Introduction
Navigating the complex regulatory landscape for medical devices in Latin America presents both challenges and opportunities for organizations seeking to enter this dynamic market. With diverse regulatory requirements across countries such as Brazil, Mexico, and Colombia, understanding the nuances of each jurisdiction is crucial for successful market entry. This article delves into essential strategies for accelerating medical device approvals, emphasizing the importance of local expertise, comprehensive documentation, and robust communication with regulatory authorities.
By leveraging technology and building strong local networks, companies can streamline their submission processes and enhance compliance, ultimately facilitating the introduction of innovative medical solutions to a region increasingly focused on improving healthcare outcomes.
Navigating the Regulatory Landscape for Medical Devices in Latin America
In Latin America, the regulatory landscape for medical devices varies significantly from one country to another, with key players including Brazil's ANVISA, Mexico's COFEPRIS, and Argentina's ANMAT, each having distinct requirements.
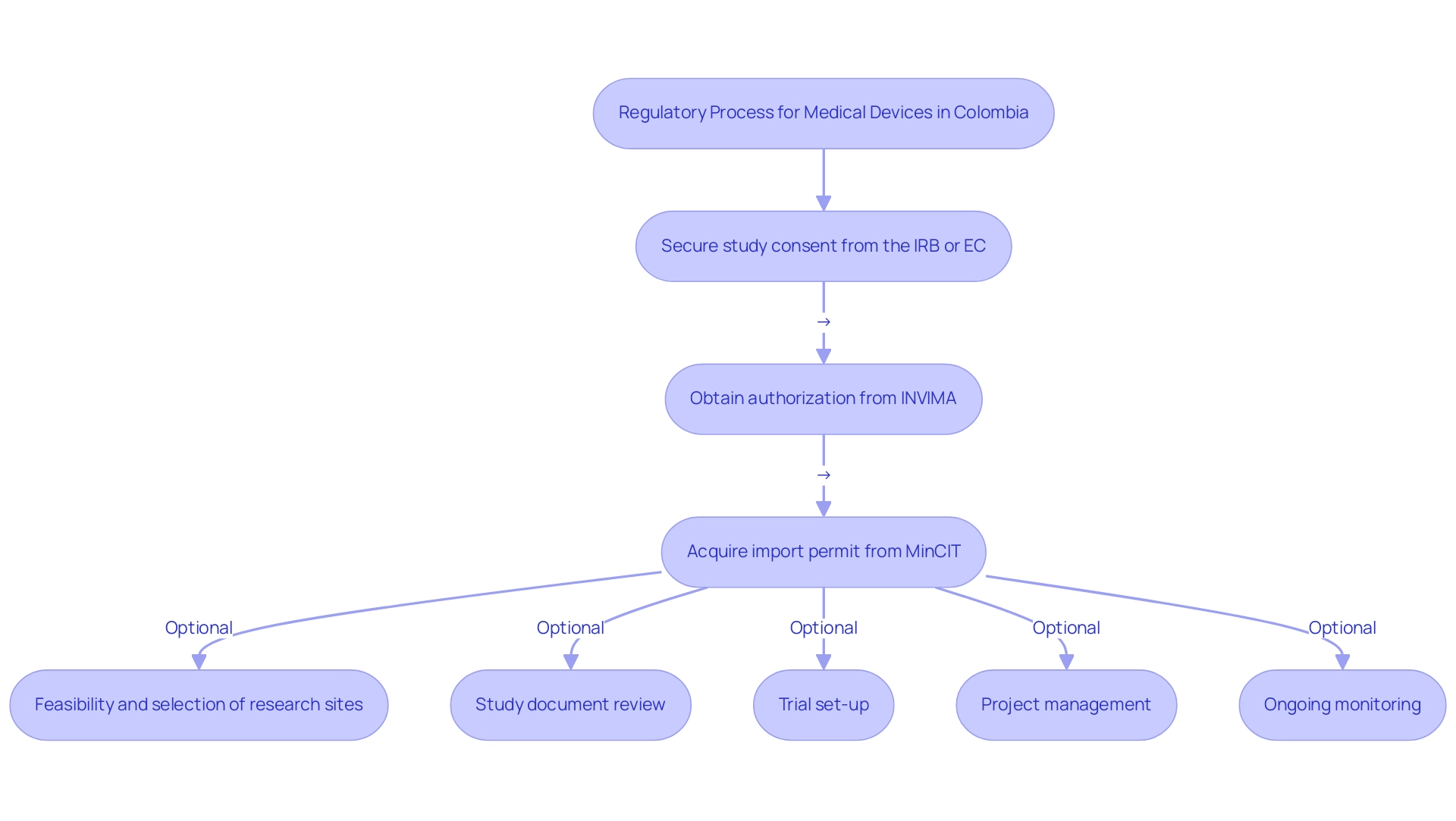
In Colombia, the process for obtaining clinical trial authorization involves several critical steps:
- Secure study consent from your site's institutional review board (IRB) or ethics committee (EC).
