Overview
This article addresses best practices for implementing advanced analytics in clinical trial design, highlighting the critical role of data-driven decision-making in enhancing both efficiency and effectiveness in research. It elucidates how predictive modeling and adaptive study designs can significantly optimize patient recruitment and resource utilization. Ultimately, these strategies lead to improved outcomes and expedited market access for new treatments, underscoring the importance of integrating advanced analytics into clinical research methodologies.
Introduction
In the rapidly evolving landscape of clinical research, advanced analytics has emerged as a transformative force, reshaping trial design and execution. By harnessing sophisticated statistical methods and real-world data, researchers can now optimize every phase of clinical trials, from patient recruitment to data monitoring. This innovative approach enhances operational efficiency and fosters a deeper understanding of patient populations and treatment effects.
As organizations confront mounting challenges in the healthcare sector, integrating advanced analytics proves essential for driving successful outcomes and expediting the development of new therapies. With the promise of artificial intelligence and machine learning on the horizon, the future of clinical trials is poised for unprecedented advancements, making it crucial for stakeholders to remain informed and adaptable in this dynamic environment.
Understanding Advanced Analytics in Clinical Trial Design
Advanced analytics in trial design employs sophisticated statistical methods and examination techniques to optimize the planning and execution of clinical studies. This innovative approach empowers researchers to uncover patterns, forecast outcomes, and make informed, data-driven decisions that significantly enhance efficiency and effectiveness. By integrating various information sources, including real-world evidence and historical study results, advanced analysis provides a comprehensive understanding of patient groups and treatment impacts.
For instance, predictive modeling is crucial in identifying the most suitable patient cohorts, thereby enhancing recruitment processes and elevating overall study design. Recent statistics indicate that utilizing advanced analytics can lead to a 30% reduction in recruitment time through more precise patient targeting, underscoring its impact on operational efficiency. Furthermore, advanced analytics supports adaptive study designs, enabling researchers to modify protocols based on interim results.
This flexibility is particularly advantageous in the rapidly evolving Medtech sector, where timely insights can accelerate the development of medical technologies and treatments. A case study illustrating this is the proactive issue management approach, wherein sponsors utilize historical trend information to anticipate and address potential challenges during clinical trials. By establishing thresholds and fostering cross-departmental information sharing, they can monitor trends over time, enhancing quality through early issue detection.
This method not only results in quicker approvals but also promotes improved resource efficiency and reduced study timelines.
As pharmaceutical firms increasingly face challenges in optimizing healthcare insights—such as information quality issues, organizational silos, regulatory compliance concerns, talent gaps, and change management—leveraging advanced analysis becomes essential. According to Accenture’s Healthcare Analytics Benchmark Survey, organizations that effectively merge their information silos experience significantly enhanced decision-making abilities, highlighting the transformative potential of advanced analytics in trial design. By 2025, the ongoing advancement of these technologies is expected to further improve research efficiency, making them indispensable tools for Medtech innovators.
Moreover, sponsors are transitioning toward entirely insourced models and seeking technology that provides direct access to real-time data, reflecting current trends in the sector.
At bioaccess®, we focus on comprehensive management services for studies, drawing on over 20 years of experience in Medtech. Our services encompass Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Our partnership with Caribbean Health Group aims to establish Barranquilla as a premier location for research studies in Latin America, supported by Colombia's Minister of Health.
This collaboration enhances our capabilities to deliver accelerated medical device research study services, achieving over a 50% reduction in recruitment time and 95% retention rates, ultimately driving success in research.
The Role of Patient-Centric Design in Clinical Trials
Patient-centric design in clinical studies is essential for aligning research with the needs and preferences of participants throughout the study process. This approach not only boosts recruitment and retention rates but also enhances the quality of data collected. Engaging patients during the design phase allows researchers to identify and proactively address potential barriers to participation.
For instance, simplifying consent forms, ensuring clear communication about study procedures, and offering flexible scheduling options can significantly enhance patient engagement.
Current statistics indicate that many individuals are more willing to engage in research when studies are designed with their convenience in mind and when they are kept informed about study results. This underscores the significance of patient-focused design in enhancing study outcomes.
Moreover, the integration of advanced technology, such as mobile health applications, plays a crucial role in facilitating real-time data collection and improving patient monitoring. This not only makes participation more convenient but also reduces the burden on participants.
