Introduction
Navigating the regulatory landscape for medical devices in Latin America presents a unique set of challenges and opportunities. With each country maintaining its own set of regulations, understanding the intricate approval processes is crucial for companies aiming to introduce their products to this diverse market. In Colombia, for instance, the pathway to approval involves a series of steps, including securing necessary permissions from ethical committees and regulatory bodies like INVIMA.
As the region moves towards harmonization of regulations, organizations can benefit from strategic approaches that not only streamline these processes but also enhance their market readiness. By fostering local partnerships, engaging with regulatory experts, and leveraging clinical trials effectively, companies can significantly reduce approval timelines and accelerate their entry into Latin America's burgeoning healthcare sector.
Navigating the Regulatory Landscape for Medical Devices in Latin America
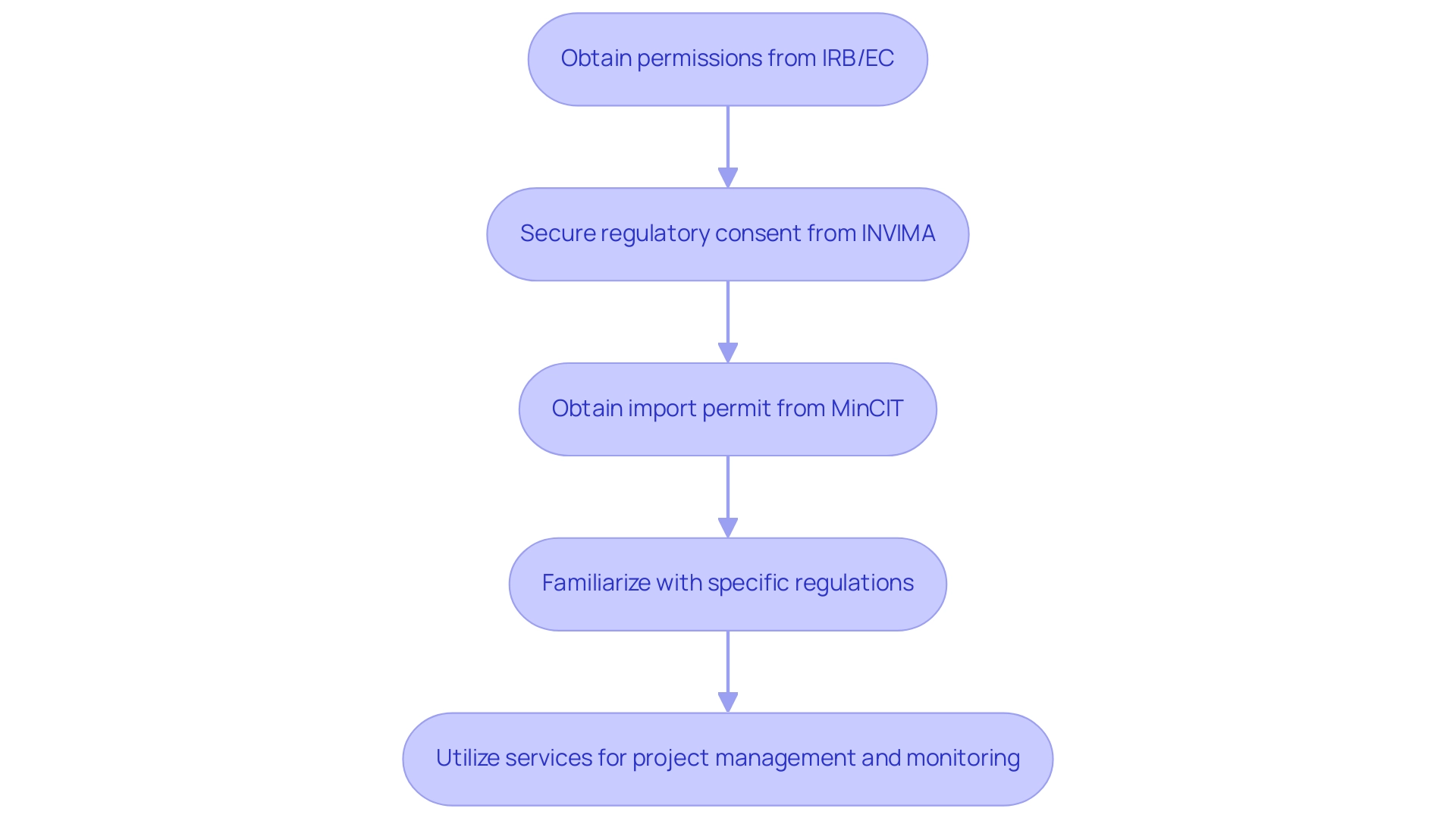
In Latin America, the oversight environment for medical devices differs by nation but is typically managed by national health authorities. In Colombia, the process for clinical trials involves several key steps:
- Obtaining necessary permissions from the institutional review board (IRB) or ethics committee (EC)
- Securing regulatory consent from INVIMA (Colombia National Food and Drug Surveillance Institute)
- Obtaining an import permit from the Ministry of Industry and Commerce (MinCIT)
INVIMA plays a crucial role in overseeing the marketing and manufacturing of health products and is classified as a Level 4 health authority by PAHO/WHO, ensuring compliance with international safety and efficacy standards. Companies must familiarize themselves with specific regulations, including:
- Registration requirements
- Safety and efficacy standards
- Post-market surveillance obligations
Furthermore, services like feasibility and selection of research locations, project management, monitoring, and reporting are crucial for navigating the authorization process efficiently. Experts like Katherine Ruiz, who have significant experience with INVIMA, can provide invaluable guidance. Moreover, numerous nations in the area are progressing towards aligning regulations, which can enable quicker endorsements if businesses are ready to comply with these changing standards.
Strategies for Accelerating Medical Device Approval in Latin America
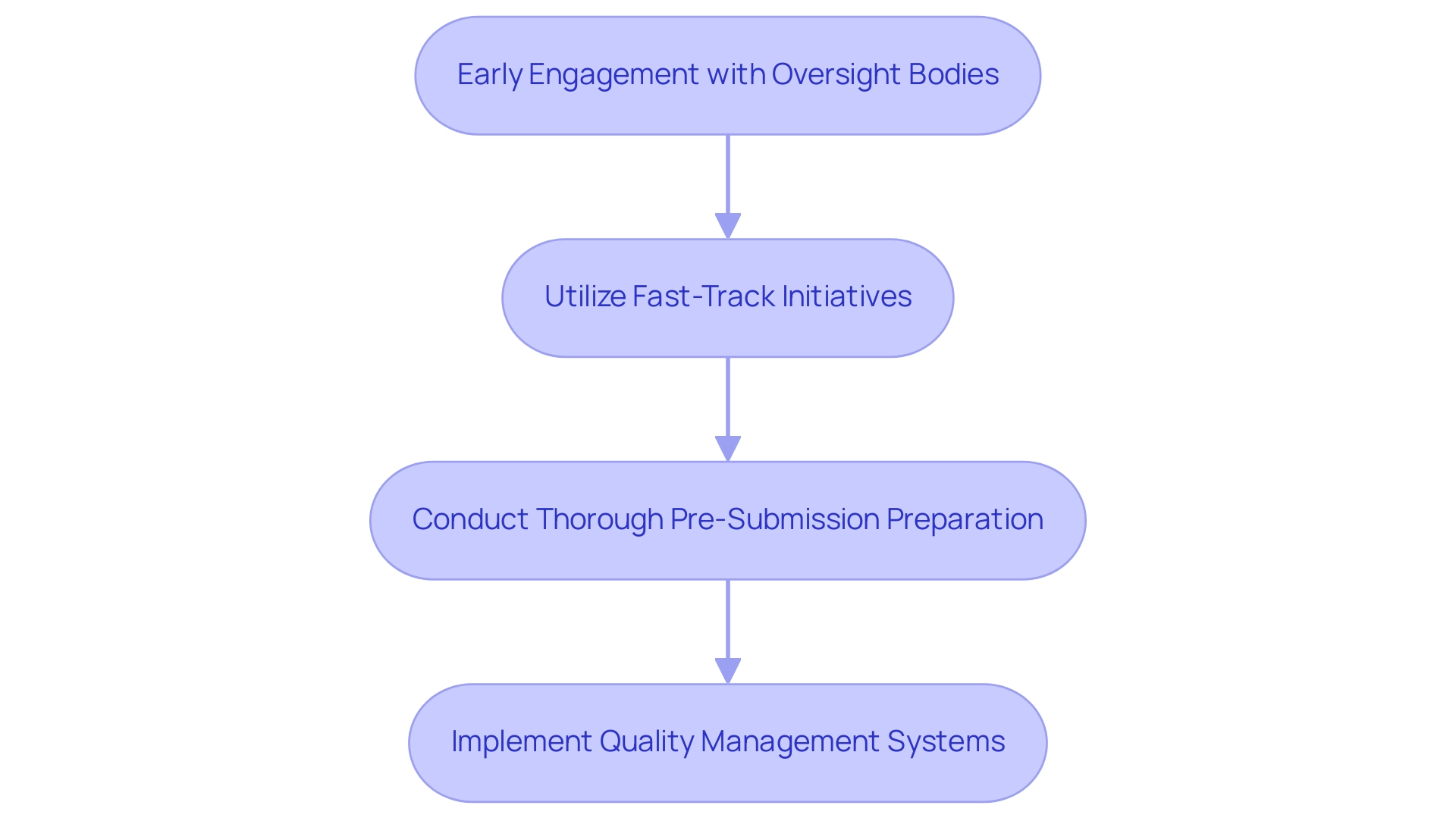
To effectively expedite the approval process for medical devices in Latin America, companies can adopt several strategic approaches:
- Early Engagement with Oversight Bodies: Initiating discussions with local oversight authorities, such as INVIMA, from the outset of the development process is crucial. This proactive step allows for clarification of requirements and expectations, facilitating smoother submissions. Cecot emphasizes that > Attention to the distribution of impacts from regulations has never been greater <, indicating the heightened focus on alignment with regulations. Joint case studies, like the collaboration between Welwaze Medical Inc. and bioaccess™ for the Celbrea® medical product launch in South America, demonstrate that firms interacting early with regulatory authorities faced timeframes shortened by up to 30%.