- Obtain authorization from Colombia's oversight agency, INVIMA.
- Acquire an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational instruments.
Comprehensive services to support this process include:
- Feasibility and selection of research sites
- Study document review to ensure adherence to regional regulations
- Trial set-up
- Project management
- Ongoing monitoring
Involving regional compliance specialists, such as Ana Criado, the Director of Affairs with extensive experience at INVIMA, and Katherine Ruiz, a medical device compliance expert, can offer invaluable guidance throughout this process. Their knowledge in biomedical engineering, health economics, and compliance is essential in navigating the validation steps efficiently.
Familiarizing yourself with local language and documentation requirements will also facilitate smoother interactions with regulatory bodies. Furthermore, remaining informed about regional harmonization initiatives, like those from the MERCOSUR bloc, can offer insights into potential alterations that may streamline the process across various nations.
Step-by-Step Strategies for Accelerating Medical Device Approvals
Accelerating medical device approvals in Latin America requires a strategic approach that incorporates several key practices:
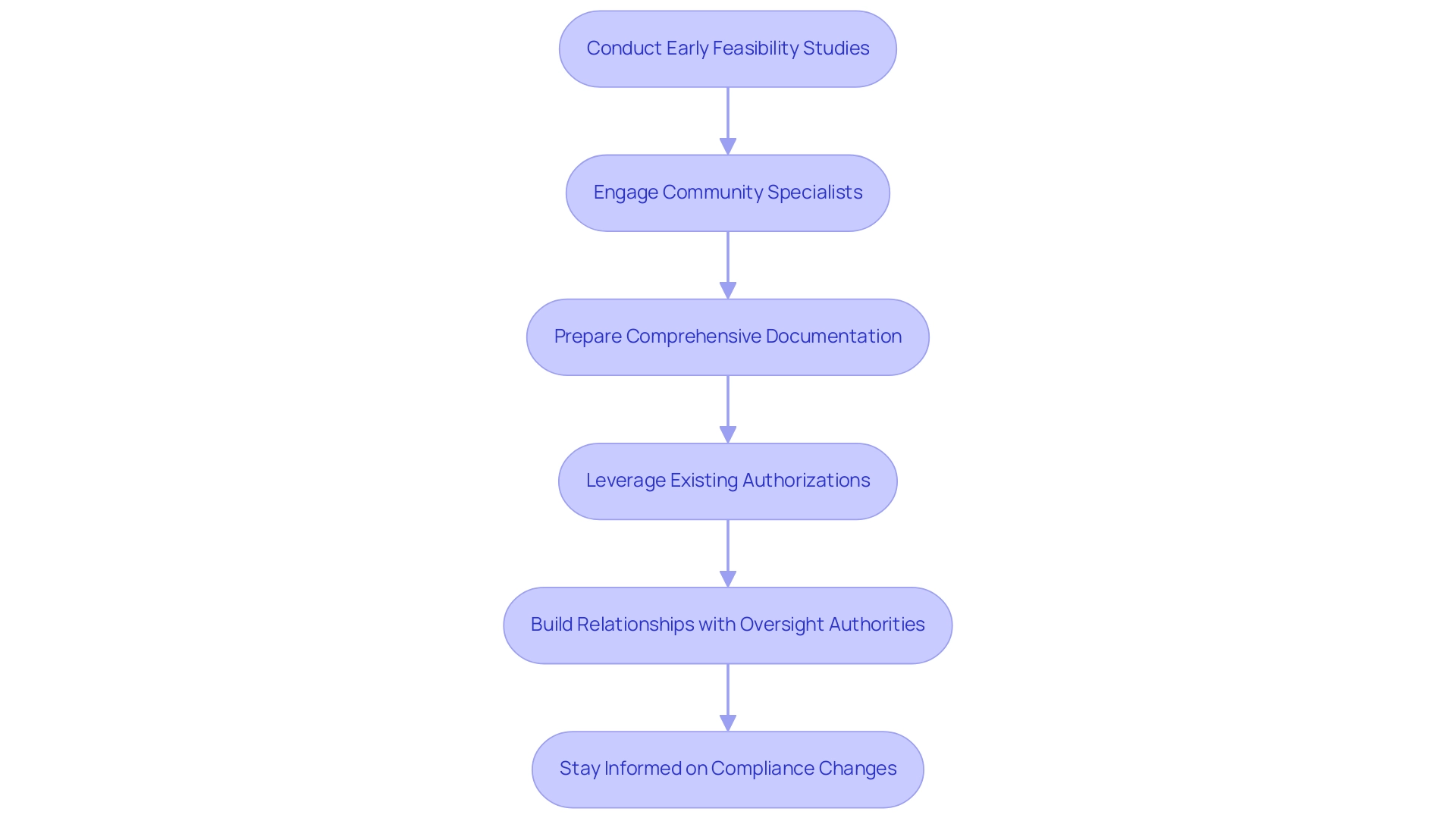
- Conduct Early Feasibility Studies: Initiating early feasibility studies is crucial for gathering critical data on the system’s performance and safety. This preemptive measure not only assists in addressing potential compliance inquiries but also highlights the device's advantages, affirming its market relevance. For example, ReGelTec's Early Feasibility Study for HYDRAFIL™ successfully treated patients in Colombia, demonstrating the effectiveness of this approach.
- Engage Community Specialists: Partnering with regional compliance consultants is essential. Their profound understanding of each country’s specific requirements can significantly streamline the validation process. Katherine Ruiz, a Regulatory Affairs specialist with considerable experience at INVIMA, emphasizes that "navigating the local regulatory environment can be intricate, but possessing local knowledge can significantly enhance the speed of obtaining permissions."
- Prepare Comprehensive Documentation: Thorough preparation of all required documentation is vital. This should encompass clinical data, risk assessments, and detailed manufacturing information. Incomplete submissions frequently lead to delays, making meticulous documentation essential for a smooth validation process.
