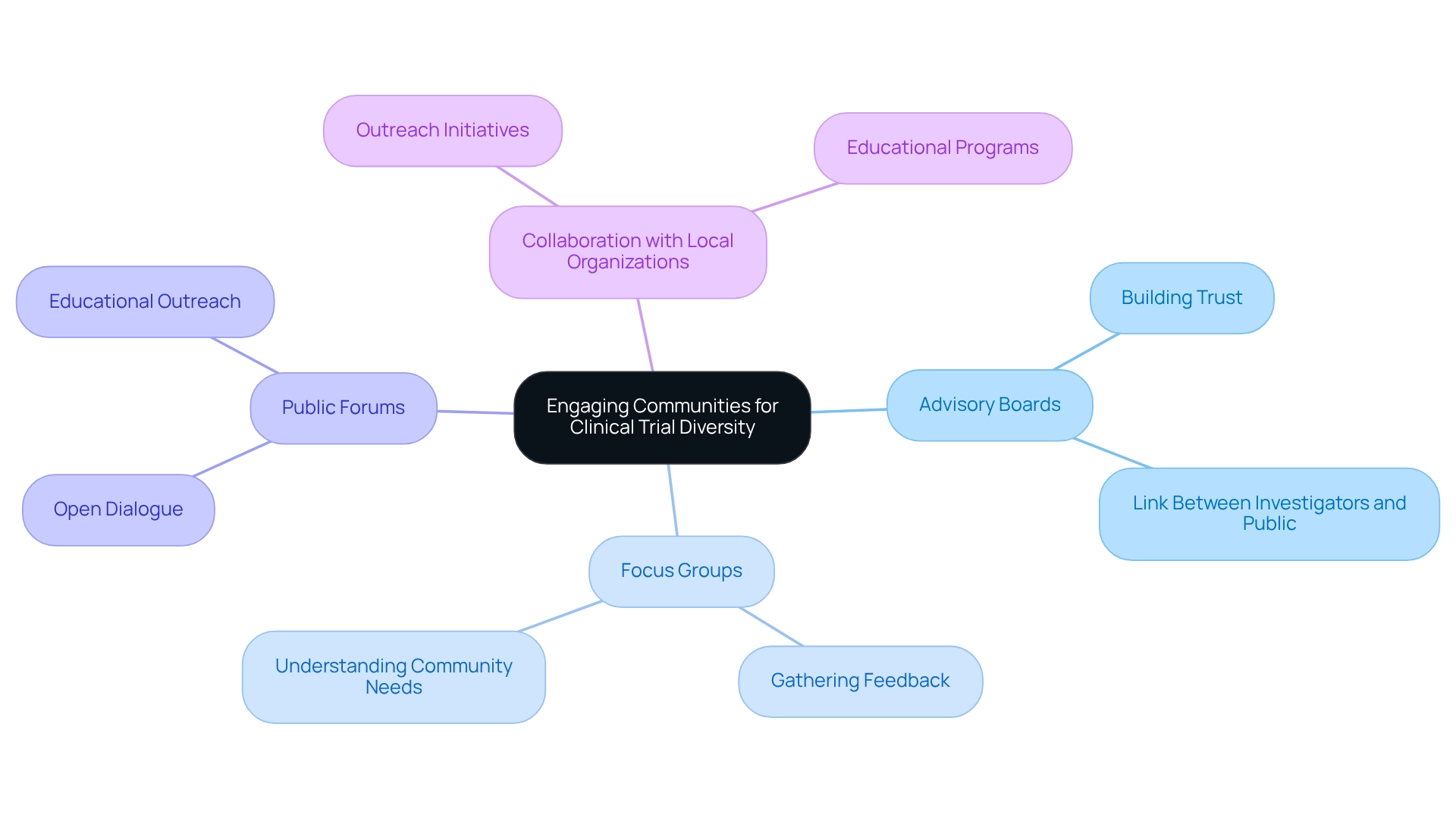
Engaging Communities to Enhance Clinical Trial Diversity
Involving groups is crucial for enhancing patient diversity in Mexican clinical trials. Researchers must actively engage local members in both the design and implementation phases of studies, ensuring their perspectives and needs are integral to the process. This can be effectively accomplished through the formation of advisory boards, focus groups, and public forums that encourage open dialogue and feedback.