- Utilize Fast-Track Initiatives: Colombia has broadened its fast-track endorsement program to encompass more product categories, significantly reducing the endorsement timeline for items addressing critical unmet medical needs. For instance, devices associated with urgent health matters have experienced time reductions for authorization by up to 50%. Leveraging these programs can offer a competitive edge in the market, as evidenced by GlobalCare Clinical Trials’ partnership with bioaccess™ to enhance ambulatory services, which has led to notable improvements in recruitment times and retention rates.
- Conduct Thorough Pre-Submission Preparation: Ensuring that all documentation—including clinical data and technical files—is meticulously prepared and adheres to local standards is vital before submission. This thorough preparation can mitigate delays and enhance the chances of acceptance. According to recent statistics, 45% of submissions that were well-prepared obtained acceptance on the first attempt.
- Implement Quality Management Systems: Establishing robust quality management systems is essential for adherence to legal requirements. Such systems not only streamline the approval process but also foster ongoing adherence to standards, thereby reducing the risk of post-approval issues. By employing these strategies, organizations can navigate the compliance landscape more efficiently, ultimately leading to faster market access for their medical devices. The partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent location for clinical trials further illustrates the significance of strategic alliances in attaining compliance success. Moreover, bioaccess™ provides extensive clinical trial management services, such as feasibility studies, site selection, compliance reviews, and project management, which are essential for ensuring successful trial outcomes and adherence to regulations.
