Introduction
Latin America is emerging as a compelling hub for clinical trials, particularly in the medical device sector, due to its unique advantages that cater to both sponsors and participants. With a rich tapestry of diverse patient populations, favorable regulatory environments, and cost-effective operational frameworks, the region is not only enhancing the inclusivity of clinical research but also streamlining processes for faster study initiation. Colombia, in particular, stands out as a prime location, supported by a robust regulatory framework and a growing market eager for innovation.
As the medical device outsourcing landscape evolves, understanding the key considerations for selecting the right Contract Research Organizations (CROs) and establishing effective communication strategies becomes essential for sponsors aiming to achieve successful trial outcomes. This article delves into the myriad benefits of conducting clinical trials in Latin America, with a focus on Colombia's strategic positioning and the critical factors influencing trial success.
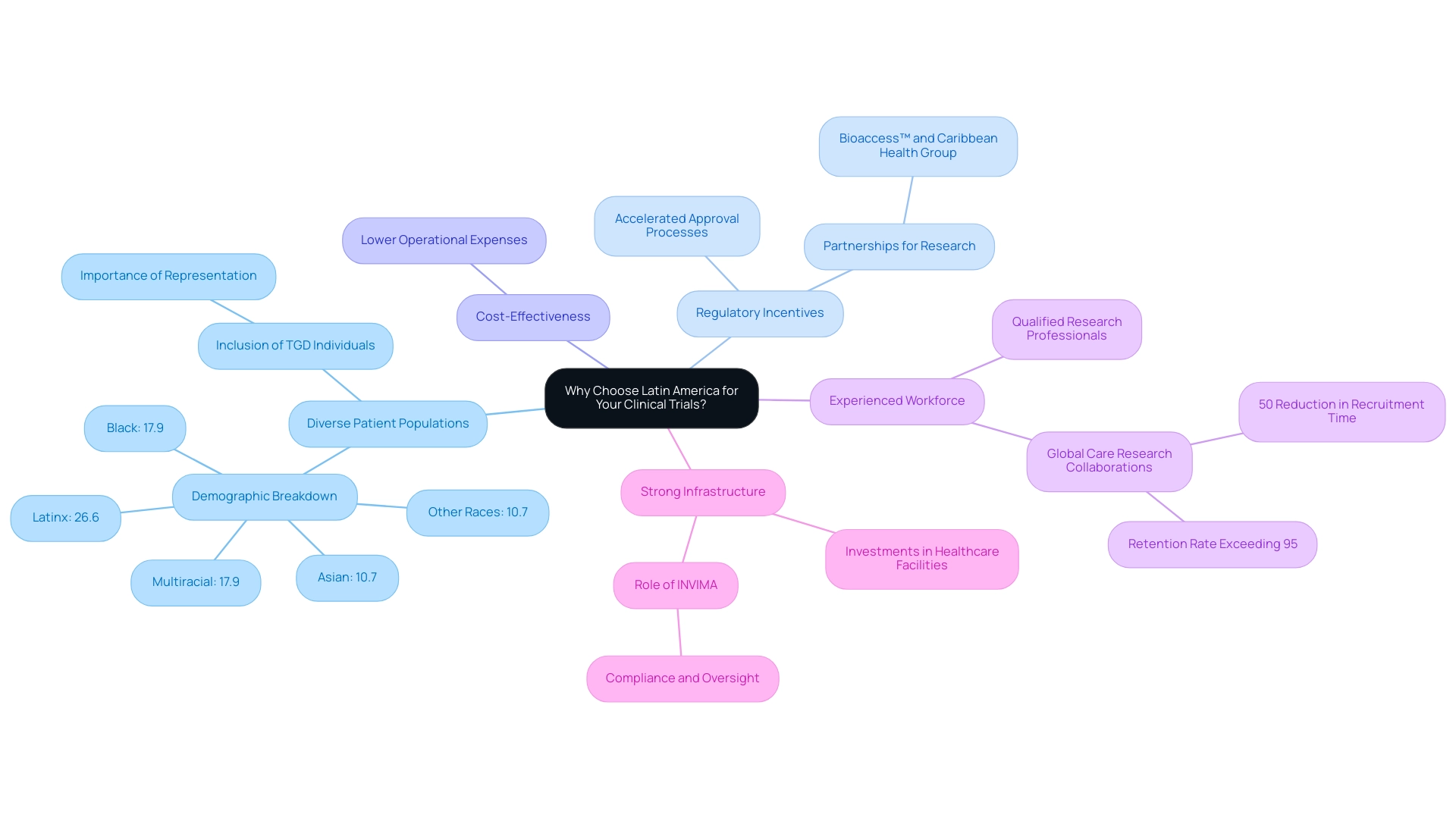
Why Choose Latin America for Your Clinical Trials?