The decentralization of medical studies has emerged as a transformative trend, leveraging digital tools to enhance participation and reduce barriers. A recent study emphasizes that decentralization can enhance access to medical studies for various populations, although it also introduces challenges that must be addressed effectively. As Joseph E. Vandigo expressed, "If medical studies are to be successful, it is essential that more individuals participate."
This highlights the necessity of involving a broader participant base to achieve meaningful results.
By prioritizing the patient experience, research studies can yield more precise and significant results, ultimately leading to enhanced healthcare outcomes. Best practices for enhancing patient engagement include:
- Personalized communication
- Flexible and remote monitoring
- Providing incentives and feedback to participants
All of which contribute to a more effective and inclusive research environment. The adoption of patient-centered study designs is essential for achieving the goals of healthcare reform, reinforcing the overall significance of this approach in the context of current healthcare objectives.
Innovative Statistical Methodologies for Enhanced Trial Design
Advanced analytics in trial design is essential for the progression of clinical study design. Innovative statistical methodologies, particularly Bayesian statistics, stand out for their capacity to integrate prior knowledge and adapt to real-time data updates. This flexibility fosters informed decision-making throughout the experimental process, sharply contrasting with traditional frequentist methods that often rely on fixed sample sizes and rigid protocols.
The implementation of adaptive study designs, a form of advanced analytics in trial design, further enhances this flexibility by allowing modifications based on interim results. Such designs can significantly improve resource utilization and expedite conclusions. For instance, if preliminary findings suggest that a treatment is ineffective, the study can be adjusted or even terminated, conserving both time and financial resources.
Recent statistics underscore the effectiveness of Bayesian methods in medical studies. A notable case study is the "Bayesian Phase 3 Case Study: BNT162b2 mRNA COVID-19 Vaccine Development," where a Bayesian framework enabled early and frequent interim analyses. The final analysis of the primary efficacy endpoint revealed a probability exceeding 98.6% that the true vaccine efficacy was greater than 30%. This method not only met the primary efficacy endpoint with a probability surpassing 99.5% but also led to Emergency Use Authorization based on robust evidence of vaccine efficacy.
Furthermore, advanced analytics in trial design, particularly through the integration of machine learning algorithms, can uncover intricate data patterns that conventional methods may overlook. This enhances both the implementation and outcomes of research studies. As Marisha Fonseca, a specialist in research analysis, states, 'Our skilled biostatisticians can assist you with intricate Bayesian analyses at any phase of your research journey.' As the landscape of medical research evolves in 2025, the adoption of these groundbreaking statistical techniques will be pivotal for improving study efficiency and effectiveness.
With over 20 years of experience in Medtech, bioaccess® plays a vital role in connecting innovative medtech companies with opportunities for conducting research studies in Latin America. This is particularly evident through its expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Such collaborations enhance the significance of these methodologies as bioaccess® partners with local health institutions, like the Caribbean Health Group, to establish Barranquilla as a premier research location in Latin America, supported by Colombia's Minister of Health.
Practical Applications of Advanced Analytics in Clinical Trials
The practical applications of advanced data analysis in research studies are both extensive and impactful, particularly illustrated by bioaccess™'s collaboration with Caribbean Health Group to position Barranquilla as a premier destination for research studies in Latin America. Endorsed by Colombia's Minister of Health, this initiative is designed to enhance the efficiency and effectiveness of clinical research in the region. A pivotal area of transformation lies in patient recruitment, where predictive analysis leverages historical data and demographic insights to pinpoint eligible participants.
This targeted strategy not only expedites the recruitment process but also cultivates a more diverse and representative sample, ultimately bolstering the validity of outcomes. Furthermore, the application of predictive analysis can reduce costs, improve success rates, and expedite the market entry of new therapies, establishing it as an indispensable tool in clinical research.
Moreover, advanced analytics significantly enhance monitoring and safety evaluations throughout the study's lifecycle. By employing real-time information analysis, researchers can swiftly identify adverse events or emerging trends that require immediate intervention, thereby ensuring patient welfare and preserving the integrity of the trial. For instance, companies utilizing integrated data systems have reported a 29% increase in decision-making speed across clinical and commercial operations, underscoring the efficiency achieved through comprehensive data integration.
Novartis has also reported an impressive return on investment exceeding 300% from its data analysis initiative, emphasizing the financial advantages of adopting advanced data techniques.