- Leverage Existing Authorizations: Utilizing existing authorizations from other jurisdictions can be advantageous. Many Latin American countries acknowledge foreign approvals, which can considerably expedite local processes. This strategy not only saves time but also enhances credibility, as it indicates a prior vetting by recognized authorities.
- Build Relationships with Oversight Authorities: Establishing robust communication channels with relevant oversight bodies, such as INVIMA, is beneficial. Regular updates and proactive consultations can foster smoother interactions and expedite the resolution of any arising issues. A collaborative relationship often leads to a more favorable approval experience, as evidenced by GlobalCare Clinical Trials' partnership with bioaccess™ to enhance clinical trial services in Colombia.
- Stay Informed on Compliance Changes: Keeping abreast of compliance changes is a necessity. Engaging in industry forums and workshops allows organizations to gain valuable insights into upcoming shifts in the regulatory landscape. This proactive stance enables companies to adapt strategies promptly, ensuring compliance and fostering efficiency in endorsements.
Statistics suggest that the typical duration for medical equipment endorsements in Latin America can vary from 6 to 18 months, depending on the nation and intricacy of the equipment. By implementing these strategies, organizations can significantly reduce this time frame.
Moreover, bioaccess™ provides extensive clinical trial management services, including trial setup, project management, compliance reviews, and reporting, which are essential in navigating these authorization processes efficiently. By utilizing these strategies and insights, organizations can significantly shorten the time needed for medical equipment authorizations. This not only speeds up the launch of innovative solutions to the market but also aligns with the changing compliance environment in Latin America, as highlighted by recent studies that emphasize the region's commitment to enhancing its medical equipment certification processes. Furthermore, Chile's commitment to universal healthcare coverage and advanced medical facilities supports high-quality clinical trials, ensuring access to diverse patient populations. This dual healthcare system (public and private) enhances health outcomes and life expectancy, making Chile an attractive destination for clinical research.