Collaboration with local organizations plays a vital role in facilitating outreach and educational initiatives. These partnerships help demystify the clinical trial process, making it more accessible and encouraging broader participation. By promoting a sense of ownership and collaboration within the group, researchers can establish trust, which is essential for improving recruitment results.
Case studies have demonstrated that effective collaborations between health centers and academic organizations, characterized by outreach and education, significantly enhance participation in studies. For instance, the case study titled "Building Trust for Effective Research Partnerships" illustrates how outreach and education can foster trust and collaboration, ultimately advancing biomedical knowledge and achieving equitable health outcomes. Moreover, utilizing various public involvement strategies simultaneously has been recognized as the most effective method for attracting underrepresented groups into studies, as emphasized by 22 interviews carried out with stakeholders between July and October 2023.
Continuous investigation is essential to identify the most effective strategies for enrolling older adults.
Engaging local participants in research design not only enhances the study but also guarantees that the research is pertinent and advantageous to the populations being examined. The effect of advisory boards on research diversity is significant, as they act as a link between investigators and the public, promoting trust and motivating involvement. As Scott Gottlieb, former FDA Commissioner, noted, "Digital technologies are one of the most promising tools we have for making health care more efficient and more patient-focused."
By emphasizing community involvement, researchers can greatly improve patient diversity in Mexican clinical trials, ultimately resulting in more thorough and relevant health solutions.