In addition to recruitment and safety, data analysis is crucial for optimizing logistics in experiments, including site selection and resource allocation. As researcher Nehal Hassan noted, "Even models that show strong predictive capabilities cannot assist research teams in achieving better results in design or execution independently," underscoring the importance of proper implementation alongside data analysis. This ensures that experiments are conducted efficiently while maximizing their potential for success.
As the landscape of medical research continues to evolve, the integration of advanced analytics in trial design will remain a fundamental component for enhancing study design and implementation, ultimately leading to improved patient outcomes and faster market access for new treatments. Bioaccess® aims to connect innovative Medtech firms with opportunities for conducting research studies in Latin America, further emphasizing the significance of medical devices.
Challenges in Implementing Advanced Analytics in Trial Design
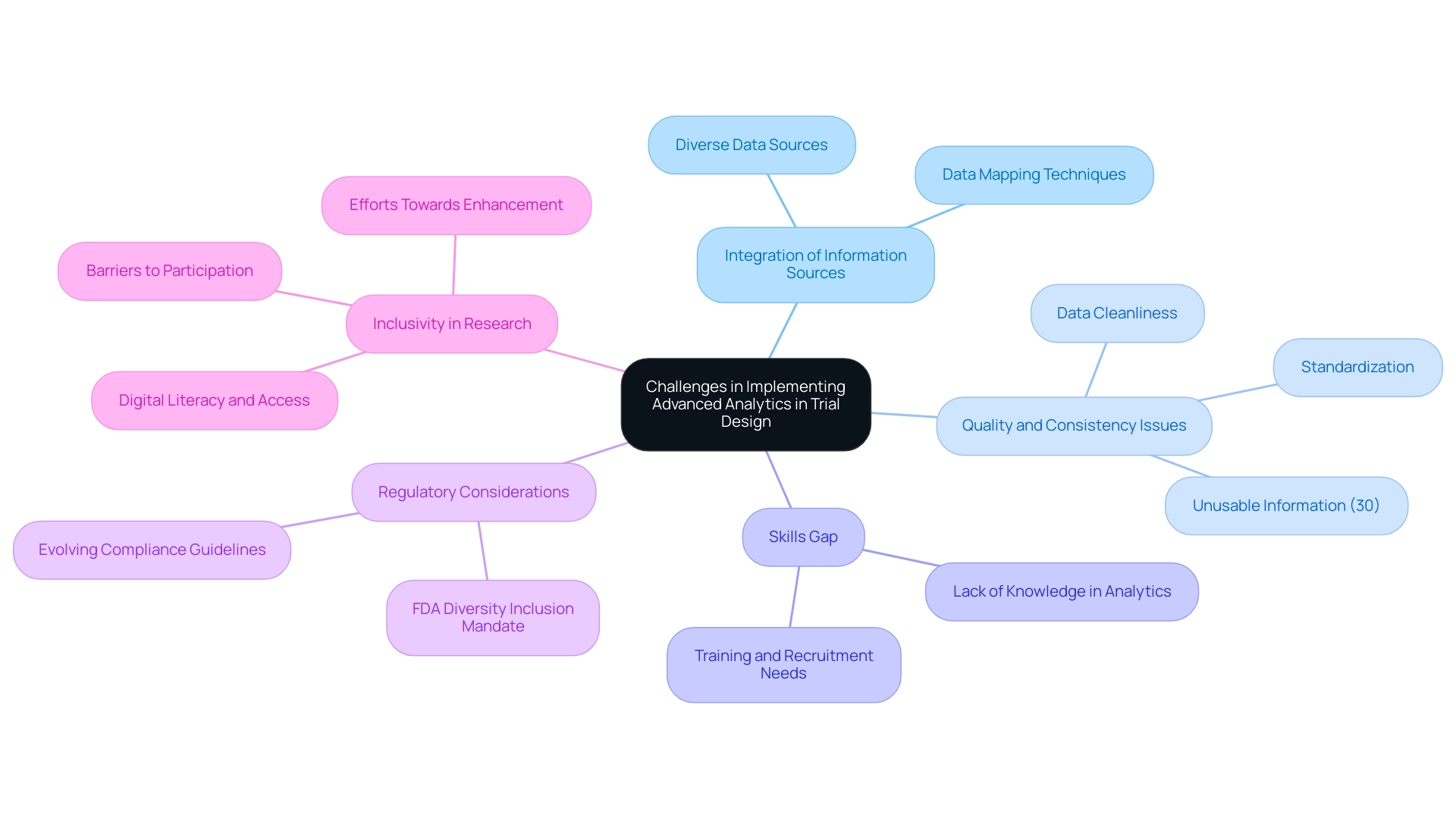
Implementing advanced analytics in trial design presents several significant challenges. A primary obstacle is the integration of diverse information sources, often resulting in quality and consistency issues. Ensuring that information is clean, standardized, and readily accessible is essential for effective analysis and decision-making.