Understanding Local Market Dynamics
Steering through the endorsement process for medical instruments requires a deep comprehension of market dynamics in each particular nation, especially in areas such as Latin America where governance structures and healthcare systems can differ greatly. Conducting detailed market research is essential to identify the unique needs and preferences of healthcare providers and patients. This research should encompass a comprehensive analysis of the competitive landscape, pricing strategies, and reimbursement policies, while also considering the role of oversight authorities like INVIMA, which oversees medical device approvals in Colombia.
Engaging with community stakeholders, including healthcare professionals and distributors, can yield critical insights into market expectations and the regulatory landscape. For instance, the recent adoption of telehealth programs, which reached 15% of hospitals in Latin America by the end of 2021, signals a growing market for innovative healthcare solutions. As noted by the GHI Team, GHI enabled the client to quantify the opportunity ahead, to determine the market leaders and to understand the willingness of adoption from key opinion leaders, further emphasizing the importance of this research.
Furthermore, a case study on the 'Best-Equipped Hospitals in Latin America' published by GHI emphasizes the differing levels of equipment resources among hospitals, acting as a benchmark for comprehending healthcare dynamics in the area and promoting enhancements in services. Comprehending the regional healthcare system, including hospitals and clinics, is essential as it directly impacts product adoption rates and review timelines.
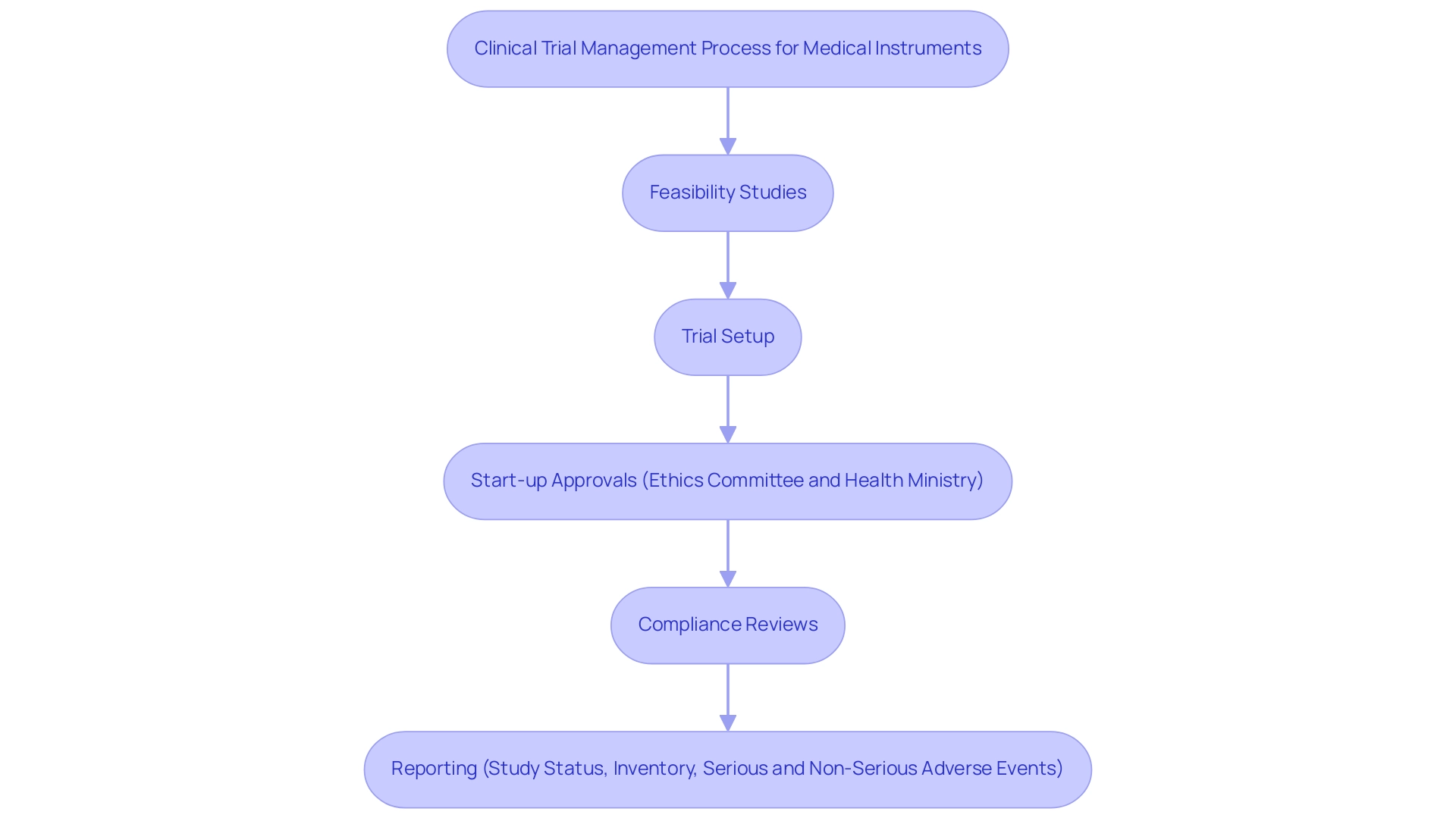