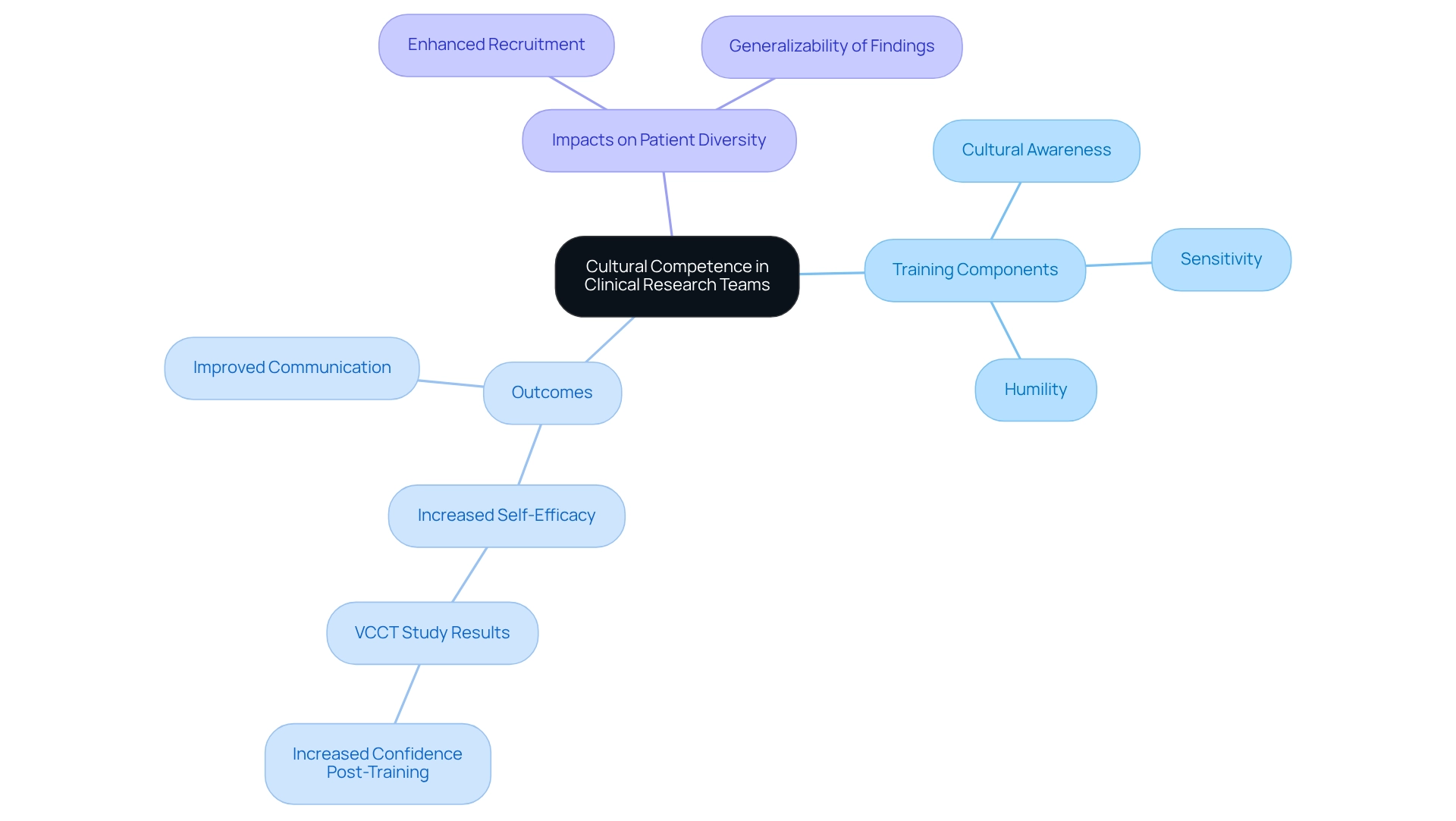
Cultural Competence in Clinical Research Teams
Cultural competence within healthcare teams is essential for effectively engaging diverse patient groups. Training focused on cultural awareness, sensitivity, and humility equips team members to better understand the unique needs and perspectives of participants from various backgrounds. Such training not only helps researchers recognize and address potential biases but also enhances communication, fostering a more inclusive environment.
For instance, a study on Veteran Cultural Competence Training (VCCT) demonstrated significant improvements in participants' confidence to provide culturally competent services, as evidenced by increased self-efficacy scores post-training. Notably, the number of professionals enrolling in VCCT surged to 1,018 in FY2022, underscoring the program's relevance and reach. Arch G. Mainous III, PhD, noted that the vast majority of physicians believe they are aware of their patients’ health beliefs, customs, and values, highlighting the critical importance of cultural competence in medical studies.
By prioritizing cultural competence, healthcare teams can markedly enhance their ability to recruit and retain participants, thus improving patient diversity in Mexican clinical trials. This approach ultimately leads to more representative and impactful study outcomes, reinforcing the notion that culturally sensitive practices are vital for the success of medical research. Statistics indicate that patient diversity in Mexican clinical trials can significantly enhance the generalizability of findings, making it imperative for research teams to invest in cultural competence training.
Moreover, addressing obstacles to mental health care that arise from cultural disparities and systemic issues affecting minority populations is essential to ensuring that all voices are acknowledged and represented in research.