Leveraging Clinical Trials to Expedite Device Approval
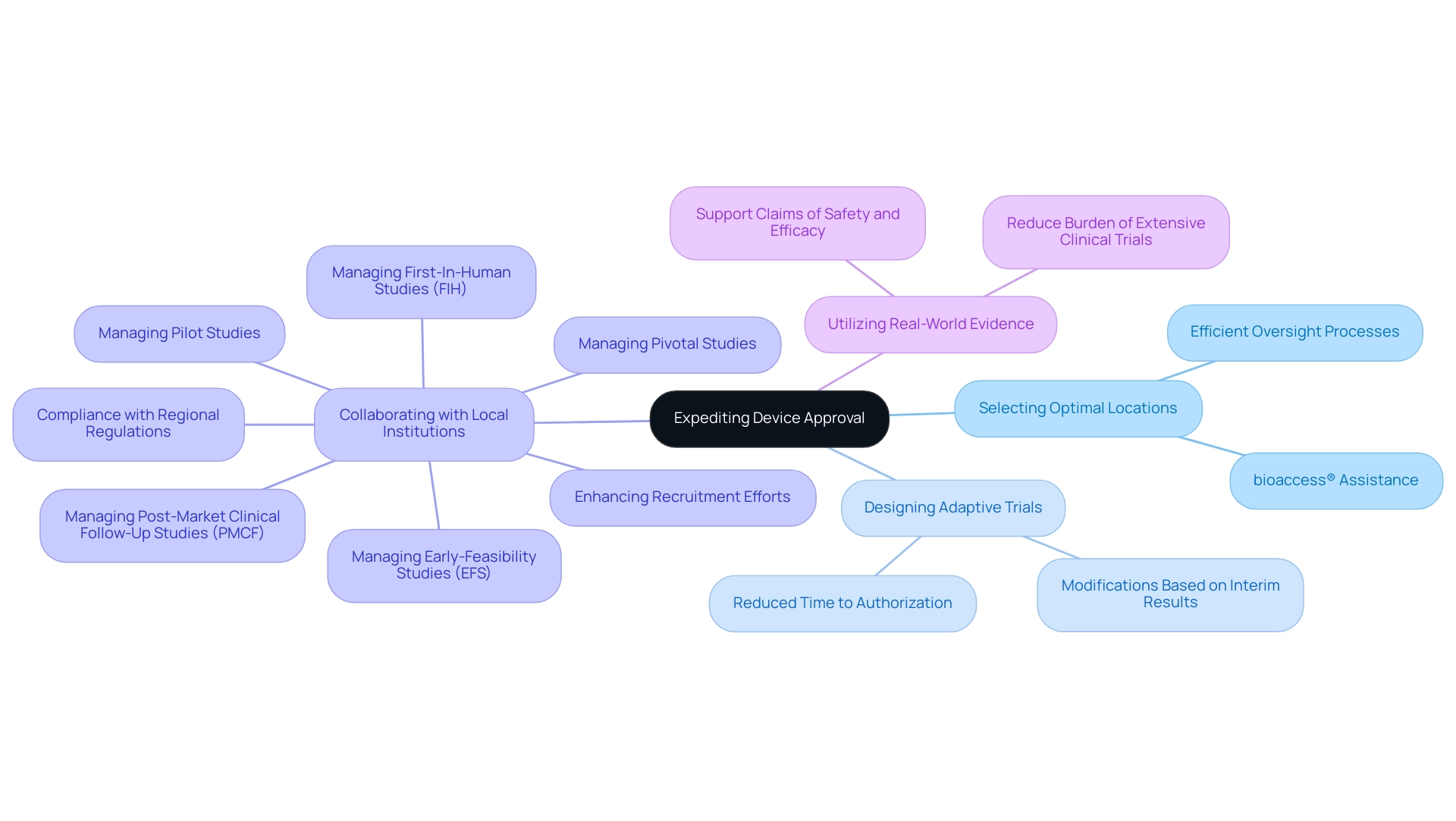
Clinical trials can significantly speed up the process for medical devices. Companies should focus on:
- Selecting Optimal Locations: Choose clinical trial sites in nations with efficient oversight processes, where bioaccess® provides strong assistance in site selection and adherence to INVIMA regulations.