Research in the field of medical devices can greatly benefit from the services provided by a Latin America CRO for Medical Device Trials. The key benefits include:
-
Diverse Patient Populations: The region is characterized by a rich demographic variety, which can significantly enhance the generalizability of study findings.
For instance, recent statistics indicate that research participants in Latin America are increasingly diverse, with 26.6% identifying as Latinx, 17.9% as Black, and 10.7% as Asian, among other identities. This diversity is crucial for ensuring that research encompasses a wide array of experiences, particularly for marginalized groups such as transgender and gender-diverse (TGD) individuals. One participant emphasized the importance of researchers seeking out hard-to-reach TGD individuals who 'are least likely to be able to' participate, highlighting the need to include their experiences and voices in the research.
-
Regulatory Incentives: Numerous nations in Latin America have established regulatory structures aimed at accelerating the approval process for research studies. This allows for faster study initiation, an attractive feature for sponsors looking to streamline their timelines. The partnership between bioaccess™ and the Caribbean Health Group, backed by Colombia's Minister of Health, illustrates this initiative, intended to position Barranquilla as a premier location for Latin America CRO for Medical Device Trials.
-
Cost-Effectiveness: The expense of carrying out experiments in Latin America can be significantly lower compared to North America and Europe. This is attributed to reduced operational expenses, including labor and site fees, thus making the Latin America CRO for Medical Device Trials a financially viable option for many sponsors.
-
Experienced Workforce: The region boasts a growing pool of qualified research professionals, including investigators and coordinators who are adept in international standards and regulations. Their knowledge enhances the overall quality of research studies and aligns with Global Care Research Collaborations' partnership with bioaccess™ to improve ambulatory services, achieving over 50% reduction in recruitment time and a retention rate exceeding 95%.
-
Strong Infrastructure: Latin American nations have made significant investments in healthcare facilities, enhancing access to research sites and improving patient recruitment abilities. This is particularly beneficial in reaching underrepresented populations and ensuring comprehensive study results.
Furthermore, INVIMA, the National Food and Drug Surveillance Institute, plays a vital regulatory role in the context of Latin America CRO for Medical Device Trials, ensuring compliance and oversight of medical device studies, classified as a Level 4 health authority by PAHO/WHO.
In summary, by capitalizing on these advantages, sponsors can effectively optimize their research strategies, leading to more successful and inclusive outcomes in their research endeavors. Moreover, the economic effects of carrying out medical studies in the country encompass job creation, enhanced healthcare access, and global acknowledgment, further reinforcing the region's attractiveness for research. The steadfast assistance from the Minister of Health emphasizes the government's dedication to promoting a strong research environment.