Furthermore, the current market dynamics indicate a shift towards more integrated healthcare solutions, reflecting the preferences of healthcare providers for devices that enhance patient care. By tailoring your submission to address these regional dynamics, particularly through comprehensive clinical trial management services such as:
- feasibility studies
- trial setup
- start-up approvals (ethics committee and health ministry)
- compliance reviews
- reporting (study status, inventory, serious and non-serious adverse events)
you can significantly enhance your chances of achieving a successful and expedited approval process. The economic impact of clinical studies on regional economies is also noteworthy, as these initiatives can create jobs, promote economic growth, and improve healthcare services.
Don't forget to subscribe to our newsletter via MailChimp for ongoing insights into market research and trends in the medical equipment sector.
Building a Strong Local Network
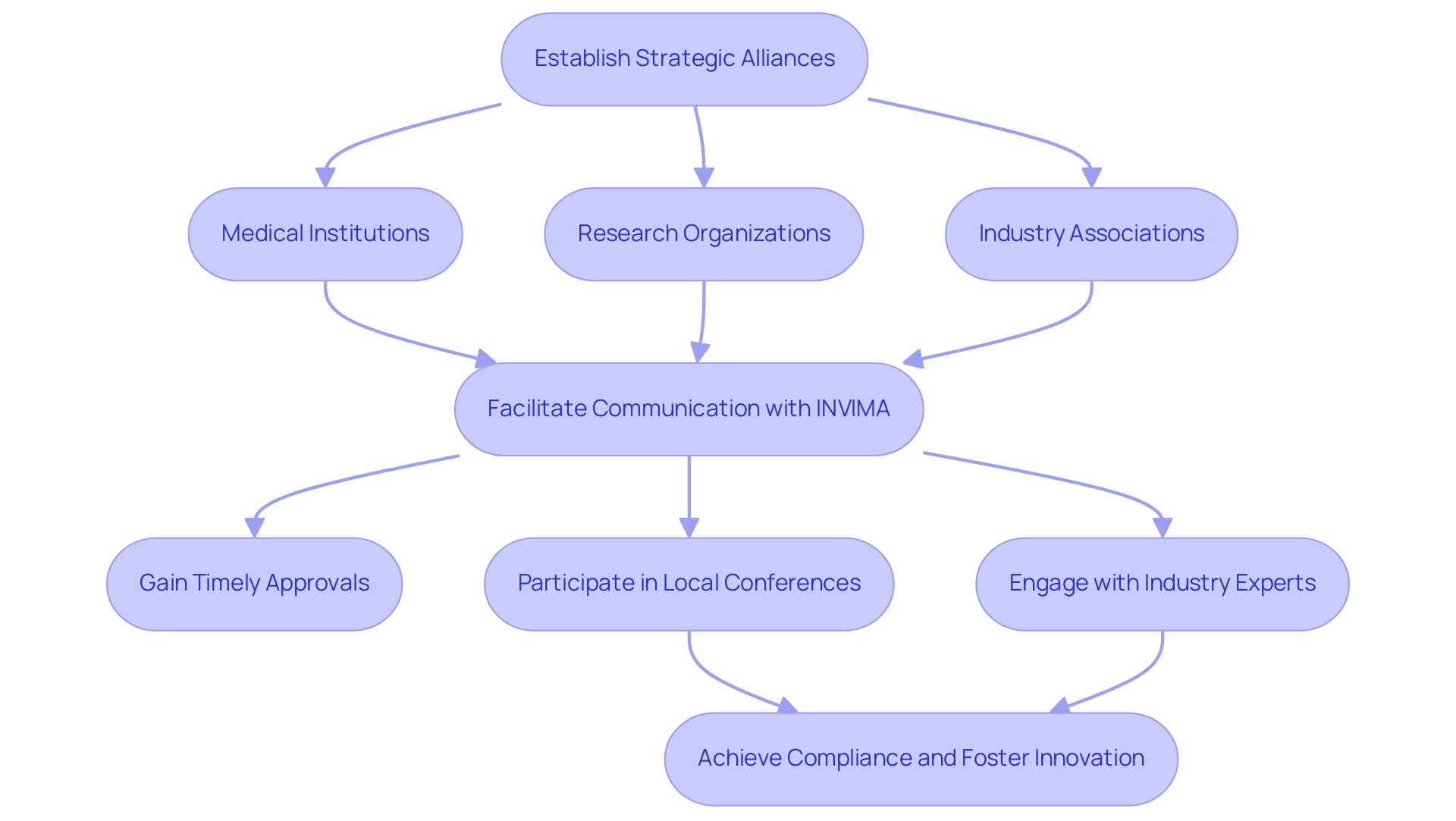
Creating a strong regional network is crucial for successfully maneuvering through the intricate oversight framework of the medical equipment sector in Colombia, especially concerning INVIMA, the National Food and Drug Surveillance Institute. Establishing strategic alliances with nearby medical institutions, research organizations, and industry associations can greatly improve your comprehension of expectations outlined by INVIMA. These collaborations facilitate valuable communication with regulatory authorities, which is crucial for timely approvals of medical devices.
INVIMA's oversight includes monitoring manufacturing and marketing practices, ensuring compliance with safety, efficacy, and quality standards. As a Level 4 health authority, INVIMA is acknowledged for its competence and efficiency in executing health regulation functions recommended by PAHO/WHO, which further emphasizes the significance of regional networks in achieving compliance.
