Overview
The article centers on best practices for fostering Medtech innovation through Colombian research, underscoring the importance of:
- Grasping the local regulatory landscape
- Leveraging technology
- Customizing clinical approaches
It asserts that by:
- Engaging local expertise
- Utilizing advanced technologies
- Tailoring research methodologies to address regional health challenges
Companies can adeptly navigate the complexities of the Colombian Medtech environment. This strategic alignment not only enhances their prospects for successful innovation but also facilitates smoother market entry.
Introduction
As the Colombian Medtech landscape undergoes a dynamic transformation, the demand for innovative medical devices is surging, driven by an expanding market projected to reach $2.21 billion by 2030. This growth is not merely a statistic; it reflects a confluence of factors, including:
- Increased healthcare spending
- A burgeoning population
- Rapid technological advancements
With a regulatory environment that is becoming increasingly accommodating, companies are now presented with unprecedented opportunities to introduce groundbreaking solutions. However, navigating this vibrant yet complex market necessitates a deep understanding of:
- The local context
- Regulatory compliance
- Effective recruitment strategies for clinical trials
As Colombia positions itself as a key player in the Medtech sector, insights into these critical elements will be essential for companies aiming to thrive in this promising arena.
Understanding the Colombian Medtech Landscape
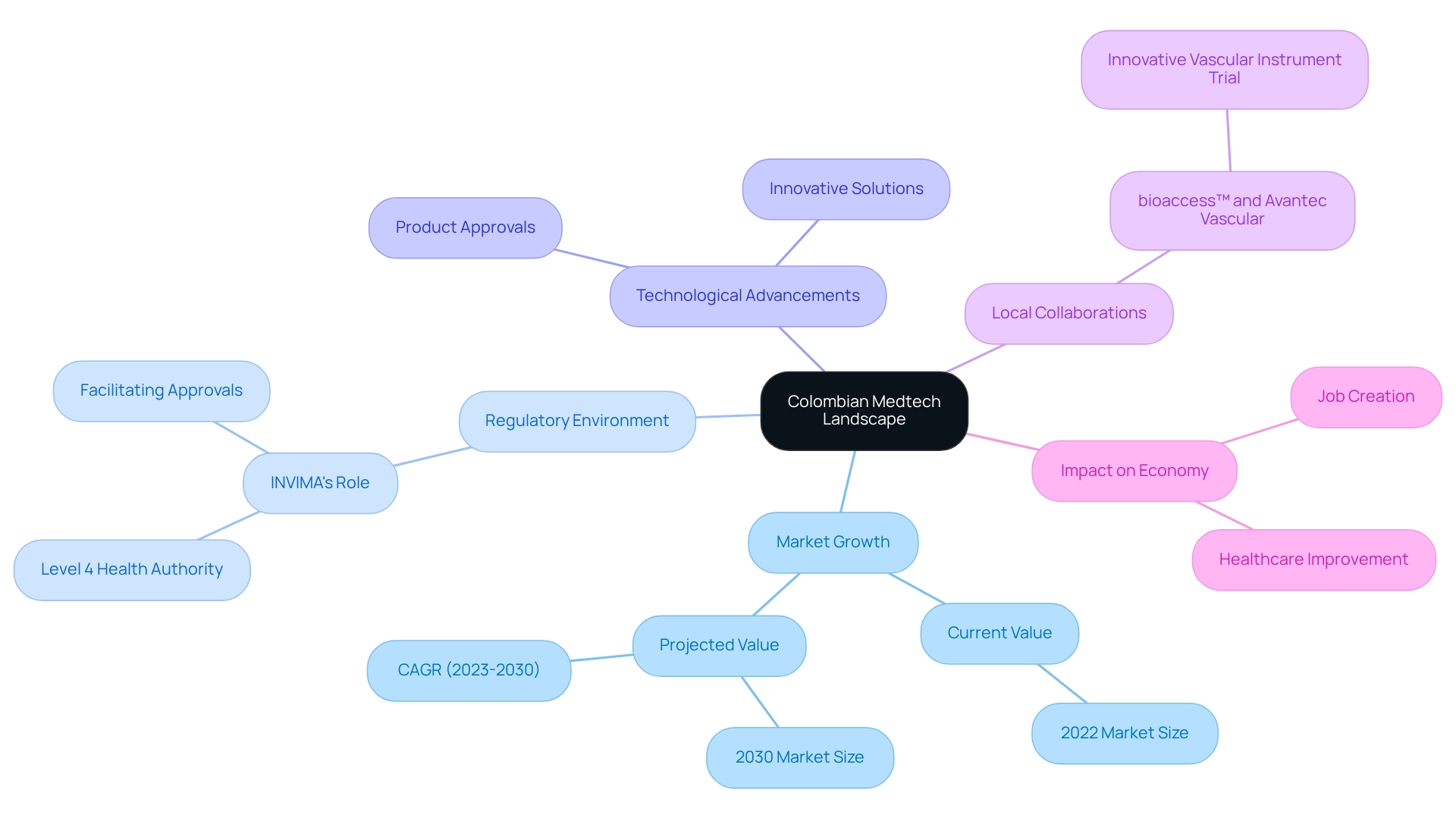
Colombia's Medtech environment is undergoing significant transformation, marked by an increasing demand for innovative medical equipment and a regulatory framework that supports such advancements. This evolution reflects the potential for Medtech innovation driven by Colombian research. Currently valued at approximately $1.42 billion in 2022, the market is projected to grow to $2.21 billion by 2030, representing a compound annual growth rate (CAGR) of 5.7% from 2023 to 2030. This upward trajectory is propelled by several factors, including heightened healthcare spending, a growing population, and rapid technological advancements.
