Overview
Post-market study strategies in Mexico are crucial for ensuring compliance and success in the medical device sector. These strategies play a vital role in monitoring product performance and safety following market approval. Understanding regulatory requirements is essential, as is the development of robust plans. Engaging stakeholders, leveraging technology, and building strategic partnerships are critical best practices that not only enhance compliance but also foster innovation. Ultimately, these efforts lead to improved patient outcomes.
Introduction
In the dynamic world of medical technology, post-market studies serve as a critical lifeline for ensuring the safety and efficacy of devices long after they hit the market. These evaluations not only fulfill regulatory requirements but also provide invaluable insights that can shape future innovations.
In Mexico, where the regulatory landscape is governed by COFEPRIS, understanding the intricacies of these studies is paramount for Medtech companies aiming for compliance and success. As the industry shifts towards a more data-driven approach, the incorporation of post-market surveillance into clinical evaluations is becoming increasingly vital.
This article delves into the fundamental aspects of post-market studies, the challenges faced, and best practices for navigating the complexities of the regulatory environment, ultimately highlighting the strategic advantages that come with effective post-market research.
Understanding Post-Market Studies: A Foundation for Compliance
Post-market assessments are crucial evaluations conducted after a medical product has received market approval, serving to monitor the product's performance in real-world environments. These investigations are vital for ensuring that medical devices consistently meet safety and effectiveness standards over time. A comprehensive understanding of the framework surrounding these analyses is essential for compliance, as they yield critical information that can influence future product development and regulatory decisions.
Furthermore, these assessments play a significant role in identifying long-term effects or rare adverse events that may not have been evident during pre-market trials. Thus, a solid grasp of post-market study strategies for Mexico is foundational for any Medtech company operating in the region.
In Mexico, the regulatory body COFEPRIS mandates post-market study strategies to ensure that medical devices remain safe and effective throughout their lifecycle. Companies must be prepared to implement these strategies as part of their surveillance obligations, which can significantly impact their reputation and market success. Recent trends indicate an increasing emphasis on integrating post-market surveillance (PMS) information into clinical evaluation reports (CERs), as highlighted by the EU Medical Device Regulation (MDR).
This shift underscores the necessity for manufacturers to actively collect and assess PMS information to keep their CERs updated, ensuring that clinical evaluations reflect the most current safety and performance details.
Moreover, regulations stipulate that for a relevant clinical trial, the responsible party must update the Enrollment information element to reflect the actual number of human subjects enrolled after reaching the primary completion date. Compliance with these regulations is not merely a legal obligation; it also serves as a strategic advantage. For instance, companies that neglect to comply with clinical trial registration and results submission requirements may face serious legal repercussions, including civil or criminal actions and monetary penalties.
Thus, the significance of post-market evaluations extends beyond compliance; they are essential for maintaining a competitive edge in the Medtech landscape.
A pertinent case analysis titled 'Postmarket Surveillance Information in Clinical Evaluation Reports' addresses the growing focus on incorporating PMS information in CERs for medical devices. It highlights the challenges manufacturers face in determining how much PMS data to include in their CERs and encourages them to actively gather and evaluate this data to ensure their clinical evaluations reflect the latest safety and performance information. By effectively managing post-market study strategies for Mexico, companies can enhance their credibility and foster trust among stakeholders, ultimately leading to greater market success.
bioaccess® aims to accelerate medical technologies through its expertise and tailored approach, offering valuable assistance to Medtech companies in navigating the complexities of post-market study strategies for Mexico, which encompass Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® is committed to ensuring that your clinical trials are conducted with the highest standards of compliance excellence and innovation.