In 2017, there was a +0.8% rise in partnerships with payer organizations compared to 2015, highlighting the increasing acknowledgment of the significance of community networks. Recent studies suggest that national policies can either promote or hinder these partnerships, affecting regional priorities. Additionally, the positive economic impacts of Medtech clinical studies, such as job creation and healthcare improvement, underscore the importance of these collaborations.
Participating in local conferences and networking events offers additional chances to connect with key stakeholders in the medical sector. Insights from industry leaders highlight the necessity of collaborating with regional experts, such as Katherine Ruiz, an affairs specialist who has successfully advised foreign manufacturers on obtaining market clearance in Colombia. Katherine's prior experience at INVIMA included obtaining import licenses for diagnostic reagents, medical devices, and investigational products for clinical studies, making her a valuable asset for addressing compliance challenges.
This collaborative method not only adds credibility to your submissions but also shows a commitment to understanding and following regional regulations. Andrew Hutchings, a Senior Fellow at the Health Foundation, emphasizes that effective collaboration is key to navigating these complexities. For instance, a study on bundled payments revealed significant associations between organizational partnerships and compliance success, illustrating how local networks can expedite the approval process and ensure successful market entry. Such strategic partnerships, within the framework of INVIMA's guidelines, can be invaluable assets in achieving compliance and fostering innovation in the medical equipment landscape.