According to Fortune Business Insights, technological advancements and a rise in product approvals globally are anticipated to drive the adoption of these innovative solutions. Key players in this expanding market include both local enterprises and international firms keen to capitalize on Colombia's strategic advantages, such as lower operational costs and expedited regulatory approvals. The regulatory landscape in Colombia, overseen by INVIMA, is becoming increasingly accommodating, which is essential for companies aiming to innovate in Medtech through Colombian research, as it facilitates smoother pathways for medical equipment approvals. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its pivotal role in overseeing medical device regulations.
Furthermore, the rising incidence of chronic diseases, with the pooled incidence of Rheumatoid Arthritis estimated at 11.0% annually, highlights the pressing need for advanced medical technologies. Insights from the 'Colombia Medical Devices Market Analysis' case examination reveal market trends and key industry developments, emphasizing the necessity of understanding these dynamics. Additionally, Horizon Databook provides customizable reports and analytics, enabling Medtech companies to tailor market insights to their specific business needs.
The significant impact of Medtech innovation through Colombian research on local economies contributes to job creation, economic growth, and healthcare improvement. For instance, bioaccess™ has partnered with Avantec Vascular to support their initial human trial of an innovative vascular instrument in Latin America, illustrating the potential for global collaboration in this sector. bioaccess™ delivers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, project management, and reporting.
As the market continues to evolve, understanding these dynamics is crucial for Medtech companies striving to effectively navigate this promising yet complex landscape.
Navigating Regulatory Compliance in Colombia
Colombia's regulatory environment for medical equipment is overseen by the National Food and Drug Surveillance Institute (INVIMA), which utilizes a structured four-tiered risk classification system. This system classifies equipment based on their potential risk to patients, thereby influencing the level of scrutiny and the approval timeline. Depending on the category of the equipment, the approval process can range from two to six months.
The review process for clinical trial applications by INVIMA involves an initial evaluation for document completeness, followed by a thorough review by specialists who examine scientific, technical, and ethical aspects prior to final approval.
To navigate this intricate environment efficiently, Medtech companies are encouraged to partner with local regulatory experts, such as bioaccess™, a prominent contract research organization that supports medical product clinical trials in Latin America. For instance, bioaccess™ recently collaborated with Welwaze Medical Inc. to facilitate the introduction of the Celbrea® medical product in Colombia, showcasing its expertise in regulatory and market access consulting. Engaging in pre-submission meetings with INVIMA can clarify specific requirements and streamline the approval process.
Moreover, with Colombia's aging population growing at 3.5% annually, outpacing total population growth (1.7%), the demand for innovative medical devices is increasingly critical. Staying informed about regulatory updates is essential, particularly as INVIMA continues to refine its processes in 2025. By leveraging local expertise and understanding the intricacies of the risk classification system, companies can significantly enhance their chances of successful market entry in Colombia. This proactive approach not only bolsters compliance but also fosters Medtech innovation through Colombian research, ultimately benefiting patients and healthcare systems alike.
Furthermore, developing an audit plan prior to conducting a quality management system audit is crucial for ensuring compliance with INVIMA's standards. Companies can also benefit from partnerships, such as with Pure Global, which facilitate a single registration process that simplifies entry into multiple markets, thereby easing the regulatory process for companies aiming to expand their market reach.