Studies indicate that nearly 30% of clinical trial information is unusable due to quality issues, underscoring the critical need for robust information management practices. Another pressing challenge is the skills gap within clinical research teams concerning advanced analytics in trial design. Many teams lack personnel with the required knowledge in scientific analysis and evaluation, which can hinder the adoption of these methodologies. Tackling this gap demands a united effort in training and recruiting skilled professionals who can navigate the intricacies of information analysis.
As the demand for advanced analytics in trial design grows, organizations must prioritize building a workforce equipped with these essential skills. Moreover, incorporating advanced analytics in trial design adds another layer of complexity due to regulatory considerations. Researchers must navigate compliance with evolving guidelines while leveraging innovative techniques. The upcoming FDA mandate for diversity inclusion strategies emphasizes the necessity for a more inclusive method in medical studies, which can further complicate data integration efforts.
As noted by Pierre Chetelat, 'The performance of medical trials is often marked by lack of diversity, barriers to participation, and consequent efforts towards enhancement.' This highlights the significance of tackling inclusivity in research. Real-world examples illustrate these challenges vividly. A recent case study on integrating healthcare information revealed that organizations often struggle to manage and process information from various sources effectively. The framework created in this study utilized efficient algorithms to identify changes in information and map entities across datasets, demonstrating the potential for enhanced information integration in medical research.
Moreover, obstacles such as digital literacy and access to technology must be tackled to ensure inclusivity in medical research. Overcoming these challenges necessitates a multifaceted approach that addresses data quality, skills development, and regulatory compliance. By concentrating on these aspects, research teams can improve their abilities and promote more efficient study designs.
Regulatory and Ethical Considerations in Advanced Analytics
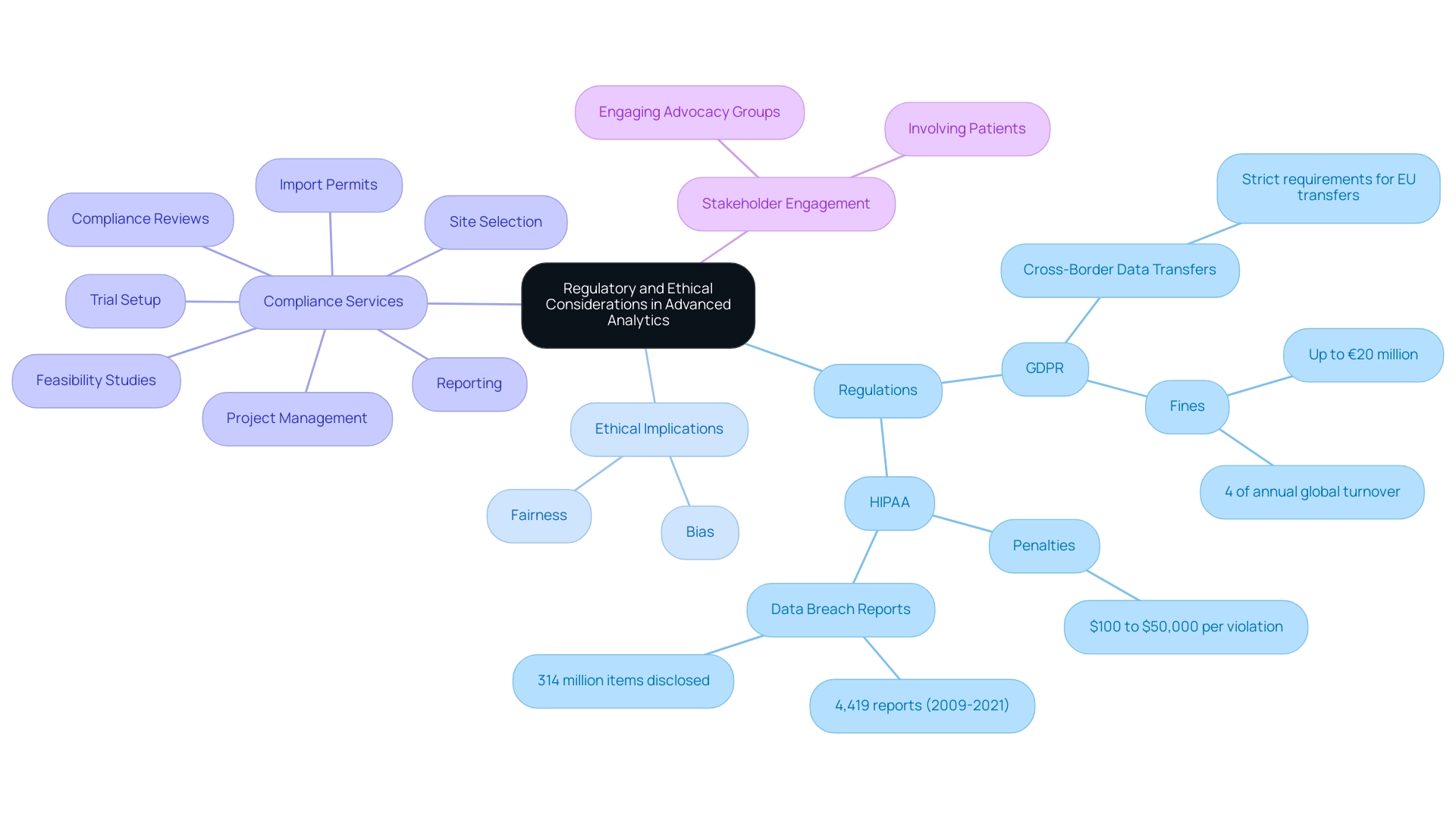