Navigating the Regulatory Landscape for Medical Devices in Mexico
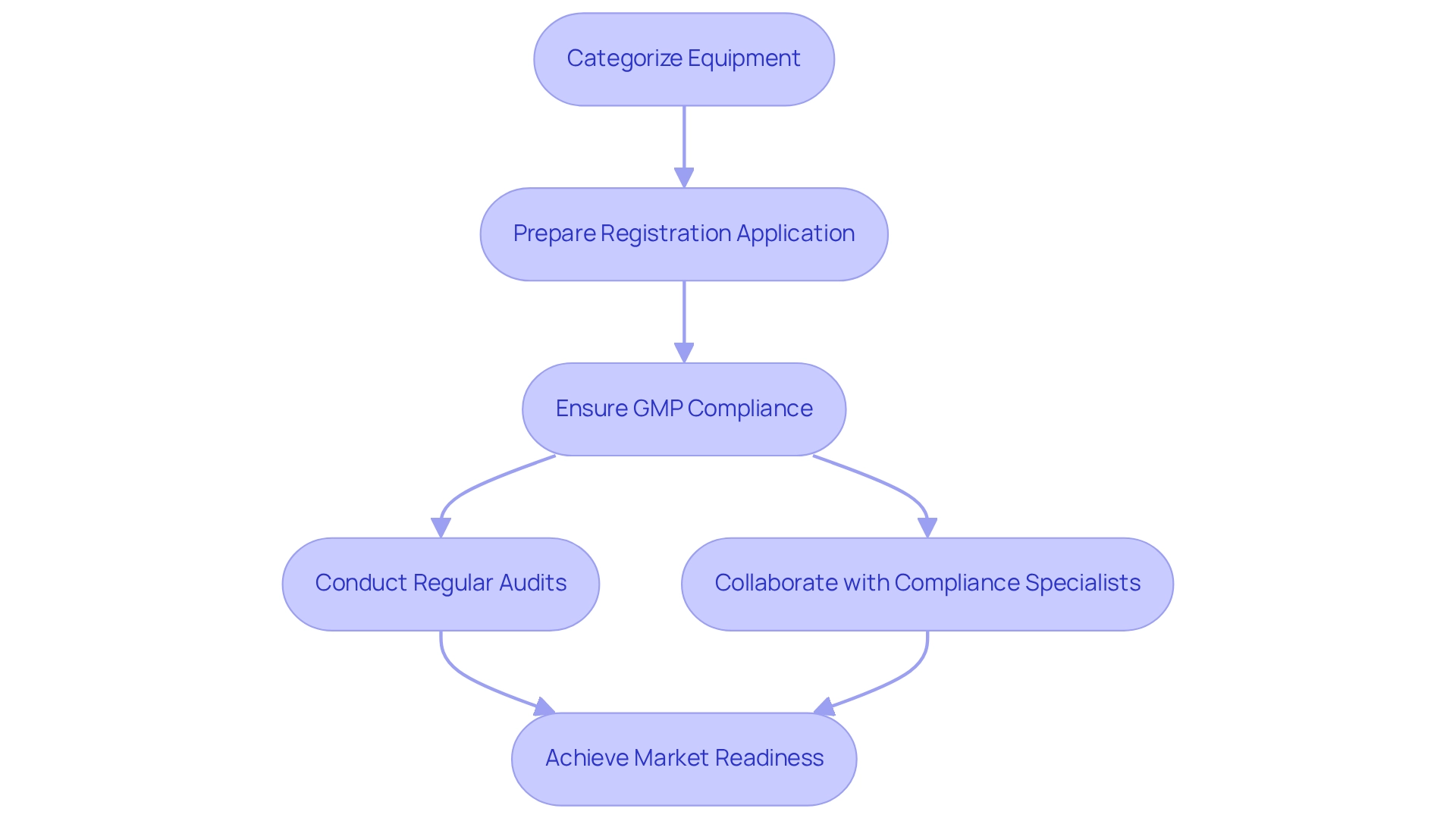
Navigating the compliance environment for medical equipment in Mexico necessitates a comprehensive understanding of the criteria set by COFEPRIS, the Federal Commission for Protection against Sanitary Risks. This agency is responsible for the approval and monitoring of medical products, ensuring they adhere to stringent safety and efficacy standards. An essential initial action for businesses is to precisely categorize their equipment, as this categorization determines the compliance route and the degree of examination involved in the approval procedure.
To effectively maneuver through this environment, companies must present a thorough registration application that encompasses detailed clinical information, production processes, and strong post-market study strategies for Mexico. Adhering to Good Manufacturing Practices (GMP) is crucial, as it guarantees product quality and conforms to compliance expectations. Regular audits are necessary to maintain adherence to these standards, thereby minimizing the risk of non-compliance.
MDSAP auditors are skilled and well-prepared, utilizing a task-oriented audit strategy that decreases the number of audits and causes less business interruption.