Effective Recruitment Strategies for Clinical Trials
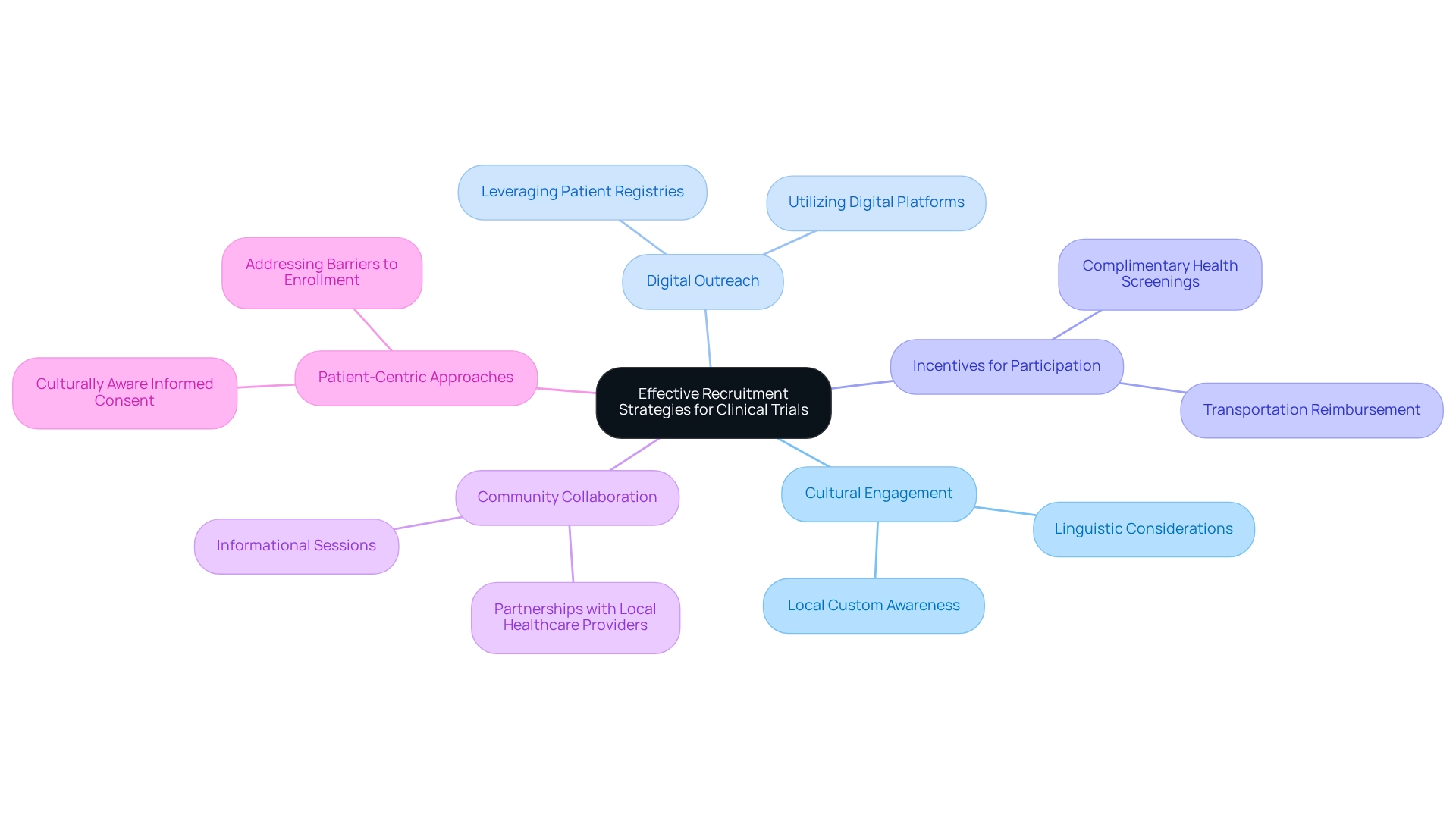
Successful recruitment approaches in Colombia must emphasize cultural subtleties and regional healthcare methods to enhance involvement in research studies. Engaging with communities through initiatives such as informational sessions and collaborations with local healthcare providers fosters trust and encourages participation. Furthermore, utilizing digital platforms for outreach and leveraging patient registries can significantly streamline the recruitment process.