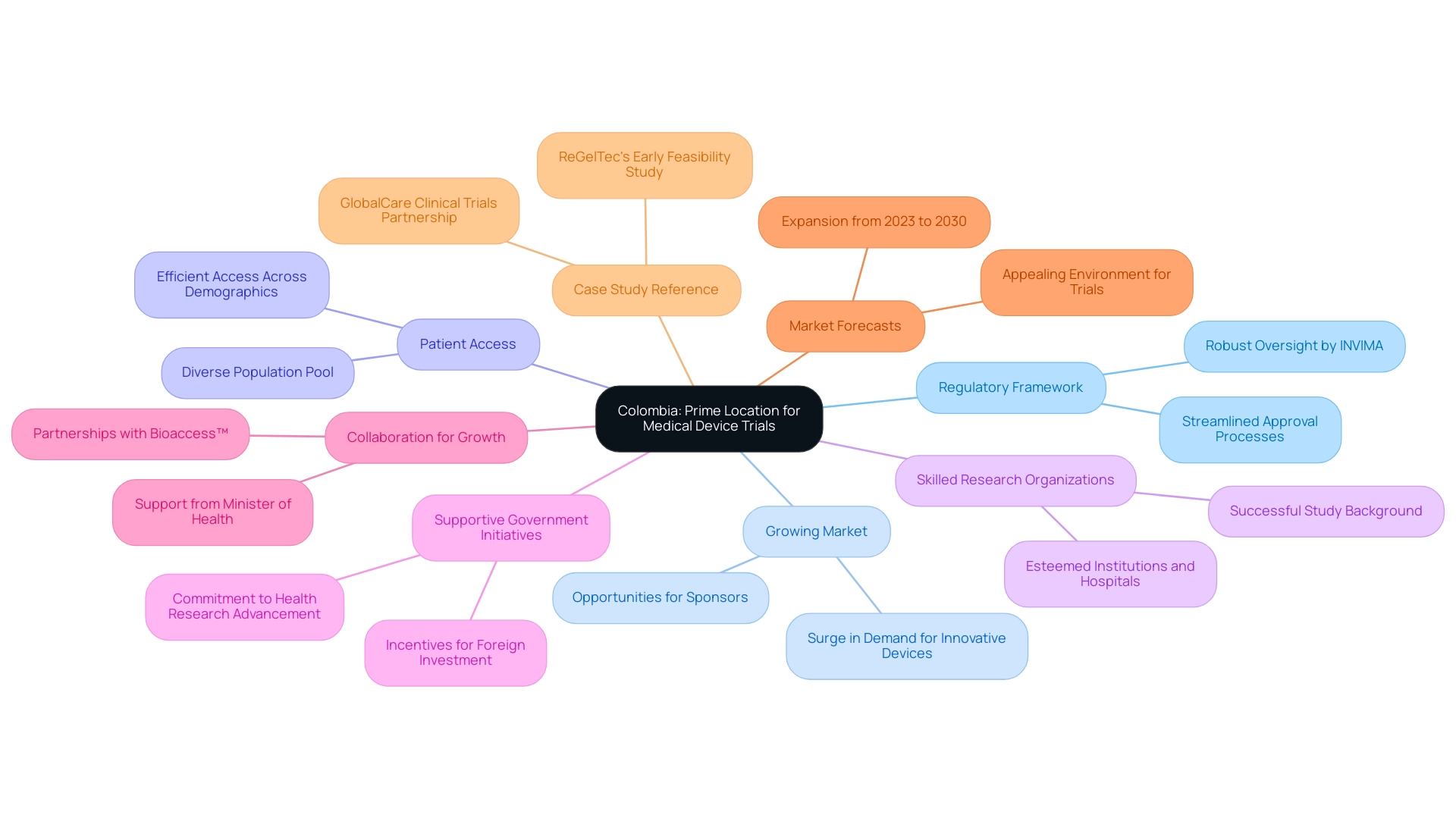
Exploring Colombia: A Prime Location for Medical Device Trials
This country is swiftly positioning itself as a top location for healthcare equipment tests, propelled by a mix of strategic benefits:
- Regulatory Framework: The country boasts a robust regulatory environment, overseen by INVIMA (National Food and Drug Surveillance Institute). Recent updates have streamlined approval processes for clinical trials, facilitating faster initiation and minimizing delays.
- Growing Market: With a surge in demand for innovative healthcare devices, the country's device sector is on the rise. Key factors driving the growth of the medical device outsourcing market, especially concerning Latin America CRO for Medical Device Trials, include a changing regulatory landscape, increased offshoring of medical device manufacturing to emerging countries, and rising demand for advanced products. This growth presents a compelling opportunity for sponsors to engage with the Latin America CRO for Medical Device Trials market, which is eager for advanced products.