Collaborating with local compliance specialists, such as Katherine Ruiz, who focuses on compliance matters for medical instruments and in vitro diagnostics in Colombia, can greatly simplify the approval process. Their insights into the latest COFEPRIS compliance requirements for 2025, including documentation for changes in packaging materials and the necessity of a stability study report covering shelf life or expiration date, can be invaluable. For instance, the recent establishment of the E14/S7B Implementation Working Group highlights the importance of standardized practices in clinical evaluations, which can also be applied to medical device assessments, ensuring consistency and reliability in the approval process. Statistics indicate that understanding the nuances of the medical device approval process in Mexico can lead to a more efficient market entry.
Companies that successfully navigate these regulations often report reduced timeframes for approval and enhanced market readiness. By utilizing expert knowledge and following established guidelines, Medtech companies can ensure compliance and pave the way for successful product launches in the Mexican market. With Bioaccess's comprehensive clinical trial management services, including expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), along with over 20 years of experience in Medtech, companies can confidently navigate the complexities of clinical trials in Latin America. Bioaccess's tailored approach further improves the ability to navigate the compliance landscape effectively.
The Role of Post-Market Clinical Follow-Up in Ensuring Compliance
Post-Market Clinical Follow-Up (PMCF) serves as a cornerstone of post-market surveillance, involving systematic data collection and analysis to evaluate the ongoing safety and performance of medical instruments. These analyses are meticulously designed to gather real-world evidence regarding a product's effectiveness and to identify any potential adverse effects that may emerge post-market introduction. In Mexico, adopting post-market study strategies is not merely a compliance requirement; it represents a best practice that significantly enhances a company's credibility and fosters trust among healthcare providers and patients.
PMCF research can manifest in various forms, including surveys, registries, and observational analyses, necessitating careful tailoring to the specific equipment and its intended application. By implementing comprehensive PMCF strategies, firms such as bioaccess®, with over 20 years of experience in Medtech, can proactively identify and address challenges, ensuring compliance while ultimately improving patient outcomes.
The importance of PMCF research within the medical device industry is paramount. These evaluations play a critical role in the regulatory landscape, facilitating continuous reassessment of safety and performance. Experts in the MedTech field emphasize that these analyses not only enhance patient safety but also contribute to the ongoing evolution of medical technologies, effectively bridging the gap between clinical trials and clinical practice.
PMCF analyses are vital for understanding the real-world impact of medical instruments and ensuring adherence to the highest safety standards.