- Designing Adaptive Trials: Consider adaptive trial designs that permit modifications based on interim results, potentially reducing time to authorization.
- Collaborating with Local Institutions: Partner with local research institutions to enhance recruitment efforts and ensure compliance with regional regulations, leveraging bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
- Utilizing Real-World Evidence: Incorporate real-world evidence from existing studies to support claims of safety and efficacy, thereby reducing the burden of extensive clinical trials.
By engaging in these practices, companies can not only accelerate their clinical trials but also contribute to local economic growth and healthcare improvement. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, noted, 'Working with bioaccess® during our first human trial in that country was instrumental in navigating the regulatory landscape effectively.' Similarly, Dr. John B. Simpson, CEO of Avinger, shared, 'The support we received from bioaccess® for our OCT-guided atherectomy clinical research was exceptional, helping us achieve our goals efficiently.
The Role of Local Partnerships in Streamlining Approvals
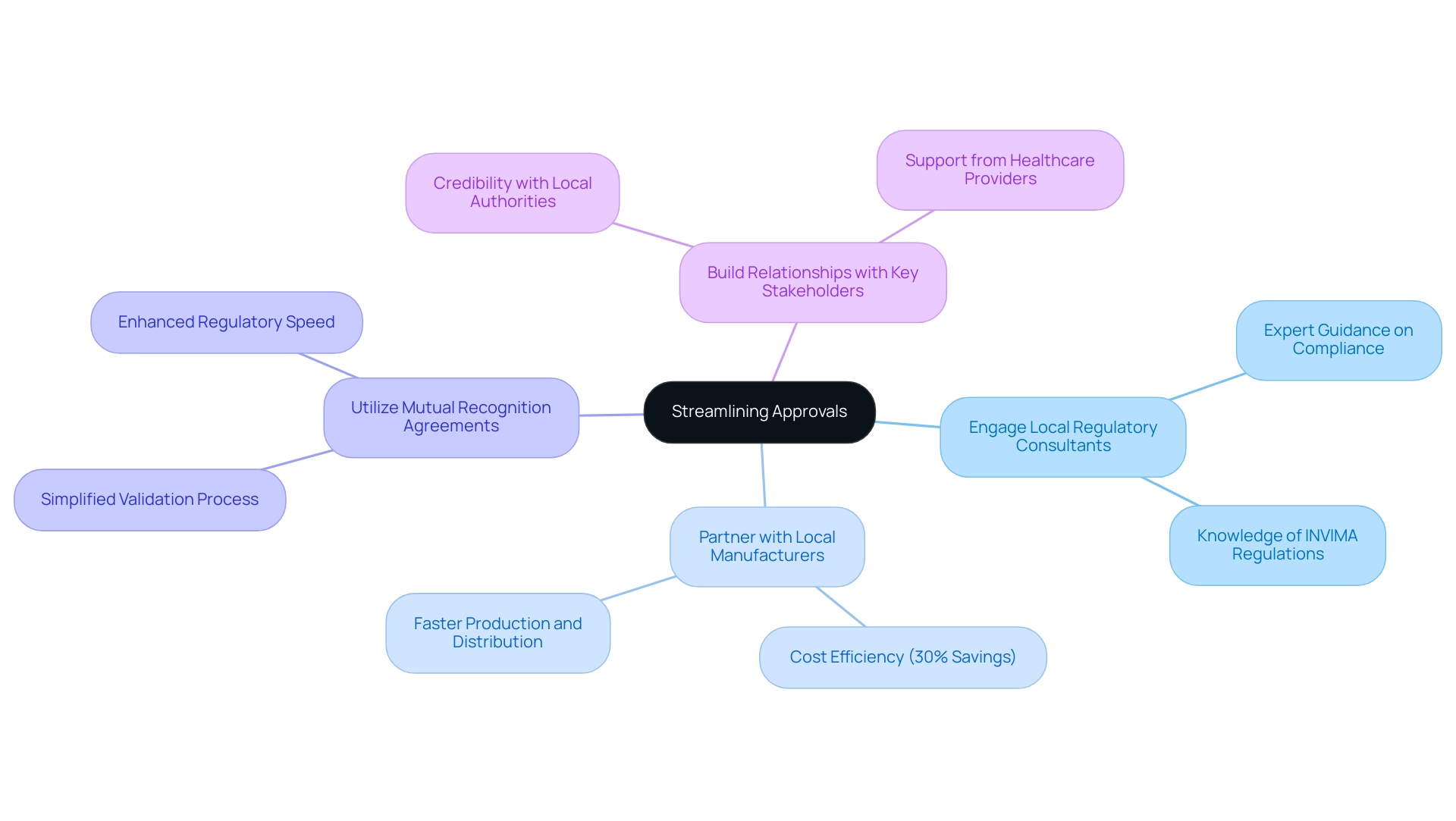
Establishing local collaborations is essential for simplifying the validation process for medical equipment in the region. Companies should:
- Engage Local Regulatory Consultants: Collaborate with experts like Katherine Ruiz, who have in-depth knowledge of local regulations and can provide guidance on compliance, especially with INVIMA, the National Food and Drug Surveillance Institute that oversees health product regulations.
- Partner with Local Manufacturers: Establish relationships with local manufacturers to facilitate quicker production and distribution of products, taking advantage of Colombia's cost efficiency—saving upwards of 30% compared to North America and Western Europe.
- Utilize Mutual Recognition Agreements: Leverage mutual recognition agreements to simplify the validation process for devices already authorized in other markets, enhancing regulatory speed.