Incentives, such as transportation reimbursement and complimentary health screenings, can further improve participation rates. Notably, experts indicate that dropout rates in Latin America are approximately one-third of those in the U.S. and EU, highlighting the potential for successful patient retention when these strategies are effectively employed.
A relevant case study demonstrates that effectively addressing linguistic and cultural differences can reduce misunderstandings and delays in ethics committee approvals. This ensures that patient rights are respected while aligning with local customs. By adopting a patient-centric approach and proactively addressing barriers to enrollment, Medtech innovation through Colombian research can lead to substantial improvements in recruitment outcomes. Insights from research professionals underscore the importance of honoring local customs and ensuring that informed consent procedures are culturally aware, resulting in more effective engagement and increased patient participation rates in studies throughout Colombia.
As Julio G. Martinez-Clark, CEO of bioaccess®, observes, "The government of Colombia appears to be the sole nation in Latin America with a proactive effort to draw more research studies as part of its strategy to evolve into a knowledge economy by 2031." This initiative aligns with bioaccess®'s expertise in comprehensive research management services, including feasibility studies, compliance evaluations, setup, import permits, project oversight, and reporting, all aimed at fostering Medtech innovation through Colombian research. Additionally, the partnership between bioaccess® and Caribbean Health Group aims to establish Barranquilla as a premier location for medical studies in Latin America, supported by the Minister of Health, thus reinforcing the firm's commitment to enhancing medical devices through efficient recruitment methods tailored for the Colombian context.
Customizing Clinical Research Approaches for Local Contexts
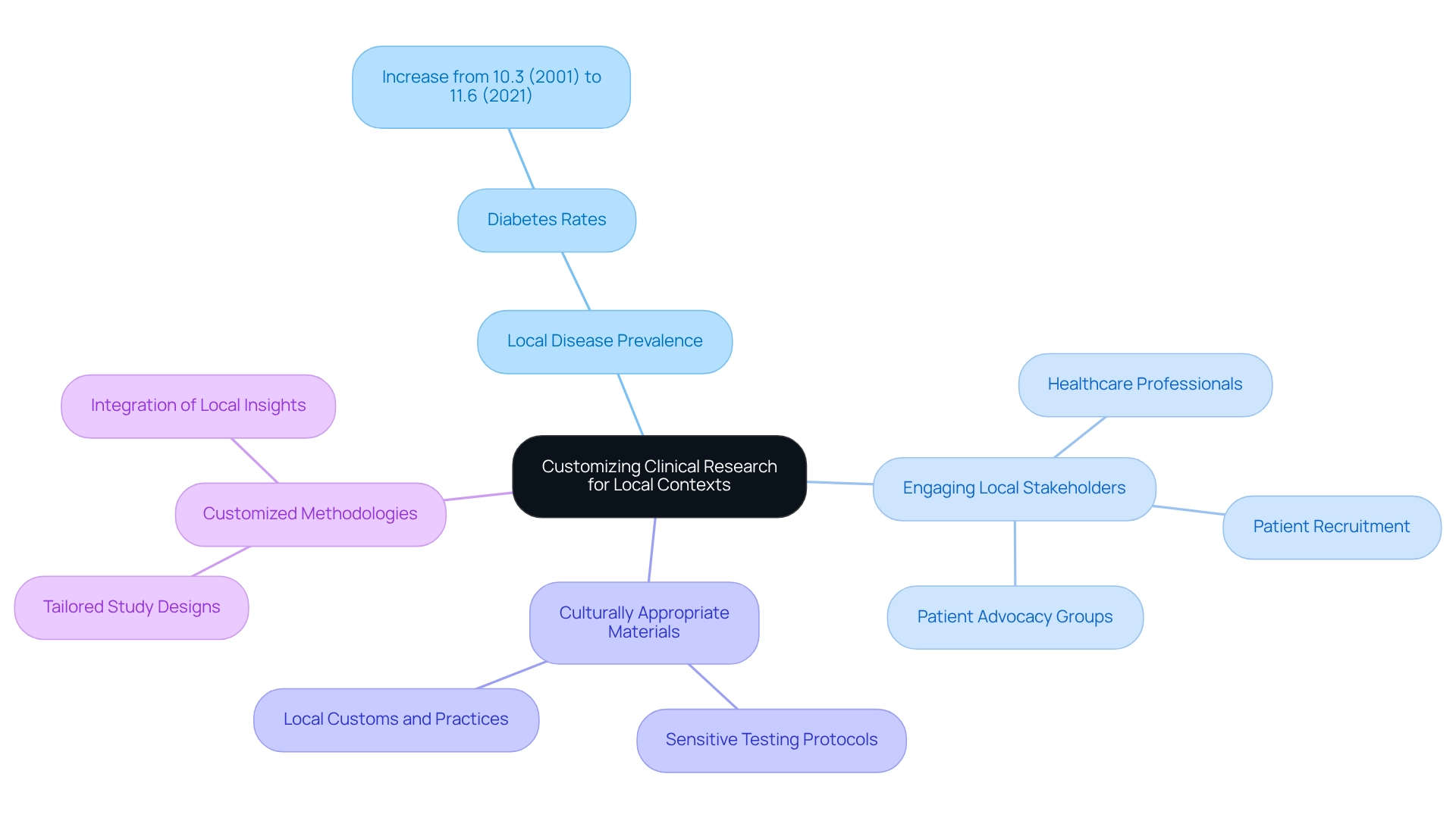
To enhance the effectiveness of clinical studies in Colombia, it is vital to customize research methodologies to address the specific health challenges and patient needs prevalent in the region. This customization may involve adapting study designs to reflect local disease prevalence, such as the rising rates of diabetes, which increased from 10.3% in 2001 to 11.6% in 2021. Employing culturally appropriate materials and ensuring that testing protocols are sensitive to local customs and practices are essential steps in this process.