Best practices for conducting PMCF analyses in 2025 underscore the need for a systematic approach that includes clear objectives, appropriate methodologies, and thorough data evaluation techniques. Real-world examples from various medical fields illustrate how effective PMCF evaluations have led to significant improvements in device safety, reinforcing the necessity of these assessments in ensuring compliance and fostering innovation in the medical device sector. In Mexico, post-market study strategies are crucial for companies navigating the complexities of the compliance landscape while upholding the highest standards of patient care.
Furthermore, bioaccess® provides a suite of services, including Early-Feasibility Studies, First-In-Human Studies, and Pivotal Studies, all specifically designed to address the unique challenges of the Latin American market.
Identifying and Overcoming Challenges in Post-Market Studies
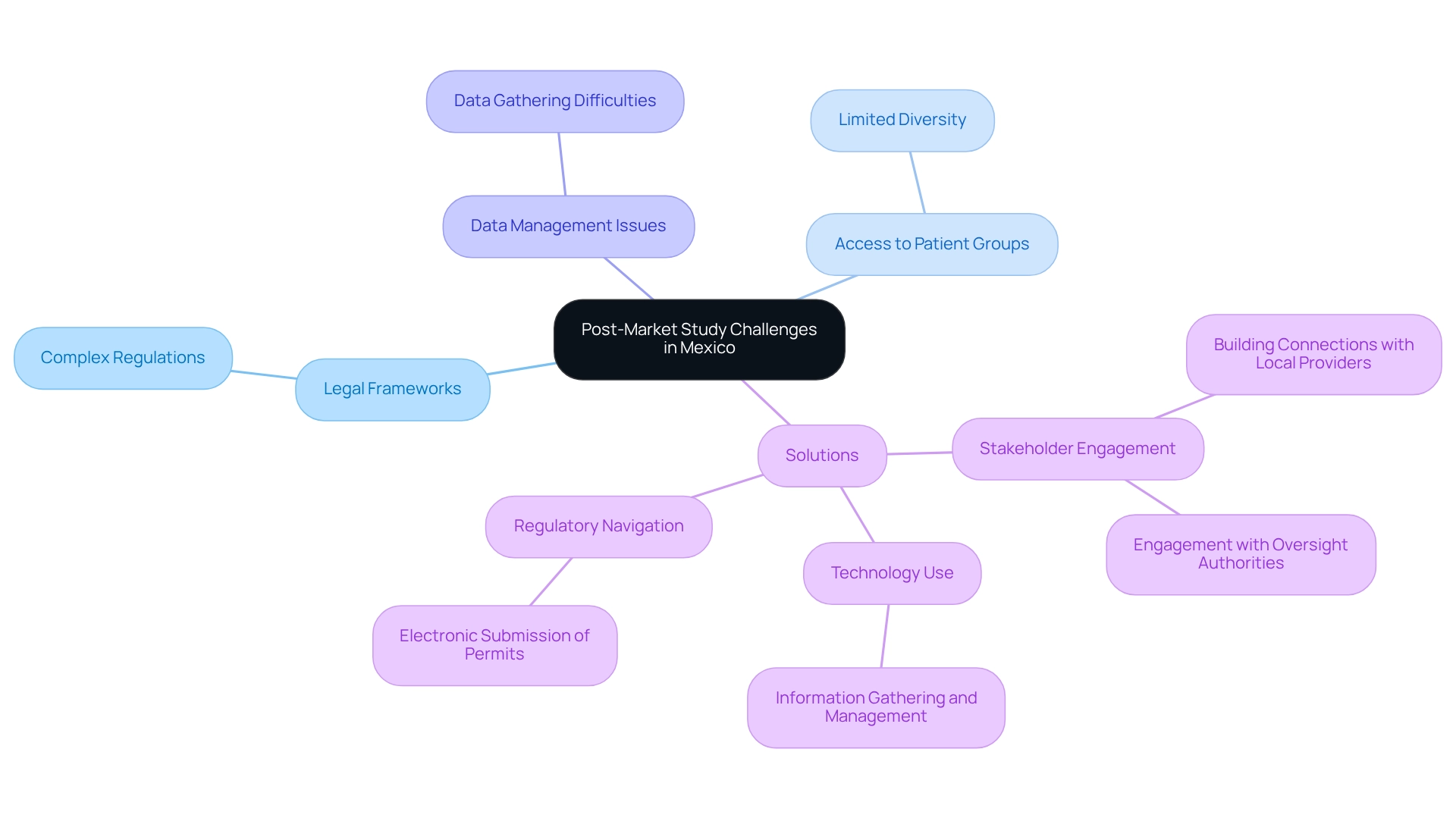
Implementing post-market study strategies for Mexico requires navigating a landscape filled with obstacles that can hinder adherence and achievement. Key challenges include:
- Complex legal frameworks
- Limited access to diverse patient groups
- Difficulties in data gathering and analysis
Cultural differences and the inequality in healthcare systems across various areas can greatly influence the practicality of these investigations.
To effectively address these challenges, companies should prioritize comprehensive planning and proactive stakeholder engagement. Building strong connections with local healthcare providers and oversight authorities is essential for enabling smoother study execution. Notably, applications for import or export permits can be submitted electronically or in person at COFEPRIS's service center, streamlining the regulatory process and enhancing efficiency.
Utilizing technology for information gathering and management not only boosts efficiency but also enhances the precision of outcomes. Organizations should remain adaptable, prepared to modify their strategies according to real-time insights and emerging trends, ensuring that their post-market analyses are both pertinent and efficient.
Statistics indicate that clinical records in Mexico must be preserved for at least five years after the final medical procedure or appointment, underscoring the significance of careful data management in post-market research. Furthermore, the composition of Research Ethics Committees (RECs) in Mexico mandates the inclusion of at least three scientists alongside community representatives, ensuring a diverse range of expertise and perspectives that enrich the ethical review process. This structure promotes transparency and safeguards the integrity of evaluations, free from external pressures.
A case analysis titled 'Financial Independence of Research Ethics Committees' emphasizes that RECs do not charge sponsors for their evaluations and are supported by the health institution, ensuring that funding does not generate conflicts of interest.
As an example of successful navigation within the Mexican Medtech landscape, Luis Armando Bravo Castillo, founder of Probiotics Prosthetic Limbs, has produced prosthetic limbs at a significantly lower cost than other companies since 2008. This illustrates how innovative companies can thrive despite regulatory challenges.