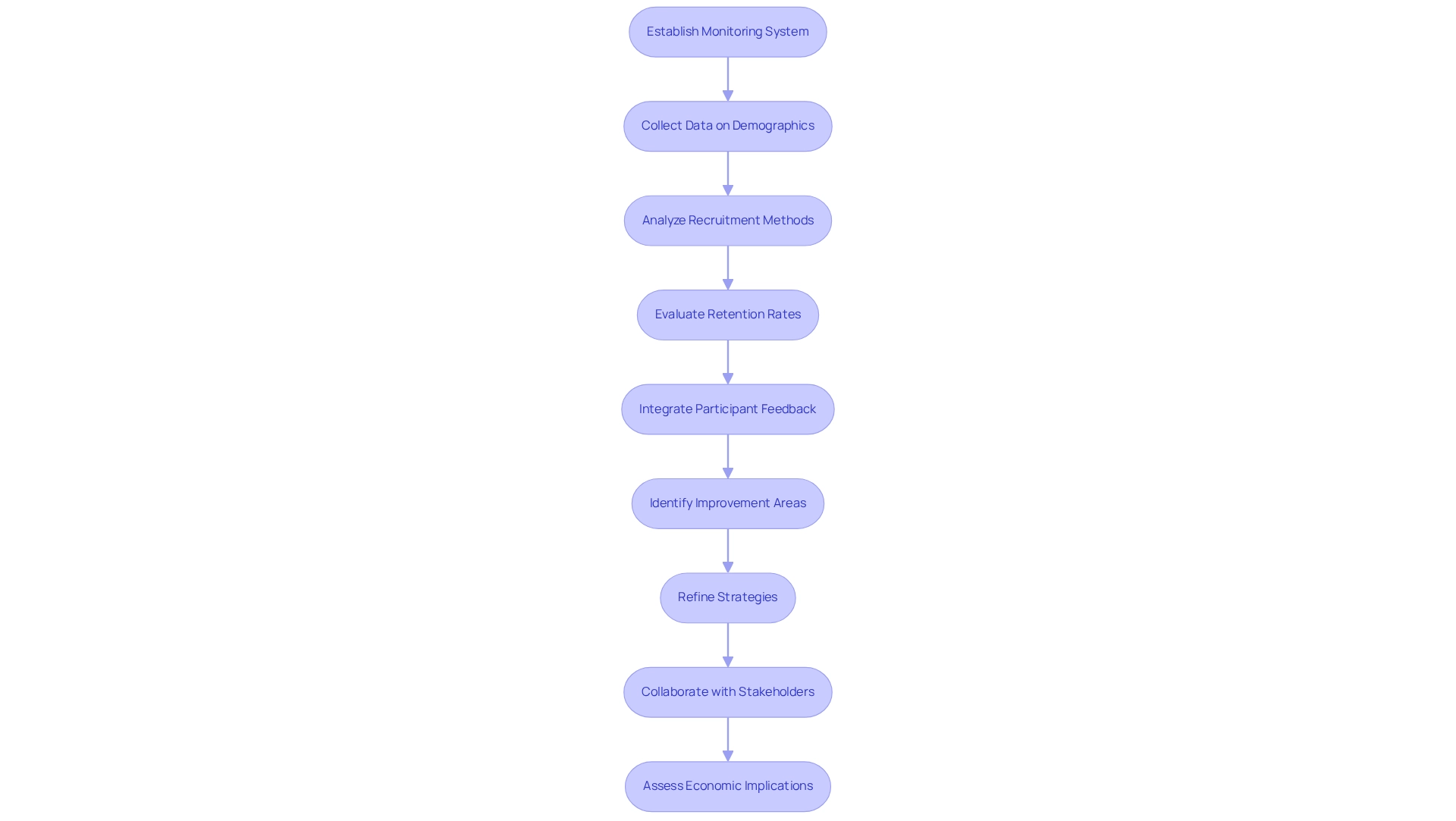
Monitoring and Adapting Diversity Strategies in Clinical Trials
To enhance the effectiveness of diversity approaches in medical studies, researchers must establish a robust system for continuous monitoring and assessment. This process entails the systematic collection and analysis of data concerning participant demographics, recruitment methods, and retention rates. Regular evaluations of these metrics empower researchers to identify areas for improvement and refine their strategies to bolster diversity efforts.
Moreover, integrating feedback from participants and local stakeholders is vital. Such insights can shed light on the effectiveness of engagement initiatives and reveal potential barriers to participation. Diverse study groups facilitate a deeper understanding of treatment effects on underrepresented communities, underscoring the critical importance of patient diversity in Mexican clinical trials.
The collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier hub for research studies in Latin America, with the endorsement of Colombia's Minister of Health. This initiative not only enhances the research landscape but also fosters a culture of continuous improvement within research teams, aligning with regulatory expectations. Research from the University of Southern California indicates that even a modest 1% increase in study diversity could yield substantial economic benefits, with projections estimating an additional $40 billion for diabetes-related research and $60 billion across the healthcare sector.