Engaging local stakeholders, including healthcare professionals and patient advocacy groups, can yield invaluable insights into the community's needs and preferences. Healthcare experts frequently highlight the significance of grasping patient viewpoints to improve recruitment and retention in research studies. As Dr. Maria Lopez, a local doctor, states, "Comprehending the distinct health issues encountered by our patients is essential for creating effective studies that genuinely meet their needs."
By integrating these insights, Medtech companies can drive innovation through Colombian research, significantly enhancing the relevance of their studies and leading to improved participant engagement and results. Moreover, culturally appropriate clinical study designs foster trust among participants and enhance the overall quality of the research. Case analyses from earlier trials in Colombia, such as ReGelTec's successful Early Feasibility Assessment on HYDRAFIL™ for addressing chronic low back pain, demonstrate that when investigations align with local health challenges, they produce more dependable data and enable smoother regulatory approvals.
With over 20 years of experience in Medtech, bioaccess® possesses the expertise to manage a comprehensive range of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. This customized approach ensures that Medtech innovation through Colombian research enables companies to advance medical devices sooner, ultimately driving innovation and improving health outcomes in the region. By tailoring research methodologies to address the unique needs of Colombian patients, bioaccess® aids in connecting innovative solutions and effective healthcare delivery.
Leveraging Technology to Enhance Trial Efficiency
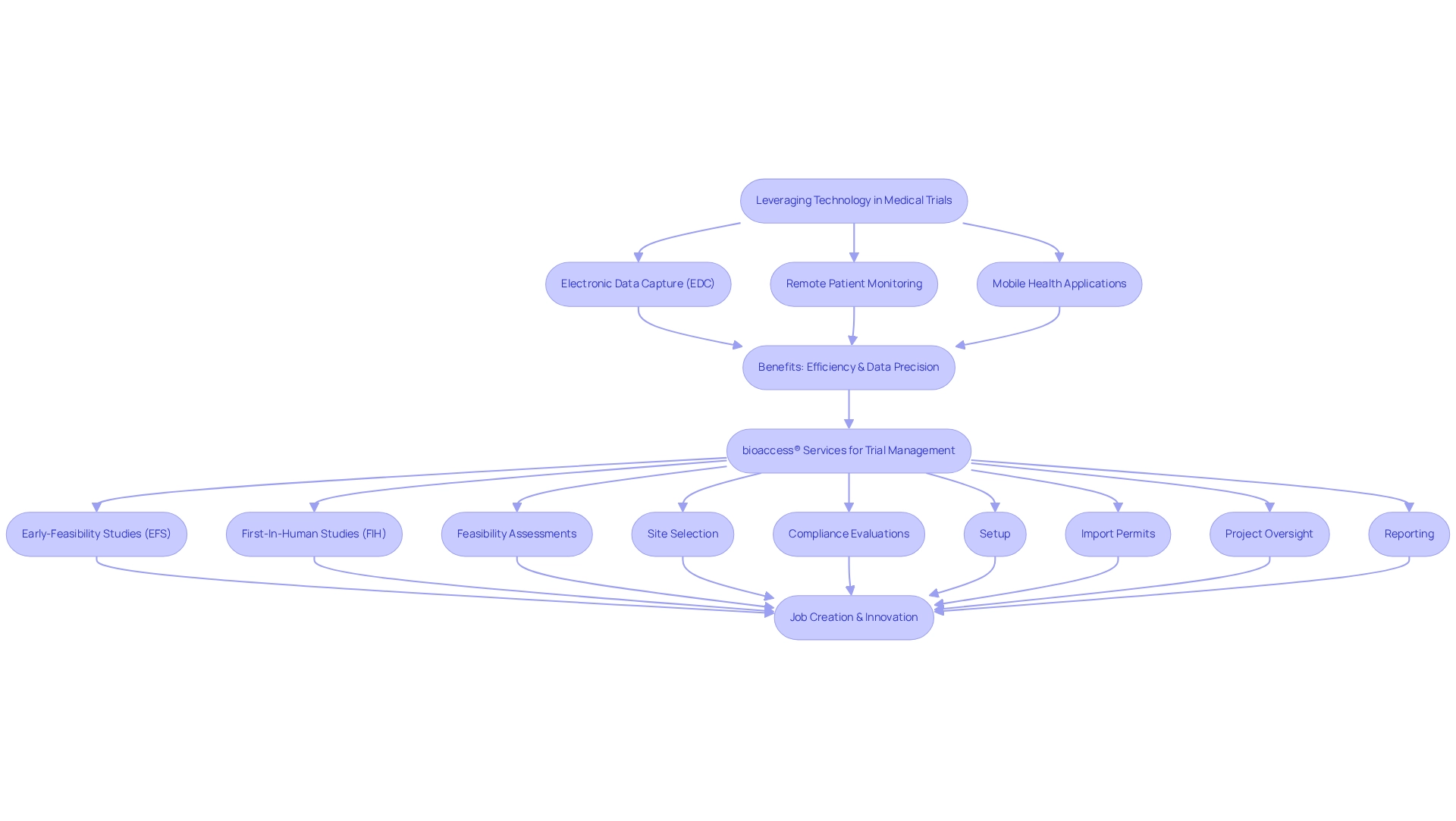
Utilizing technology in medical trials is paramount for achieving medtech innovation through Colombian research, enhancing efficiency and ensuring data precision. Electronic data capture (EDC) systems, remote patient monitoring devices, and mobile health applications play a crucial role in streamlining data collection processes and fostering patient engagement. A prime example is the Apple Heart Study, which effectively executed remote procedures with 420,000 participants, showcasing the potential of digital tools in medical research.