By understanding these dynamics and implementing post-market study strategies for Mexico, companies can navigate the complexities of post-market research, ultimately leading to successful outcomes and enhanced patient care. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research, organizations can leverage comprehensive clinical trial management services to ensure their projects are conducted efficiently and effectively. Bioaccess®'s 20+ years of experience and tailored approach distinctly equip them to tackle these challenges, guaranteeing that their clients attain successful results in post-market study strategies for Mexico.
Best Practices for Conducting Successful Post-Market Studies
To implement effective post-market study strategies for Mexico, companies must adhere to several best practices.
- Develop a Comprehensive PMCF Plan: Establish a detailed plan that outlines clear objectives, methodologies, and timelines for data collection and analysis. This plan should conform to compliance standards, specifically addressing safety and efficacy inquiries relevant to the medical instrument in question.
As emphasized by the European Union MDR requirements, effective PMS and vigilance are essential for monitoring device performance and ensuring adherence to compliance standards.
- Engage Stakeholders Early: Actively involve healthcare providers, patients, and oversight organizations during the planning phase. Early engagement not only garners valuable insights but also fosters collaboration, enhancing recruitment efforts and improving the overall quality of data collected.
As Sumatha Kondabolu, a QA/RA expert, underscores, engaging stakeholders is crucial for ensuring compliance with regulatory standards. This approach is particularly relevant in the diverse healthcare systems across Latin America, where local insights can significantly impact study outcomes.
- Utilize Technology: Embrace digital tools for effective information collection, management, and analysis. Implementing electronic information capture systems can streamline processes and improve accuracy, while advanced analytics can provide crucial insights into equipment performance and user experience. Analyzing PMS information aids in recognizing issues, identifying new applications for devices, and guiding essential measures to reduce risks and enhance product design. bioaccess® utilizes its expertise in clinical trial management to incorporate these technologies efficiently, ensuring robust information handling throughout the study.
- Monitor and Adjust: Maintain continuous oversight of research progress and remain flexible to modify strategies based on emerging data and participant feedback. This proactive strategy facilitates the prompt resolution of unexpected challenges, ensuring the project remains aligned with its objectives. A comprehensive understanding of efficacy and safety, particularly for higher-risk devices, necessitates the implementation of post-market study strategies for Mexico.
bioaccess®'s experience in managing various study types, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, equips them to navigate these challenges adeptly.
- Communicate Findings: Share results with all stakeholders, including oversight organizations and healthcare providers. Transparent communication not only builds trust but also enhances the company's reputation within the market, reinforcing its commitment to safety and efficacy in medical device performance. By fostering open dialogue, companies can strengthen relationships with local stakeholders, which is vital for successful market access in the Latin American Medtech landscape.
Building Strategic Partnerships for Effective Post-Market Research
Building strategic partnerships is crucial for maximizing the effectiveness of post-market study strategies in Mexico. Partnering with local healthcare institutions, research organizations, and regulatory experts not only enriches research design but also enhances execution through shared insights and resources. Research highlights that a recommendation from a trusted friend is up to 50 times more likely to result in a purchase than a low-impact recommendation, underscoring the critical importance of trust in partnerships.
- Identify Key Partners: Seek organizations that align with your objectives and values. Potential partners may include hospitals, universities, and other contract research organizations (CROs) that possess experience in the Latin American market. For instance, bioaccess™ has successfully collaborated with Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America, supported by Colombia's Minister of Health. This alignment is essential for fostering a collaborative environment.
- Establish Clear Roles and Responsibilities: Clearly delineate the roles of each partner involved in the research. This clarity ensures accountability and streamlines communication, reducing the likelihood of misunderstandings and enhancing overall collaboration.
- Foster Open Communication: Regular communication is vital for discussing progress, addressing challenges, and sharing findings. Maintaining transparency strengthens relationships and facilitates effective problem-solving, which is essential in the dynamic landscape of clinical research.
- Utilize Shared Resources: Capitalize on the strengths and assets of each partner to enhance research capabilities. This may involve accessing diverse patient populations, utilizing advanced data management systems, or tapping into regulatory expertise, all of which can significantly enhance research outcomes. For example, GlobalCare Clinical Trials' partnership with bioaccess™ has led to an impressive reduction in recruitment time by over 50% and achieved 95% retention rates, showcasing the benefits of leveraging shared resources.