Regulatory and ethical considerations are paramount when implementing advanced analytics in trial design. Researchers must navigate a complex landscape of regulations governing privacy, patient consent, and the utilization of real-world evidence. Adhering to regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) is essential for safeguarding patient information and maintaining public trust.
Notably, violations of GDPR can result in fines of up to €20 million or 4% of annual global turnover, while HIPAA violations may incur civil and criminal penalties ranging from $100 to $50,000 per violation. Between 2009 and 2021, the OCR received 4,419 reports of breaches involving 500 or more medical records from US healthcare organizations, revealing approximately 314 million medical information items disclosed without written consent. This underscores the urgent need for information security and compliance in healthcare research.
At bioaccess®, we provide comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Our commitment to information protection is paramount, ensuring that all processes comply with applicable regulations and that client concerns are addressed transparently through our grievance and information protection procedures. Ethically, the implications of advanced analytics in trial design must be meticulously considered, particularly regarding bias and fairness.
It is crucial to ensure that algorithms employed in analysis do not inadvertently disadvantage specific patient populations. Engaging with stakeholders—including patients and advocacy groups—can help identify potential ethical concerns and foster a more inclusive approach to study design. For instance, a case study on cross-border information transfers in medical trials illustrates the stringent requirements imposed by GDPR for transferring personal information outside the EU, highlighting the necessity for organizations to implement additional measures to ensure compliance when conducting international studies.
This complexity in information management strategies emphasizes the importance of understanding relevant laws and each party's role in research to enhance security and utilization. Furthermore, bioaccess is well-versed in the regulatory landscape governed by INVIMA, ensuring compliance with Colombian regulations. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, leads our efforts in navigating these intricate requirements.