Furthermore, the integration of artificial intelligence (AI) for data analysis accelerates the identification of trends and outcomes, facilitating more agile decision-making. This technological advancement not only reduces operational costs but also significantly elevates the overall quality of research outcomes. The COVID-19 pandemic has underscored this transition, leading to a swift adoption of digital methodologies, as illustrated in the case analysis 'Impact of COVID-19 on Digital Technology Adoption in Clinical Trials.'
This crisis demonstrated that maintaining research integrity and participant safety is achievable through innovative solutions, indicating a lasting shift towards more digital methods in medical studies.
In Latin America, bioaccess® provides comprehensive management services for studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These services are vital for managing the complexities of medical device research in the region, ultimately contributing to job creation, economic development, and fostering Medtech innovation through Colombian research.
However, it is essential to acknowledge the ongoing challenges related to the digital divide, which affects representation in medical research, particularly among underserved populations. To illustrate the broader implications of technology in clinical studies, Wilhelm and colleagues discuss methods to utilize the Internet, phones, and apps to provide cognitive-behavioral therapy to individuals with mental health disorders.
To fully leverage these advancements, Medtech companies must prioritize training for their teams, ensuring that these technologies are seamlessly integrated into the workflow. By doing so, they can enhance trial efficiency and ultimately facilitate the rapid progress of medical products in the market.
