Introduction
As the landscape of clinical research continues to evolve, Panama has emerged as a vital hub for first-in-human studies, attracting Medtech companies seeking to navigate the complexities of early-phase trials. With its strategic geographic location, a robust regulatory framework, and a growing clinical research infrastructure, Panama offers a unique environment for innovation in healthcare. However, the journey is not without its challenges, including regulatory hurdles and communication barriers that can hinder progress.
This article delves into the key factors that make Panama an attractive destination for clinical trials, explores the intricacies of selecting a suitable Contract Research Organization (CRO), and highlights the importance of understanding the regulatory environment. Through effective collaboration with local stakeholders and a focus on performance metrics, sponsors can unlock the full potential of their research endeavors in this promising region.
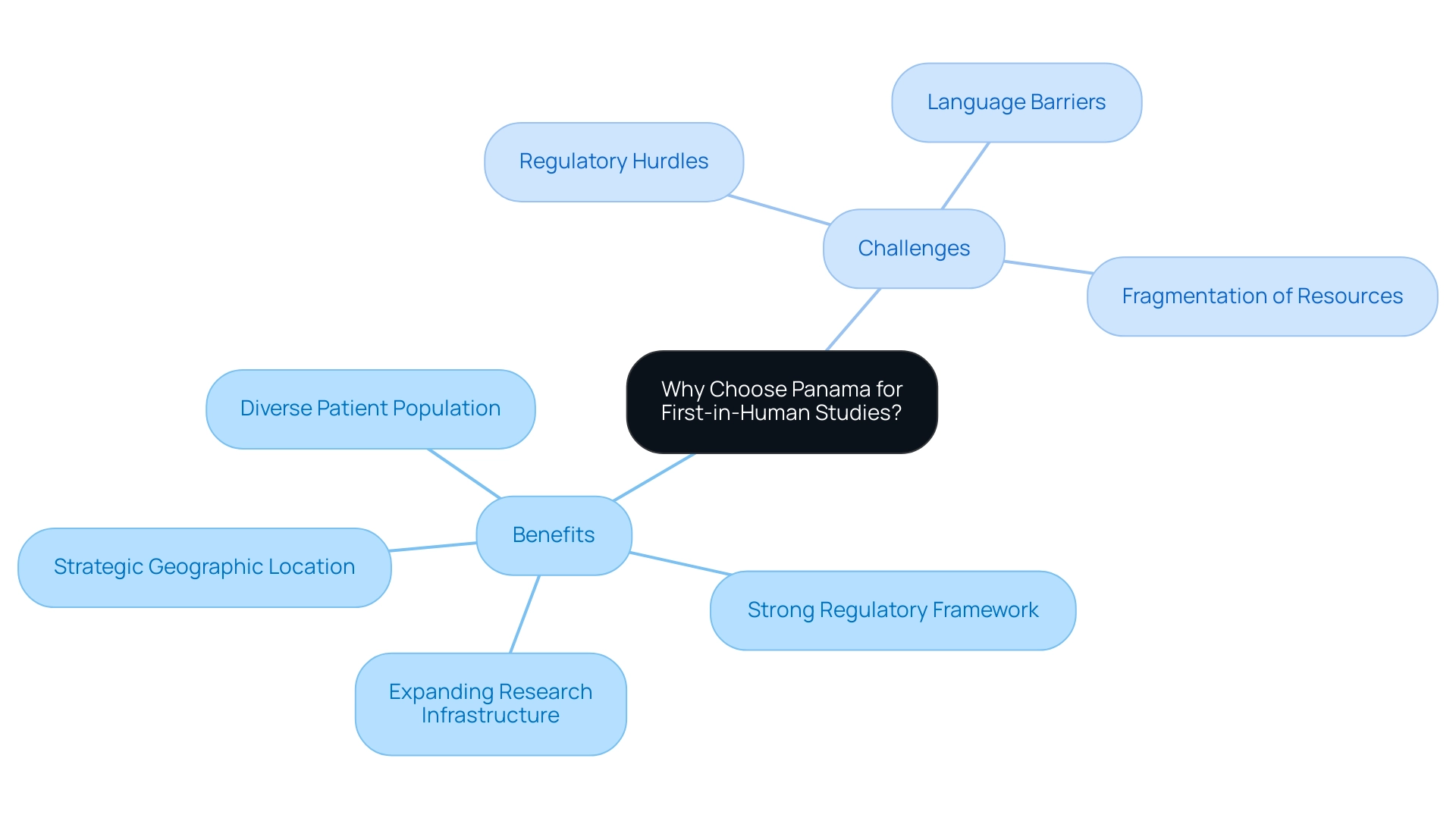
Why Choose Panama for First-in-Human Studies?