- Patient Access: Colombia’s varied and large population provides a valuable pool of potential research participants. The country's healthcare system enables efficient access to patients across various demographics, enhancing the feasibility of trials conducted by a Latin America CRO for Medical Device Trials.
- Skilled Research Organizations: The existence of esteemed research institutions and hospitals with a background of executing successful studies enhances the quality and dependability of research results. This experience fosters confidence among sponsors regarding the integrity of the data collected by a Latin America CRO for Medical Device Trials.
- Supportive Government Initiatives: The Colombian government actively encourages foreign investment in health research, offering incentives and assistance to sponsors seeking to carry out studies in the country. These initiatives reflect a commitment to advancing the Latin America CRO for Medical Device Trials.
- Collaboration for Growth: A significant partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading destination for conducting Latin America CRO for Medical Device Trials. This partnership, backed by the nation's Minister of Health, enhances the country's attractiveness as a Latin America CRO for Medical Device Trials.
- Market Forecasts: The healthcare equipment sector in the country is anticipated to undergo considerable expansion from 2023 to 2030, creating an appealing environment for conducting trials with a Latin America CRO for Medical Device Trials.
- Case Study Reference: Notable examples include ReGelTec's successful Early Feasibility Study utilizing HYDRAFIL™ for chronic low back pain treatment, and GlobalCare Clinical Trials' partnership with bioaccess™ to enhance ambulatory services, achieving over a 50% reduction in recruitment time and a 95% retention rate. Testimonials from industry leaders, including Dushyanth Surakanti of Sparta Biomedical and Dr. John B. Simpson of Avinger, further highlight the favorable experiences and results from clinical research carried out in the country.
By choosing this location for testing, sponsors can utilize these benefits while aiding in the improvement of healthcare knowledge within the framework of Latin America CRO for Medical Device Trials. As the healthcare device outsourcing market continues to evolve, driven by factors such as changing regulations and increasing offshoring, this country emerges as a pivotal player in this landscape.
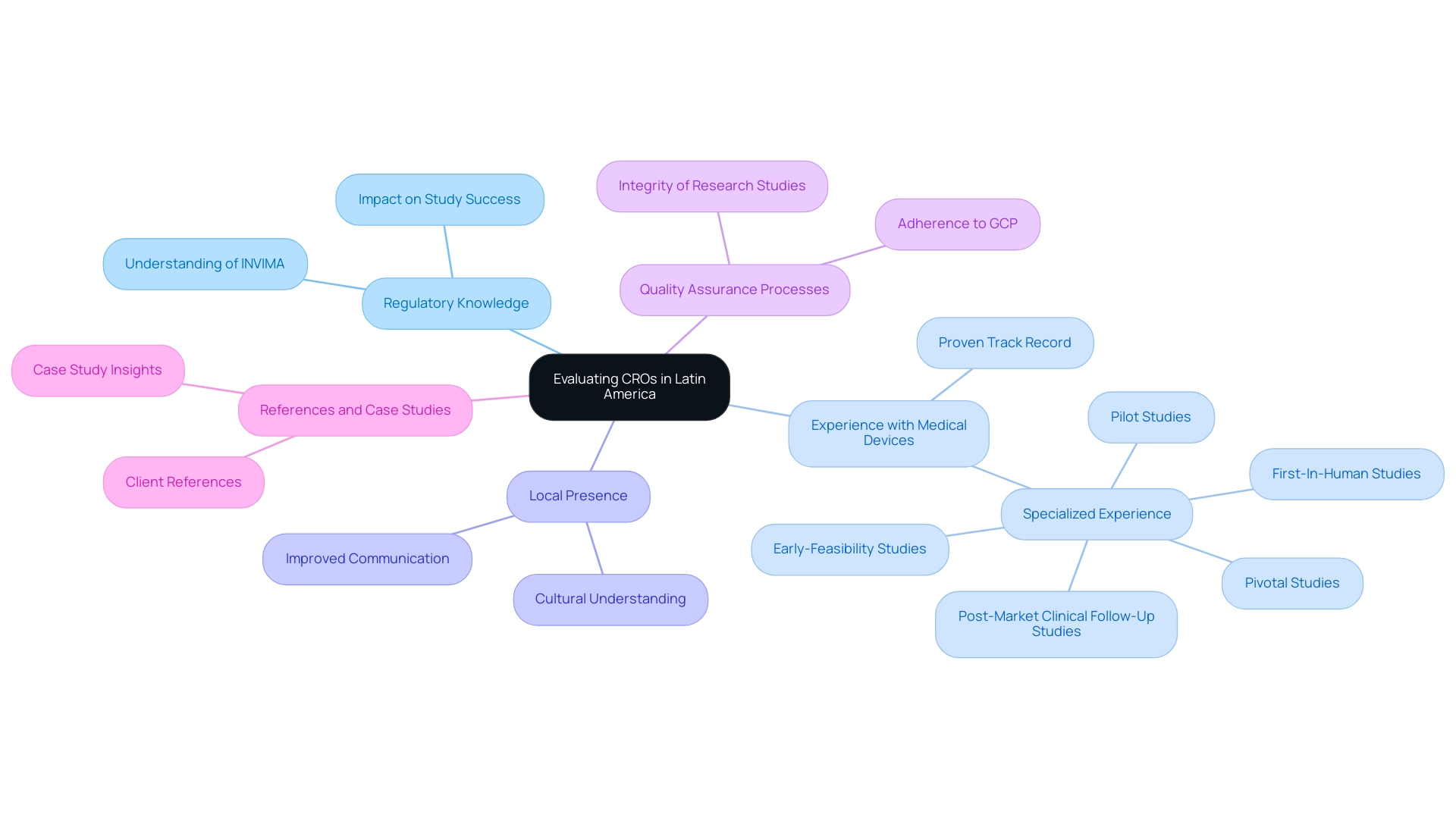
Evaluating CROs in Latin America: Key Considerations
When evaluating Contract Research Organizations (CROs) in Latin America for healthcare device studies, it is essential to consider several key factors that can greatly impact study success:
- Regulatory Knowledge: It is imperative that the CRO possesses an in-depth understanding of local regulatory requirements, particularly those set forth by INVIMA, the National Food and Drug Surveillance Institute of the country. Their capacity to maneuver this regulatory environment effectively is crucial, particularly considering the intricacies linked to device evaluations. As highlighted by industry leaders, including Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, “the expertise of bioaccess® during our first human study in Colombia was instrumental in ensuring compliance and achieving our research objectives.”
- Experience with Medical Devices: Choosing a Latin America CRO for Medical Device Trials that has a proven track record of overseeing studies for medical devices is essential. bioaccess® has over 20 years of specialized experience in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring adherence to compliance standards and enhancing the overall chance of success.
- Local Presence: A CRO that maintains a local presence can provide invaluable advantages, including improved communication and a nuanced understanding of cultural factors that may affect patient recruitment and retention rates. This local insight is particularly beneficial in tailoring strategies that resonate with the target population.
- Quality Assurance Processes: It is essential to examine the CRO’s quality assurance protocols to confirm adherence to Good Clinical Practice (GCP) and other relevant international standards. Strong quality assurance practices are essential to upholding the integrity of research studies and are a hallmark of bioaccess®'s operational approach.
- References and Case Studies: Requesting references from former clients and reviewing pertinent case studies can provide insight into the CRO’s past performance and reliability. For example, Dr. John B. Simpson, CEO of Avinger, Inc., shared his positive experience with bioaccess® during OCT-guided atherectomy research in Cali, Colombia, highlighting the effective management and support that contributed to the study's success.
Furthermore, statistics on the performance of Latin America CRO for Medical Device Trials indicate that organizations with strong regulatory knowledge and local presence have a 30% higher success rate in studies compared to those lacking these attributes. By meticulously evaluating these factors, sponsors can select a CRO that not only aligns with their objectives but also enhances the likelihood of achieving successful study outcomes. The importance of regulatory knowledge, as highlighted by industry leaders, cannot be overstated, as it forms the backbone of informed decision-making in the CRO selection process. Furthermore, bioaccess® provides extensive services such as study setup, project oversight, compliance evaluations, and additional offerings, guaranteeing a meticulous strategy for study management.