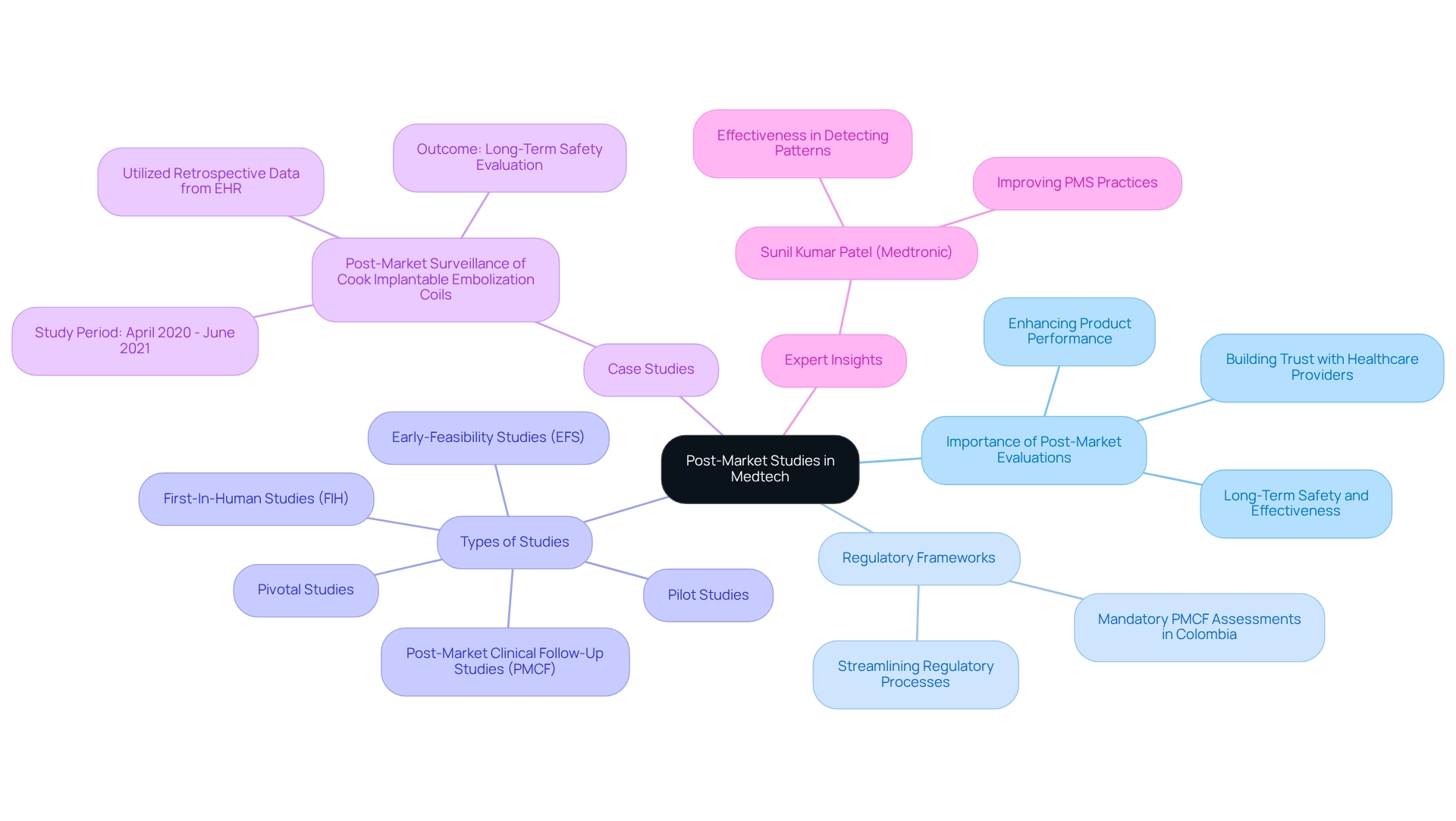
The Role of Post-Market Studies in Medtech Success
Post-market evaluations are essential to the life cycle of medical instruments, providing crucial insights into their long-term safety and effectiveness. These investigations are vital for recognizing uncommon negative occurrences and guiding necessary adjustments to enhance equipment performance. In Colombia, regulatory frameworks mandate companies to perform post-market clinical follow-up (PMCF) assessments, which are critical for fostering medtech innovation through Colombian research while ensuring ongoing adherence to safety standards.
By actively monitoring equipment performance and gathering real-world evidence (RWE), Medtech companies can not only enhance their products but also build trust among healthcare providers and patients. The importance of post-market research is underscored by recent findings, where technical success was achieved in 96.7% of instances, surpassing the performance target of 85% according to SIR guidelines. This statistic emphasizes the effectiveness of rigorous post-market surveillance in enhancing product safety and efficacy. Notably, only one participant indicated they had never performed post-market clinical research evaluations, suggesting a strong industry commitment to these essential assessments.