Panama has positioned itself as a leading destination for first-in-human studies, owing to its strategic geographic location, strong regulatory framework, and expanding research infrastructure. With over 50 active research sites currently in operation—and a significant increase projected for 2024—Panama offers an accessible and diverse patient population, particularly in urban centers, which is critical for participant recruitment.
However, Medtech companies still face challenges such as:
- Regulatory hurdles
- Language barriers
- Fragmentation of resources that can impede progress.
The Panamanian Ministry of Health has implemented guidelines to streamline the approval process for research trials, enhancing efficiency for sponsors. Additionally, collaborations such as the one between Greenlight Guru and bioaccess™ aim to expedite the entry of Medtech innovations into the region, while GlobalCare Clinical Trials has demonstrated successful ambulatory service expansion in Colombia, achieving over 50% reduction in recruitment time and a retention rate exceeding 95%.
As Eduardo Miranda Martinez, a telemedicine patient, highlights, “for example, if a patient cannot keep his appointment, there is no way to contact the doctor to reschedule,” signaling a critical area for improvement in patient communication.
The regulatory clarity and supportive environment for clinical research in Panama, including tax incentives and fruitful partnerships with local institutions, further enhance its appeal as a prime location for first-in-human trials. This infrastructure not only allows for effective research execution but also advances healthcare access through innovative telemedicine initiatives, particularly benefiting underserved Indigenous communities.
To fully realize this potential, there is an urgent need for a solution-driven approach that addresses the challenges faced by Medtech companies in the region.
Key Factors to Consider When Selecting a CRO in Panama
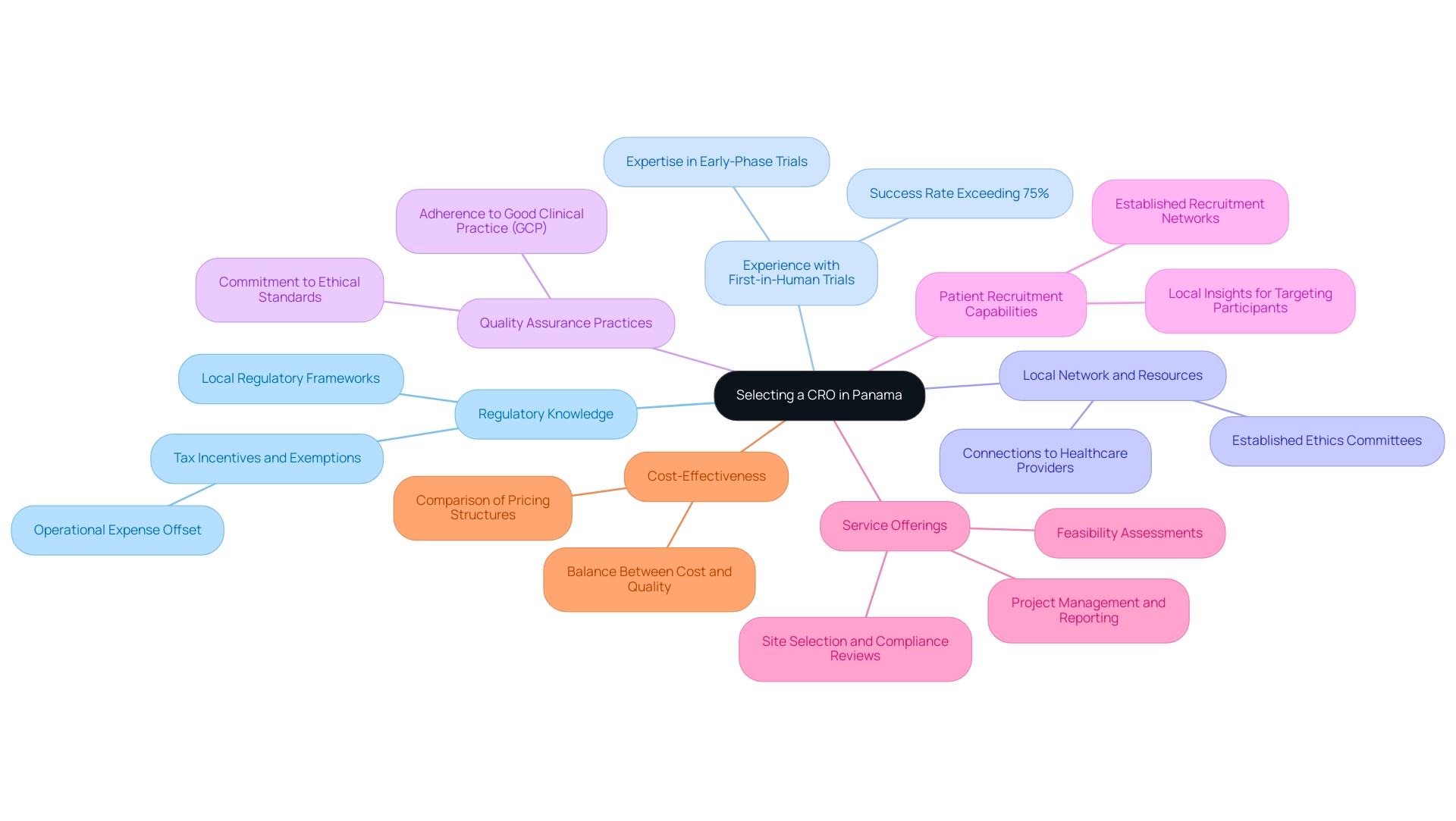
Selecting a Contract Research Organization (CRO) in Panama requires careful consideration of several critical factors to ensure the success of first-in-human studies:
- Regulatory Knowledge: It is essential to partner with a CRO that possesses in-depth knowledge of local regulatory frameworks. Their ability to navigate the approval process efficiently can significantly expedite timelines, which is vital for maintaining project momentum. As Julio G. Martinez-Clark notes, "The Panamanian government offers various tax incentives and exemptions for research and development activities, further offsetting the operational expenses for foreign sponsors."