For any queries or concerns regarding data protection, clients can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
Future Trends in Advanced Analytics for Clinical Trials
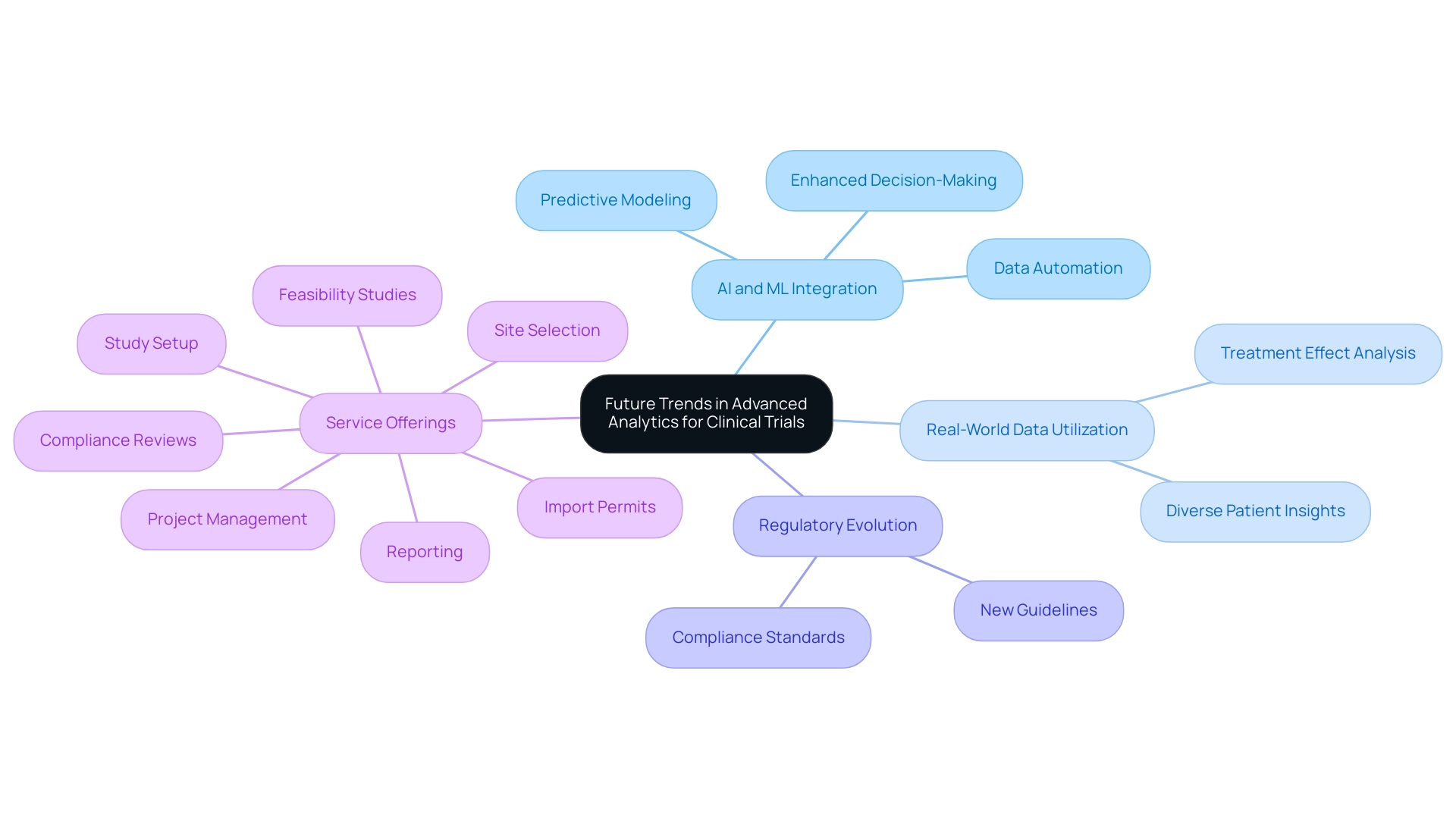
The terrain of advanced analytics in trial design is poised for a significant evolution, propelled by rapid technological advancements and innovative methodologies. A prominent trend is the increasing integration of artificial intelligence (AI) and machine learning (ML) into the planning and execution of studies. These technologies are revolutionizing the field by enhancing predictive modeling, automating data analysis, and streamlining decision-making processes, ultimately resulting in more efficient outcomes.
Moreover, the utilization of real-world data (RWD) is expected to expand considerably, providing deeper insights into diverse patient populations and treatment effects. This shift toward incorporating RWD will facilitate the creation of more robust and broadly applicable results, thereby enhancing the relevance of studies in real-world contexts. As regulatory bodies evolve to incorporate these innovations, it is crucial for researchers to remain vigilant regarding new guidelines and best practices, ensuring compliance and maintaining ethical standards throughout their investigations.
In 2025, the impact of AI and ML on study design is anticipated to be substantial, particularly with the adoption of advanced analytics in trial design. Bioaccess®, with over 20 years of experience in the Medtech sector, exemplifies how organizations can leverage these technologies to refine study designs. Their comprehensive research management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
This extensive service offering not only supports the successful execution of medical studies but also contributes to job creation and economic development within local communities.