Establishing Effective Communication with Your CRO
Establishing effective communication with your selected Contract Research Organization (CRO) is crucial for the success of clinical studies. A recent survey conducted by Endo revealed that most sites reported positive feedback about their relationships with CROs, highlighting the importance of these partnerships. Here are several best practices to enhance collaboration:
- Set Clear Expectations: At the beginning of your partnership, it is essential to define roles, responsibilities, and mutual expectations to prevent misunderstandings. As Carrie Lewis, Senior Director of Strategy, Oversight, and Training at Endo Pharmaceuticals Inc., asserts,
Expectations must be revisited as needed and must include proper goals and metrics. This aligns with the need for senior management buy-in to ensure governance charters are established with flexible KPIs. - Regular Updates: Schedule consistent meetings to discuss progress, challenges, and key milestones. These interactions ensure that all parties remain informed and aligned with project objectives, fostering a collaborative atmosphere.
- Utilize Technology: Take advantage of project management tools and communication platforms that facilitate real-time updates and document sharing. This technological integration promotes transparency and efficiency among team members.
- Encourage Feedback: Create an environment where team members feel comfortable providing feedback. This openness allows for continuous improvement in processes and communication, ultimately enhancing project outcomes.
- Crisis Management Protocols: Establish comprehensive protocols to address potential issues or crises that may arise during the proceedings. Both parties should be well-informed of the procedures to ensure effective responses, particularly during challenging times.
Additionally, leveraging the CRO's service capabilities such as feasibility and selection of research sites, principal investigator (PI) reviews, compliance checks on study documents, setup approvals, import permits, and robust project management can significantly enhance collaboration. Furthermore, regular reporting on study status, inventory, and serious and non-serious adverse events is essential for maintaining transparency and accountability throughout the research. By implementing these strategies, sponsors can significantly enhance their collaboration with CROs, which in turn results in more successful study outcomes.
The case study on best practices for successful FSP management emphasizes that building trust and maintaining open communication are key elements that enhance overall project outcomes, especially during uncertain times.
Monitoring and Evaluating Trial Progress
To effectively monitor and evaluate the progress of clinical studies, it is essential to follow a structured approach that incorporates the best practices of 2024:
- Establish Key Performance Indicators (KPIs): Begin by defining specific KPIs that are vital for measuring performance. This includes metrics such as patient recruitment rates, which recent statistics show have improved by 25% in the past year due to enhanced engagement strategies implemented by leaders in the field like bioaccess®. Additionally, focus on data collection timelines and compliance metrics that ensure adherence to protocols.
- Regular Data Review: Conduct systematic reviews of the collected data at predetermined intervals. This practice is crucial for identifying trends, addressing discrepancies, and pinpointing areas that require immediate attention, thereby allowing for proactive management of potential issues. Utilizing subscription-level features, such as automatic reports and smart alerts, can streamline this process.
- Adjust Protocols as Needed: Be flexible and ready to adjust trial protocols in response to interim findings or unforeseen challenges that may emerge during the study. For example, the partnership between bioaccess™ and Caribbean Health Group demonstrates how customized strategies can result in successful outcomes in establishing Barranquilla as a leading location for Latin America CRO for Medical Device Trials. Their adaptability in navigating the clinical landscape demonstrates the value of responsiveness in clinical trials.
- Engage with Investigators: Foster open lines of communication with principal investigators. Their insights are invaluable for understanding site performance and enhancing patient engagement, which is essential for optimizing recruitment and retention. Regular feedback, as demonstrated in collaborations such as that of GlobalCare Clinical Trials with bioaccess™, can help refine strategies and enhance overall study effectiveness, achieving over a 50% reduction in recruitment time and a retention rate exceeding 95%.