- Experience with First-in-Human Trials: Choose a CRO that has proven expertise in carrying out first-in-human trials. Their familiarity with the unique challenges and complexities of early-phase trials can provide invaluable support throughout the research. Statistics indicate that first-in-human trials in Panama have a success rate exceeding 75%, highlighting the significance of choosing a skilled CRO.
- Local Network and Resources: A CRO with established connections to local healthcare providers, ethics committees, and regulatory bodies can enhance operational efficiency. These relationships often lead to quicker approvals and smoother interactions with key stakeholders.
- Quality Assurance Practices: Assess the CRO’s commitment to quality assurance and adherence to Good Clinical Practice (GCP) guidelines. A rigorous approach to quality ensures that studies are conducted to the highest ethical and operational standards, fostering confidence among sponsors.
- Patient Recruitment Capabilities: Assess the CRO's approaches for patient recruitment, as timely enrollment is essential for the success of clinical studies. A CRO with local insights and established recruitment networks can more effectively target potential participants, thereby accelerating the process.
- Service Offerings: Consider the specific services provided by the CRO, including feasibility assessments, site selection, compliance reviews, study arrangements, import permits, project management, and reporting. These services are essential for a comprehensive understanding of the CRO's capabilities.
- Cost-Effectiveness: Finally, it is important to compare the pricing structures and services offered by various CROs. Finding a balance between cost and quality is essential to maximize the value of your investment in research. The Neurotronic research project, which encompasses various sites in Panama and Paraguay for addressing Type 2 diabetes, hypertension, and obesity, serves as a relevant example, demonstrating the importance of choosing the appropriate CRO to guarantee successful results.
By thoroughly assessing these factors, sponsors can choose a CRO that not only aligns with their research goals but also plays a crucial role in the successful implementation of first-in-human initiatives in Panama, leveraging bioaccess®'s 20+ years of expertise in Medtech and comprehensive management services.