Furthermore, GlobalCare Clinical Trials' partnership with bioaccess™ has demonstrated remarkable improvements in study efficiency, achieving over a 50% reduction in recruitment duration and 95% retention rates. By referencing the case study titled 'Economic Implications of Clinical Trial Diversity,' which suggests that enhancing patient diversity in Mexican clinical trials could lead to significant economic gains, clinical trial teams can ensure compliance while effectively positioning themselves to meet their goals for patient diversity in Mexican clinical trials.
Conclusion
Emphasizing patient diversity in clinical trials transcends ethical obligation; it is essential for enhancing the safety and efficacy of medical treatments. By including diverse participants, clinical research can accurately reflect the varied responses of different populations, particularly in culturally rich regions like Mexico. Addressing the underrepresentation of various demographics allows researchers to enhance the applicability of their findings and contribute to equitable healthcare outcomes.
Despite the challenges posed by socioeconomic disparities, historical mistrust, and cultural barriers, innovative strategies can effectively bridge these gaps. Engaging community leaders, employing culturally relevant recruitment strategies, and fostering trust through transparent communication are critical steps toward increasing participation from diverse populations. By prioritizing these approaches, clinical trial sponsors can improve recruitment and ensure that their research is comprehensive and applicable to all segments of society.
Ultimately, integrating diverse patient populations into clinical trials serves as a pathway to advancing medical knowledge and promoting health equity. As the landscape of clinical research evolves, a commitment to inclusivity will enrich the research process and ensure that the benefits of medical advancements are accessible to everyone. Moving forward, concerted efforts to build trust, engage communities, and monitor diversity strategies will be vital for the success of clinical trials in achieving meaningful and representative outcomes.
Frequently Asked Questions
Why is patient diversity important in Mexican clinical trials?
Patient diversity is crucial in Mexican clinical trials as it allows for a comprehensive understanding of how different populations respond to medical treatments and interventions. It enhances the evaluation of the safety and effectiveness of new medical technologies.
What challenges exist in achieving patient diversity in Mexican clinical trials?
Significant challenges include socioeconomic disparities, cultural barriers, lack of awareness about clinical trials among underrepresented populations, restricted access to healthcare information, and historical skepticism towards medical studies.
How can these challenges be addressed to improve patient diversity in clinical trials?
A multifaceted approach is needed, which includes building trust through community engagement, increasing awareness of the benefits of clinical trials, and designing studies that consider the unique cultural and social dynamics of the Mexican population.
What role does Bioaccess play in promoting patient diversity in clinical trials?
Bioaccess provides comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, and project oversight, which help to create an inclusive research environment and attract more studies to Mexico.
What statistics highlight the underrepresentation of certain populations in medical studies?
A global study found that 85.6% of participants were White, indicating significant underrepresentation of Black individuals and Hispanic or Latino participants relative to their population percentage in the U.S.
What historical context has influenced the current landscape of clinical trials in Mexico?
The North American Free Trade Agreement (NAFTA), enacted in 1993, significantly boosted Mexico's economy and attracted foreign pharmaceutical investment, which has laid the groundwork for the current testing landscape.
How does patient diversity contribute to healthcare outcomes?
Ensuring that medical studies reflect the diverse demographics of the population leads to more equitable healthcare outcomes and enhances the significance of research findings, benefiting all segments of society.
What is the forecast for the Mexican medical study market regarding patient diversity?
The Horizon Databook forecasts a shift towards more inclusive methodologies in the Mexican medical study market from 2018 to 2030, underscoring the growing importance of patient diversity in clinical trials.
How does the Colombian government’s approach serve as a model for Mexico?
The Colombian government is actively pursuing research studies as part of its strategy to cultivate a knowledge-based economy, which can serve as a model for Mexico to enhance its research frameworks and attract more studies.
What educational initiatives can improve participation in clinical trials?
Educational programs aimed at increasing awareness of the benefits and safety of medical studies can empower individuals from underrepresented groups to participate, thereby enhancing diversity in research.