- Evaluate Partnership Outcomes: Upon completion of the research, conduct a thorough assessment of the partnership's effectiveness. Gathering feedback from all parties involved helps identify areas for improvement and lays the groundwork for strengthening future collaborations.
Strategic partnerships not only enhance the credibility of post-market study strategies in Mexico but also foster trust among stakeholders. As Diamond aptly stated, "It’s important to have partners that fill in your gaps, and that is what we have created." Furthermore, companies that engage in co-selling strategies report that nine out of ten find this approach easier than traditional reseller models, highlighting the efficiency of collaborative efforts.
Additionally, brand trust can be enhanced through strategic partnerships, as consumers may trust a brand more if it collaborates with a company they already trust. By recognizing and fostering the right collaborations, organizations can navigate the complexities of post-market study strategies in Mexico more efficiently, ultimately leading to successful outcomes in the medical equipment sector.
Leveraging Data Analysis for Enhanced Compliance and Outcomes
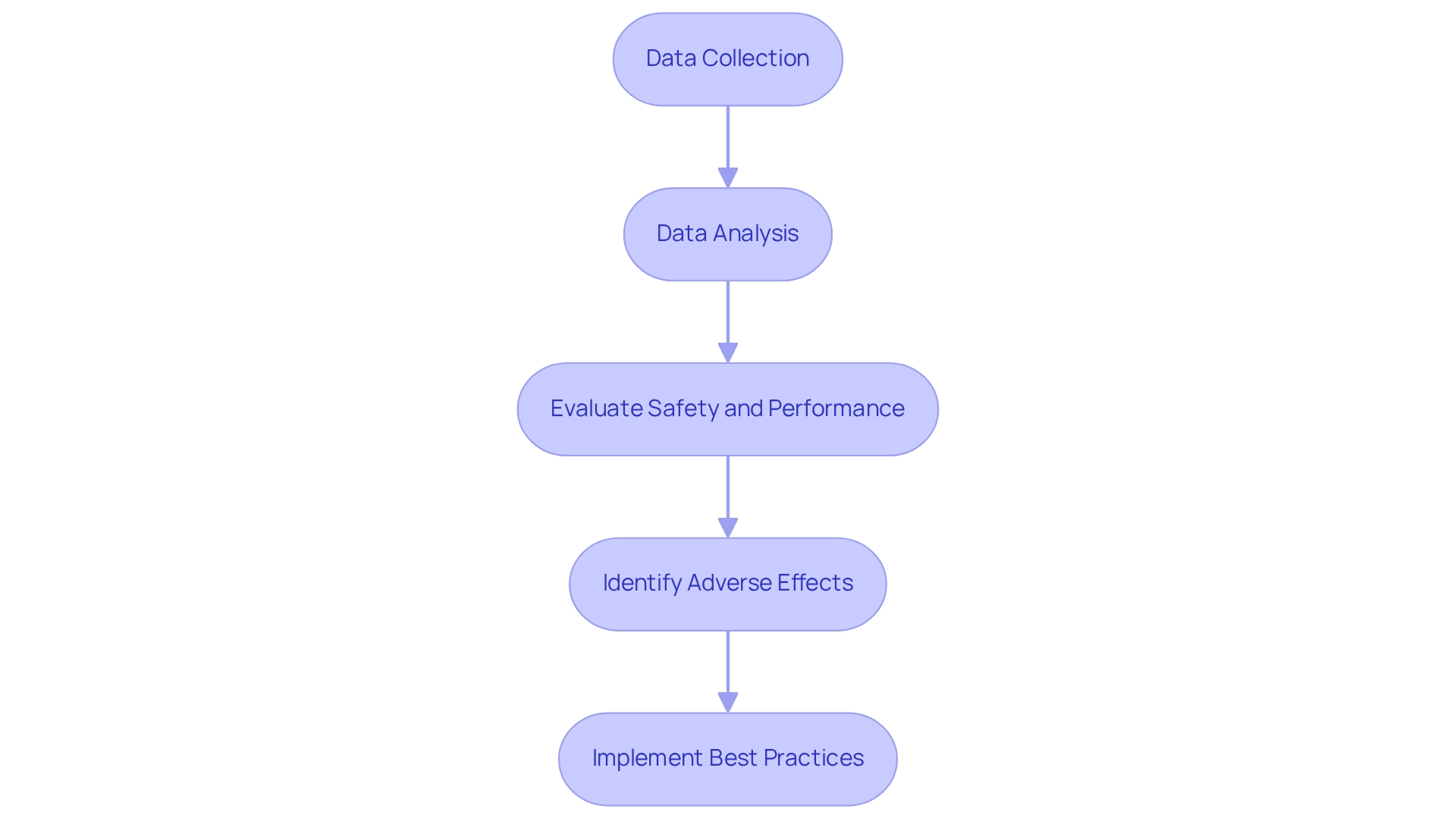
Analysis is essential to the success of post-market study strategies for Mexico, enabling companies to derive actionable insights from the information gathered. By utilizing efficient information analysis, organizations can improve compliance and optimize medical equipment performance in various important ways:
- Identify Trends and Patterns: Through comprehensive analysis, companies can uncover trends related to equipment performance, safety issues, and patient outcomes. This vital information can direct essential changes to product design or usage protocols, ensuring that items meet evolving needs.