Understanding the Regulatory Environment in Panama
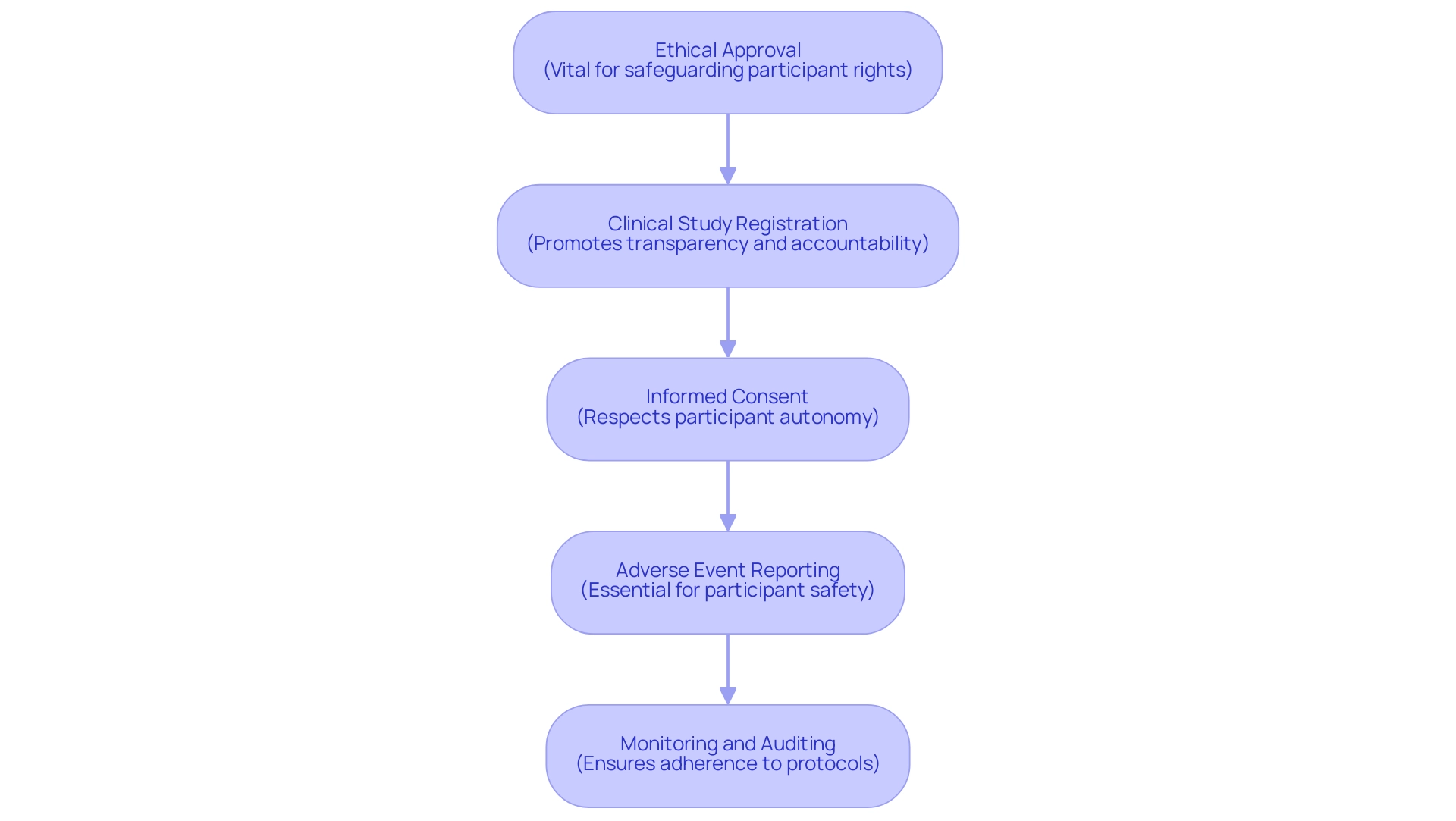
Navigating the regulatory environment for research studies in Panama necessitates a thorough understanding of the framework established by the Ministry of Health. Our thorough management services for research encompass feasibility assessments, site selection, compliance reviews, setup, import permits, project oversight, and reporting, ensuring that all facets of your research are handled efficiently. Key regulations that must be adhered to include:
- Ethical Approval: Before starting, all research studies must obtain permission from an ethics committee. This step is vital for safeguarding the rights and welfare of participants, ensuring that ethical standards are upheld throughout the research. According to a recent report, ethics committee approvals in Panama have increased by 15% over the past year, reflecting a growing commitment to ethical research practices.