Leveraging Technology for Regulatory Submissions
Incorporating technology into the submission process can greatly enhance efficiency, especially when paired with comprehensive clinical trial management services. Our offerings include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
All designed to streamline your research efforts. We guarantee that trial setup is carefully organized to satisfy compliance standards, and we support with acquiring essential import permits for research instruments.
Utilize electronic submission platforms that allow for streamlined documentation and tracking of submissions. These platforms often provide templates and checklists to ensure that all required information is included, reducing the likelihood of errors or omissions.
Additionally, consider using project management tools to coordinate efforts among team members and stakeholders. These tools can facilitate communication, track progress, and ensure that deadlines are met.
With Katherine Ruiz, a Regulatory Affairs expert in medical devices and in vitro diagnostics, guiding you through the approval processes, embracing technology not only improves the accuracy of submissions but also accelerates the overall approval process, allowing for faster market access. Reporting on study status and adverse events is also a critical component of our services, ensuring transparency and compliance throughout the trial.
Conclusion
Navigating the regulatory landscape for medical devices in Latin America is undeniably complex, yet it presents significant opportunities for organizations willing to invest the necessary resources and expertise. By understanding the distinct regulatory requirements of key countries like Brazil, Mexico, and Colombia, companies can strategically position themselves for successful market entry. The importance of early feasibility studies, comprehensive documentation, and strong local partnerships cannot be overstated, as these elements are crucial in overcoming potential hurdles in the approval process.
Moreover, leveraging technology to streamline regulatory submissions enhances efficiency and compliance, ultimately facilitating faster access to market for innovative medical solutions. Engaging local experts and building a robust network can further expedite the approval process, ensuring that organizations remain informed about evolving regulations and market dynamics.
In conclusion, the commitment to understanding and adapting to the unique regulatory environments in Latin America will not only accelerate medical device approvals but also contribute to improved healthcare outcomes in the region. By embracing these strategies, organizations can effectively introduce their products to a market that is increasingly focused on advancing healthcare solutions, thereby reinforcing their position as leaders in the medical device industry.
Frequently Asked Questions
How does the regulatory landscape for medical devices differ in Latin America?
The regulatory landscape for medical devices varies significantly across Latin American countries, with key players such as Brazil's ANVISA, Mexico's COFEPRIS, and Argentina's ANMAT, each having distinct requirements.
What are the steps for obtaining clinical trial authorization in Colombia?
The process involves three critical steps: securing study consent from the institutional review board (IRB) or ethics committee (EC), obtaining authorization from Colombia's oversight agency INVIMA, and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational instruments.
What services can support the clinical trial authorization process in Colombia?
Comprehensive services include feasibility and selection of research sites, study document review for regional regulation adherence, trial setup, project management, and ongoing monitoring.
Who are key regional compliance specialists in Colombia, and what expertise do they offer?
Ana Criado, Director of Affairs with experience at INVIMA, and Katherine Ruiz, a medical device compliance expert, provide invaluable guidance in navigating the validation steps, leveraging their knowledge in biomedical engineering, health economics, and compliance.
Why is it important to familiarize oneself with local language and documentation requirements?
Familiarizing with local language and documentation requirements facilitates smoother interactions with regulatory bodies and helps ensure compliance during the approval process.
What are some strategies for accelerating medical device approvals in Latin America?
Key strategies include conducting early feasibility studies, engaging community specialists, preparing comprehensive documentation, leveraging existing authorizations, building relationships with oversight authorities, and staying informed on compliance changes.
How long does the medical equipment endorsement process typically take in Latin America?
The typical duration for medical equipment endorsements can vary from 6 to 18 months, depending on the country and the complexity of the equipment.
What role does market research play in the endorsement process for medical devices?
Detailed market research helps identify unique needs and preferences of healthcare providers and patients, analyze the competitive landscape, and understand oversight authority roles, which are crucial for successful product adoption.
How can establishing a strong regional network benefit the medical equipment sector in Colombia?
Creating strategic alliances with medical institutions, research organizations, and industry associations enhances understanding of INVIMA's expectations and facilitates communication with regulatory authorities, leading to timely approvals.
What technological tools can enhance the submission process for medical devices?
Utilizing electronic submission platforms and project management tools can streamline documentation, track submissions, coordinate efforts among team members, and ensure compliance throughout the trial process.