- Ensure Compliance with Regulations: Regular data reviews against established standards are essential for maintaining adherence. This proactive strategy allows organizations to detect potential issues early, preventing them from escalating into significant compliance violations that could jeopardize market access. The FDA's Sentinel System, established under the FDAAA in 2007, illustrates a regulatory framework for active signal detection and regular assessment of new medical products, highlighting the significance of post-market study strategies for Mexico. In Latin America, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial as it oversees medical device regulation and classification, ensuring adherence to safety standards.
- Utilize Advanced Analytics: The application of advanced analytics techniques, including machine learning and predictive modeling, can significantly enhance data interpretation. These sophisticated methods reveal hidden insights that inform better decision-making and strategic planning. For instance, minimizing observer bias in preclinical studies is crucial for ensuring objective measurement of outcomes, as highlighted in recent case studies.
- Engage in Continuous Improvement: Data-driven findings should fuel continuous improvement initiatives within the organization. A steadfast commitment to quality not only enhances product safety and efficacy but also ultimately benefits patients by ensuring that instruments perform as intended. As Estelle Russek-Cohen noted, collaborative efforts and constructive feedback are vital for improving the content and outcomes of clinical research.
- Report Findings Effectively: Clear and concise reporting of results is crucial for communicating with stakeholders, including regulatory authorities. Effective reporting fosters transparency and reinforces trust in the organization’s dedication to safety and compliance, which is vital in the highly regulated Medtech landscape. Present educational materials, like the DATA 3100: Introduction to Statistical Methods at Penn LPS Online, can assist organizations in grasping statistical techniques pertinent to data examination in post-market evaluations.
By emphasizing data examination in post-market study strategies for Mexico, companies can ensure compliance while fostering innovation and enhancing the overall quality of medical devices. With bioaccess's comprehensive clinical trial management services, including feasibility assessments, site selection, and project oversight, along with expertise in Early-Feasibility Research (EFS), First-In-Human Research (FIH), and other crucial evaluations, organizations can effectively navigate the complexities of post-market study strategies for Mexico. With over 20 years of experience in Medtech, bioaccess is well-equipped to support your clinical research needs.
Key Takeaways for Successful Post-Market Study Strategies
To ensure successful post-market study strategies in Mexico, consider the following key takeaways:
-
Understand Regulatory Requirements: Familiarizing yourself with COFEPRIS regulations is crucial, as compliance is essential throughout the research lifecycle. Comprehending these regulations aids in obtaining smoother approvals and boosts the credibility of the research.
-
Develop a Robust PMCF Plan: A comprehensive Post-Market Clinical Follow-up (PMCF) plan should outline clear objectives, methodologies, and timelines for information collection and analysis. This organized method assists in systematically tackling the research objectives and ensures that all essential information is captured effectively.
-
Engage Stakeholders: Early involvement of healthcare providers, patients, and regulatory bodies fosters collaboration and enhances information quality. As Seth Godin aptly states, 'Build it, and they will come' only works in the movies. In reality, social media is about 'build it, nurture it, engage them and they may come and stay.' Engaging these stakeholders can provide valuable insights and enhance the relevance of the study outcomes.
-
Leverage Technology: Utilizing digital tools for information collection and management can significantly enhance accuracy and streamline processes. Technology enables real-time information access and assists in effective monitoring and reporting.