- Build Relationships with Key Stakeholders: Foster connections with local health authorities, clinical research organizations, and healthcare providers to enhance credibility and support during the approval process.
This collaborative approach not only complements the high-quality healthcare system ranked among the top five worldwide but also optimizes patient recruitment from the country's population, over 95% of whom are covered by universal healthcare. Additionally, companies can benefit from R&D tax incentives, which offer significant financial advantages, further solidifying the country's position as a favorable destination for clinical trials.
Staying Updated: Regulatory Changes in Key Latin American Markets
To stay informed about compliance changes in key Latin American markets, companies should:
- Subscribe to Compliance Newsletters: Sign up for newsletters from oversight organizations, such as INVIMA, which manages medical equipment regulations in Colombia and is acknowledged as a Level 4 health authority by PAHO/WHO. This is essential considering the compliance challenges many Medtech companies encounter.
- Attend Industry Conferences: Participate in conferences and workshops focused on medical equipment regulations to network with peers and gain insights into upcoming changes, including successful collaborations like that of Greenlight Guru and bioaccess™ that aim to enhance clinical trials in Latin America. These events can also address common challenges such as language barriers and fragmented resources.
- Engage with Compliance Consultants: Regularly consult with compliance experts, such as Katherine Ruiz, who specializes in medical device regulations in Colombia, to receive tailored advice and updates on the latest developments. Their insights can assist in navigating the complexities of the compliance environment.
- Monitor Industry Publications: Follow reputable industry publications that cover regulatory news and trends to remain informed about the evolving landscape in Latin America, particularly in response to the unique challenges and innovative solutions being implemented in the region. This proactive approach can help Medtech companies bridge the gaps in clinical research and innovation.