Final Evaluation: Upon concluding the experiment, conduct a thorough evaluation of the study's outcomes against the initial objectives and KPIs. This final assessment not only gauges success but also records lessons learned, offering a basis for improving future experiments.
By diligently tracking and assessing progress through these steps, sponsors can enhance the efficiency and effectiveness of their studies, ultimately leading to better patient outcomes and more successful research initiatives. Additionally, bioaccess® specializes in various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® offers comprehensive services, including compliance reviews, trial setup, import permits, project management, and detailed reporting, ensuring a robust framework for clinical trial success.
Conclusion
Latin America, and Colombia in particular, presents an array of strategic advantages for conducting clinical trials, especially in the medical device sector. The region's diverse patient populations enhance the generalizability of research findings, while regulatory incentives streamline approval processes, allowing for faster study initiation. Cost-effectiveness, a skilled workforce, and strong healthcare infrastructure further bolster the appeal of conducting trials in this vibrant region.
Colombia stands out as a prime location due to its favorable regulatory environment, growing market for innovative medical devices, and robust support from government initiatives. The presence of experienced research institutions and a commitment to enhancing clinical research capabilities only add to its attractiveness for sponsors. By leveraging these factors, sponsors can not only achieve successful trial outcomes but also contribute to the advancement of medical knowledge in the region.
Selecting the right Contract Research Organizations (CROs) is crucial for trial success. Evaluating their regulatory knowledge, experience with medical devices, local presence, and quality assurance processes can significantly enhance the likelihood of achieving desired outcomes. Establishing effective communication with CROs through clear expectations, regular updates, and feedback mechanisms further fosters collaboration and drives project success.
In conclusion, the unique advantages of conducting clinical trials in Latin America, particularly in Colombia, cannot be overstated. As the medical device outsourcing market continues to evolve, sponsors are encouraged to explore the opportunities presented by this dynamic landscape, ultimately benefiting from an inclusive and efficient clinical research environment.
Frequently Asked Questions
What are the benefits of using a Latin America CRO for Medical Device Trials?
The benefits include diverse patient populations, regulatory incentives for faster study initiation, cost-effectiveness, an experienced workforce, and strong healthcare infrastructure. These factors enhance the generalizability of findings and improve recruitment and retention rates.
How does the diversity of patient populations in Latin America impact research?
The diverse demographics in Latin America enhance the generalizability of study findings by including a wide array of experiences, particularly from marginalized groups such as transgender and gender-diverse individuals.
What regulatory advantages do Latin American countries offer for medical device trials?
Many Latin American nations have established regulatory frameworks that expedite the approval process for research studies, allowing for quicker study initiation and reduced delays.
Why is cost-effectiveness a significant factor for conducting trials in Latin America?
Conducting experiments in Latin America can be significantly less expensive compared to North America and Europe due to lower operational costs, including labor and site fees.
What qualifications do research professionals in Latin America possess?
The region has a growing pool of qualified research professionals, including investigators and coordinators who are knowledgeable about international standards and regulations, enhancing the quality of research studies.
How has the Colombian government supported medical device trials?
The Colombian government encourages foreign investment in health research by offering incentives and assistance to sponsors, reflecting a commitment to advancing the medical device trials sector.
What role does INVIMA play in medical device trials in Latin America?
INVIMA, the National Food and Drug Surveillance Institute, ensures compliance and oversight of medical device studies, serving as a crucial regulatory body in the region.
What should sponsors consider when evaluating CROs in Latin America?
Sponsors should consider the CRO's regulatory knowledge, experience with medical devices, local presence, quality assurance processes, and references from past clients.
How can effective communication enhance collaboration with a CRO?
Setting clear expectations, scheduling regular updates, utilizing technology for real-time communication, encouraging feedback, and establishing crisis management protocols can significantly improve collaboration with a CRO.
What practices should be followed to monitor and evaluate clinical study progress?
Establishing key performance indicators (KPIs), conducting regular data reviews, adjusting protocols as needed, engaging with investigators, and performing a final evaluation of study outcomes are essential practices for effective monitoring.