Case studies indicate that AI-ML platforms are already enhancing diagnostic processes and aiding medical decisions, particularly in pathology and healthcare. This trend underscores the potential for AI-ML tools to make significant contributions to biomarker discovery, research studies, and productive analytics, while also accelerating translational research and virtual education initiatives.
Looking ahead, the integration of AI and ML into research studies will not only streamline workflows but also foster a culture of innovation within the Medtech sector. By harnessing these advanced technologies, organizations like bioaccess® can utilize advanced analytics in trial design to optimize their processes, ultimately leading to expedited advancements in medical devices and improved patient outcomes. Furthermore, with 30% of business owners anticipating AI to generate website copy for their companies, it is clear that AI adoption is emerging as a significant trend across various sectors, including clinical research.
The work of professionals like Dr. Sergio Alvarado, Clinical Trial Manager at bioaccess®, emphasizes the importance of innovative medical research and the potential of AI to enhance healthcare outcomes in Latin America.
Conclusion
The integration of advanced analytics in clinical trials is revolutionizing clinical research by enhancing efficiency and effectiveness. Techniques such as predictive modeling for patient recruitment and adaptive trial designs facilitate streamlined processes and a deeper understanding of patient populations.
Despite these advantages, challenges persist, including:
- Data quality
- Skill shortages
- Regulatory compliance
Organizations must prioritize robust data management and invest in training to effectively harness analytics. Addressing ethical considerations and ensuring inclusivity in trials is also crucial for fostering trust among participants.
Looking ahead, the incorporation of artificial intelligence and machine learning will further refine trial design and execution, bolstering predictive capabilities and decision-making. Emphasizing patient-centric designs and leveraging real-world data will render trials more relevant and accessible to diverse populations.
In conclusion, leveraging advanced analytics is imperative for organizations aiming to excel in the competitive healthcare landscape. By embracing these innovative methodologies, stakeholders can drive successful outcomes and accelerate the development of new therapies, ultimately enhancing healthcare delivery for patients. Remaining informed and adaptable in this evolving environment is essential for maximizing the potential of advanced analytics in clinical trials.
Frequently Asked Questions
What is advanced analytics in trial design?
Advanced analytics in trial design involves sophisticated statistical methods and examination techniques that optimize the planning and execution of clinical studies, allowing researchers to uncover patterns, forecast outcomes, and make informed, data-driven decisions.
How does advanced analytics improve clinical study efficiency?
By integrating various information sources, such as real-world evidence and historical study results, advanced analytics provides a comprehensive understanding of patient groups and treatment impacts, leading to enhanced efficiency and effectiveness in clinical studies.
What role does predictive modeling play in clinical trials?
Predictive modeling helps identify the most suitable patient cohorts, improving recruitment processes and overall study design, which can lead to a 30% reduction in recruitment time through more precise patient targeting.
What are adaptive study designs and why are they important?
Adaptive study designs allow researchers to modify protocols based on interim results, providing flexibility that is particularly advantageous in the rapidly evolving Medtech sector, enabling timely insights that can accelerate the development of medical technologies and treatments.
How does advanced analytics support proactive issue management in clinical trials?
Sponsors use historical trend information to anticipate and address potential challenges during clinical trials by establishing thresholds and fostering cross-departmental information sharing, which enhances quality through early issue detection.
What challenges do pharmaceutical firms face that make advanced analytics essential?
Pharmaceutical firms encounter challenges such as information quality issues, organizational silos, regulatory compliance concerns, talent gaps, and change management, making the leveraging of advanced analytics crucial for optimizing healthcare insights.
What is the expected impact of advanced analytics technologies by 2025?
The ongoing advancement of these technologies is anticipated to further improve research efficiency, making them indispensable tools for Medtech innovators.
What services does bioaccess® provide in clinical studies?
Bioaccess® offers comprehensive management services including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), with a focus on accelerating medical device research study services.
How does patient-centric design enhance clinical studies?
Patient-centric design aligns research with participant needs and preferences, boosting recruitment and retention rates, and improving the quality of data collected by addressing barriers to participation proactively.
What best practices enhance patient engagement in clinical studies?
Best practices for enhancing patient engagement include personalized communication, flexible and remote monitoring, and providing incentives and feedback to participants, contributing to a more effective and inclusive research environment.