Conclusion
Navigating the regulatory landscape for medical devices in Latin America requires a comprehensive understanding of the distinct approval processes in each country. As highlighted, Colombia exemplifies the intricate steps involved, including securing approvals from ethical committees and regulatory bodies such as INVIMA. Companies must familiarize themselves with specific regulations, safety standards, and post-market surveillance to ensure compliance and successful market entry.
Strategic approaches can significantly streamline the approval process. Early engagement with regulatory bodies, utilization of fast-track programs, meticulous pre-submission preparation, and the implementation of robust quality management systems are essential practices that can reduce approval timelines and enhance market readiness. Furthermore, leveraging clinical trials through optimal site selection, adaptive designs, and collaboration with local institutions can expedite the journey from development to market.
Local partnerships play a pivotal role in navigating the complexities of the regulatory environment. By collaborating with local regulatory consultants, manufacturers, and stakeholders, companies can enhance their credibility and optimize patient recruitment. Engaging with mutual recognition agreements and capitalizing on R&D tax incentives can further facilitate a smoother approval process, positioning Colombia as an attractive destination for clinical trials.
Staying informed about regulatory changes is crucial for sustained success in the region. Companies should actively subscribe to regulatory newsletters, attend industry conferences, and engage with regulatory experts to remain updated on evolving standards. This proactive approach will not only help Medtech companies overcome challenges but also enable them to seize the opportunities presented by the dynamic healthcare landscape in Latin America.
Frequently Asked Questions
How is the oversight environment for medical devices managed in Latin America?
The oversight environment for medical devices in Latin America varies by country but is typically managed by national health authorities.
What are the key steps for conducting clinical trials in Colombia?
The key steps for conducting clinical trials in Colombia include obtaining permissions from the institutional review board (IRB) or ethics committee (EC), securing regulatory consent from INVIMA (Colombia National Food and Drug Surveillance Institute), and obtaining an import permit from the Ministry of Industry and Commerce (MinCIT).
What role does INVIMA play in Colombia's medical device regulation?
INVIMA oversees the marketing and manufacturing of health products in Colombia and is classified as a Level 4 health authority by PAHO/WHO, ensuring compliance with international safety and efficacy standards.
What regulations must companies be aware of when working with INVIMA?
Companies must familiarize themselves with registration requirements, safety and efficacy standards, and post-market surveillance obligations.
How can companies expedite the approval process for medical devices in Latin America?
Companies can expedite the approval process by engaging early with oversight bodies, utilizing fast-track initiatives, conducting thorough pre-submission preparation, and implementing quality management systems.
What benefits does early engagement with regulatory authorities provide?
Early engagement with regulatory authorities allows for clarification of requirements and expectations, which can lead to smoother submissions and potentially shorten approval timeframes by up to 30%.
What is the significance of fast-track initiatives in Colombia?
Fast-track initiatives in Colombia have been expanded to include more product categories, significantly reducing the endorsement timeline for devices addressing critical unmet medical needs.
Why is thorough pre-submission preparation important?
Thorough pre-submission preparation ensures that all documentation adheres to local standards, which can mitigate delays and enhance acceptance chances, as 45% of well-prepared submissions obtain acceptance on the first attempt.
What strategies can companies use to enhance their clinical trials?
Companies can enhance their clinical trials by selecting optimal locations, designing adaptive trials, collaborating with local institutions, and utilizing real-world evidence.
How can local collaborations simplify the validation process for medical devices?
Local collaborations can simplify the validation process by engaging regulatory consultants, partnering with local manufacturers, utilizing mutual recognition agreements, and building relationships with key stakeholders.
What steps can companies take to stay informed about compliance changes in Latin America?
Companies can subscribe to compliance newsletters, attend industry conferences, engage with compliance consultants, and monitor industry publications to stay informed about regulatory changes.