-
Monitor and Adapt: Continuous monitoring of progress is vital. Be ready to modify approaches according to new information and input, ensuring that the research stays in line with its goals and compliance needs.
-
Build Strategic Partnerships: Collaborating with local organizations can enhance study capabilities and resource sharing. These partnerships provide access to local expertise and facilitate smoother navigation of the regulatory landscape.
-
Utilize Information Analysis: Employing effective information analysis techniques is essential for deriving actionable insights. This practice ensures ongoing compliance and contributes to improved patient outcomes by informing future medical device enhancements.
-
Ensure Information Security: At bioaccess®, we prioritize data protection and client trust. If you have any queries or concerns regarding the processing of your information, you can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®'). We are committed to addressing your concerns in accordance with applicable law, ensuring transparency and compliance throughout the clinical trial process.
By implementing these post-market study strategies for Mexico, companies can effectively navigate the complexities of post-market studies, ensuring compliance while enhancing the overall quality of patient care. bioaccess® specializes in providing clinical research services for the medical technology sector, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® aims to help advanced medical devices reach the market sooner through their expertise and customized approach.
Conclusion
Post-market studies are not merely regulatory requirements; they are vital for ensuring the ongoing safety and efficacy of medical devices in real-world settings. In Mexico, where COFEPRIS governs the regulatory landscape, comprehending the complexities of these evaluations is essential for Medtech companies aiming for compliance and a competitive edge. By developing comprehensive Post-Market Clinical Follow-up (PMCF) plans, engaging stakeholders early, and leveraging technology, organizations can enhance their study outcomes while adhering to regulatory standards.
The challenges inherent in conducting post-market studies—such as navigating intricate regulations and accessing diverse patient populations—can be effectively mitigated through strategic planning and collaboration. Establishing partnerships with local healthcare institutions and regulatory experts can facilitate the smoother execution of studies and enrich data quality. Furthermore, effective data analysis plays a pivotal role in identifying trends and ensuring compliance, ultimately informing necessary product enhancements and improving patient outcomes.
Emphasizing best practices in post-market studies not only bolsters compliance but also fosters innovation within the Medtech industry. By prioritizing transparency, continuous improvement, and strategic partnerships, companies can adeptly navigate the regulatory complexities, paving the way for successful advancements in medical devices. As the landscape evolves, the commitment to rigorous post-market research will remain a cornerstone of patient safety and product excellence.
Frequently Asked Questions
What are post-market assessments?
Post-market assessments are evaluations conducted after a medical product has received market approval, aimed at monitoring the product's performance in real-world environments to ensure ongoing safety and effectiveness.
Why are post-market assessments important?
They are crucial for identifying long-term effects or rare adverse events that may not have been evident during pre-market trials, and they provide critical information that can influence future product development and regulatory decisions.
What role does COFEPRIS play in post-market assessments in Mexico?
COFEPRIS, the regulatory body in Mexico, mandates post-market study strategies to ensure that medical devices remain safe and effective throughout their lifecycle, requiring companies to implement these strategies as part of their surveillance obligations.
How do post-market surveillance (PMS) and clinical evaluation reports (CERs) relate?
There is an increasing emphasis on integrating PMS information into CERs, as required by the EU Medical Device Regulation (MDR), ensuring that clinical evaluations reflect the most current safety and performance information.
What are the consequences of neglecting compliance with clinical trial registration and results submission?
Companies that fail to comply may face serious legal repercussions, including civil or criminal actions and monetary penalties.
What are some post-market study strategies that Medtech companies should consider?
Companies should consider strategies such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How can companies enhance their credibility in the Medtech landscape?
By effectively managing post-market study strategies and actively gathering and evaluating PMS data, companies can enhance their credibility and foster trust among stakeholders.
What is the significance of understanding the compliance environment for medical equipment in Mexico?
A comprehensive understanding of COFEPRIS's criteria is essential for navigating the approval process, ensuring adherence to safety and efficacy standards, and minimizing the risk of non-compliance.
What is the benefit of collaborating with local compliance specialists?
Collaborating with local compliance specialists can simplify the approval process by providing insights into the latest COFEPRIS compliance requirements and helping navigate complex regulations.
How does Bioaccess assist Medtech companies in Mexico?
Bioaccess offers expertise in clinical trial management, including various study types, and helps companies navigate the complexities of post-market study strategies and compliance requirements in Mexico.