Moreover, expert insights highlight that robust post-market surveillance strategies are crucial for maintaining a competitive edge in the rapidly evolving Medtech landscape. As noted by industry leader Sunil Kumar Patel from Medtronic, "The results underscore CPA’s effectiveness in detecting temporal patterns, improving PMS practices, and streamlining regulatory processes to enhance medical device safety." These investigations not only improve patient outcomes but also simplify regulatory procedures, thus enabling faster adjustments to market demands.
A notable case analysis is the post-market surveillance of Cook Implantable Embolization Coils, which aimed to verify the ongoing safety and performance of various coil models. Conducted between April 2020 and June 2021, this research utilized retrospective data from an Electronic Health Record (EHR) database, successfully extracting RWE that informed long-term safety evaluations. Such methodologies exemplify innovative approaches to obtaining evidence that can expedite regulatory decision-making and enhance post-market surveillance efforts.
In 2025, the evolution of Colombia's PMCF criteria will underscore the necessity for Medtech innovation through Colombian research, ensuring that companies remain informed and compliant. Investing in thorough post-market research is not merely a regulatory requirement; it is a strategic necessity that can significantly impact the success and longevity of medical products in the marketplace. At bioaccess®, with over 20 years of experience in Medtech, our goal is to help advance medical devices sooner, providing valuable solutions for companies in the Medtech industry.
We specialize in managing a range of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
ensuring that our clients navigate the complexities of clinical trials effectively.
Conclusion
The Colombian Medtech landscape is on the brink of significant growth, fueled by rising healthcare spending, a growing population, and swift technological advancements. With the market projected to expand substantially by 2030, it is essential for companies to grasp the complexities of regulatory compliance, develop effective recruitment strategies for clinical trials, and recognize the critical role of post-market studies to seize this opportunity.
Navigating the regulatory environment, primarily overseen by INVIMA, necessitates a strategic approach to ensure successful market entry. Collaborating with local experts and remaining updated on evolving regulations will empower Medtech companies to streamline their product approvals and foster innovation. Furthermore, adapting clinical research methodologies to the local context is vital for enhancing participant engagement and addressing the unique health challenges faced by Colombian patients.
In addition, harnessing technology to boost trial efficiency and improve data accuracy is indispensable in today's digital landscape. By embracing innovative solutions, Medtech companies can not only accelerate their research processes but also contribute to better healthcare outcomes across the region.
Ultimately, achieving success in Colombia's Medtech sector hinges on a thorough understanding of market dynamics, a commitment to regulatory excellence, and a dedication to culturally sensitive practices. With the right strategies in place, companies can position themselves as leaders in this rapidly evolving landscape, driving advancements that will benefit both patients and the healthcare system alike.
Frequently Asked Questions
What is the current value of Colombia's Medtech market and its projected growth?
The Medtech market in Colombia is currently valued at approximately $1.42 billion in 2022 and is projected to grow to $2.21 billion by 2030, representing a compound annual growth rate (CAGR) of 5.7% from 2023 to 2030.
What factors are driving the growth of the Medtech market in Colombia?
The growth of the Medtech market in Colombia is driven by heightened healthcare spending, a growing population, rapid technological advancements, and an increasing demand for innovative medical equipment.
How is the regulatory environment for medical equipment in Colombia structured?
The regulatory environment is overseen by the National Food and Drug Surveillance Institute (INVIMA), which utilizes a structured four-tiered risk classification system to classify equipment based on their potential risk to patients.
What is the typical approval timeline for medical equipment in Colombia?
The approval process for medical equipment can range from two to six months, depending on the risk category of the equipment.
How can Medtech companies navigate the regulatory environment in Colombia effectively?
Medtech companies can navigate the regulatory environment by partnering with local regulatory experts, such as bioaccess™, and engaging in pre-submission meetings with INVIMA to clarify specific requirements.
What role does INVIMA play in the clinical trial application process?
INVIMA conducts an initial evaluation for document completeness followed by a thorough review by specialists who examine scientific, technical, and ethical aspects before granting final approval for clinical trial applications.
Why is there an increasing demand for innovative medical devices in Colombia?
The demand for innovative medical devices is increasing due to Colombia's aging population, which is growing at 3.5% annually, outpacing the total population growth of 1.7%.
What are the benefits of developing an audit plan prior to a quality management system audit?
Developing an audit plan is crucial for ensuring compliance with INVIMA's standards, which can enhance a company's chances of successful market entry in Colombia.
How can partnerships facilitate market entry for Medtech companies in Colombia?
Partnerships, such as with Pure Global, can facilitate a single registration process that simplifies entry into multiple markets, easing the regulatory process for companies aiming to expand their market reach.