- Clinical Study Registration: Sponsors are obligated to register their studies with the local regulatory authority, a process that promotes transparency and accountability in clinical research activities. A case analysis involving a recent oncology trial demonstrated how proper registration facilitated smoother interactions with regulatory bodies and enhanced public trust.
- Informed Consent: Acquiring informed consent from participants is a non-negotiable requirement. This process involves offering detailed information about the purpose, associated risks, and potential benefits, enabling participants to make well-informed decisions. As emphasized by Dr. Juan Pérez, a regulatory expert, 'Informed consent is not merely a formality; it is the basis of ethical medical practice that respects participant autonomy.'
- Adverse Event Reporting: It is imperative for Clinical Research Organizations (CROs) to establish a robust system for the timely reporting of adverse events to regulatory authorities. This protocol is crucial for maintaining participant safety and ensuring regulatory compliance. A recent survey revealed that 80% of CROs in Panama have implemented advanced reporting systems to enhance compliance, including detailed reports on serious and non-serious adverse events, research status, and inventory management.
- Monitoring and Auditing: Regular monitoring and auditing of clinical trials are mandated to confirm adherence to established protocols and regulatory standards, further enhancing the integrity of research. The case analysis on a cardiovascular experiment demonstrated how stringent oversight resulted in the detection and correction of protocol deviations, highlighting the significance of these practices.
Katherine Ruiz, our Regulatory Affairs expert, brings invaluable experience to the table. With a background in advising foreign manufacturers on obtaining market clearance for innovations in Colombia and expertise in navigating the complexities of regulatory compliance, she is dedicated to driving global health improvement through international collaboration and innovation in Medtech. Familiarity with these regulations empowers sponsors and CROs to collaborate effectively within the existing legal framework, thereby ensuring that all research is conducted ethically and efficiently. Given the backdrop of recent events, including the sentencing of former President Ricardo Martinelli for corruption, maintaining rigorous ethical standards in clinical trials is essential to rebuild public trust and foster accountability within the research environment.
Building Effective Collaborations with Local Stakeholders
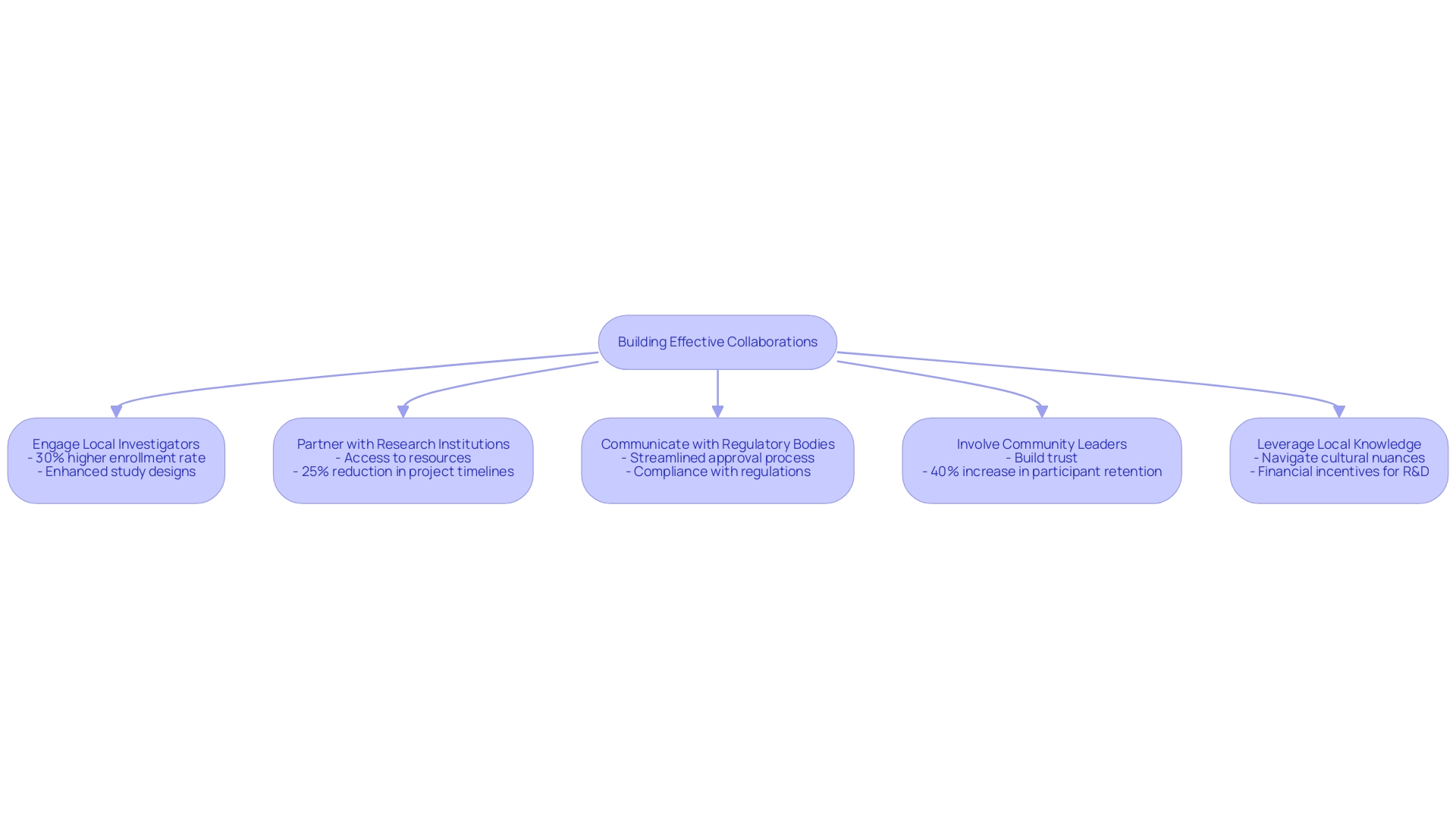
To ensure the success of first-in-human trials in Colombia, fostering effective collaborations with local stakeholders is paramount. Here are several strategies to consider:
- Engage Local Investigators: Collaborating with seasoned local investigators not only enriches the understanding of patient demographics but also significantly enhances recruitment strategies. Statistics indicate that research involving local investigator participation has a 30% higher enrollment rate compared to those lacking it. These investigators provide invaluable insights that can result in more effective study designs, particularly in a country where the healthcare system ranks among the top five globally.
- Partner with Research Institutions: Establishing partnerships with local universities and research institutions can drive innovation and provide access to critical resources, such as advanced laboratory facilities and skilled research staff. For instance, a recent partnership between a leading CRO and the University of Panama resulted in a 25% reduction in project timelines due to shared resources and expertise. In Colombia, clinical research benefits from a healthcare system where hospitals are rigorously certified, enhancing the overall quality and efficiency of the studies.
- Communicate with Regulatory Bodies: Maintaining transparent communication with regulatory authorities, such as INVIMA and local ethics committees, is essential for addressing concerns early on and ensuring compliance with local regulations. Such proactive engagement can streamline the approval process, which in Colombia typically takes only 90-120 days, facilitating smoother project execution.
- Involve Community Leaders: Engaging community leaders is crucial for building trust and improving participant recruitment. Their involvement ensures that the study is well-received and understood within the community context, which can lead to higher enrollment rates. For example, a recent study in Colombia achieved a 40% increase in participant retention after involving local leaders in the outreach process. This engagement is vital in a country where approximately 95% of the population is covered by universal healthcare.
- Leverage Local Knowledge: Utilizing the expertise of local stakeholders allows researchers to navigate cultural nuances effectively. This understanding is vital for enhancing participant engagement, which is crucial for retention and the overall quality of data collected. Moreover, the R&D tax incentives offered in Colombia, such as a 100% tax deduction, a 25% tax discount, and significant government grants, can offer extra financial assistance for innovative research projects.
By actively partnering with local stakeholders, sponsors can foster a supportive atmosphere that not only enhances the chances of success for their research studies but also addresses the essential need for diversity in research. As Karamat emphasizes, it is important that you go both ways while ensuring that this diversity is being applied in experiments. These strategic partnerships are essential in breaking down obstacles and enhancing access to research within the region. Furthermore, utilizing extensive research management services, encompassing project oversight and monitoring, can further improve the efficiency and effectiveness of these investigations.
Evaluating CRO Performance and Success Metrics
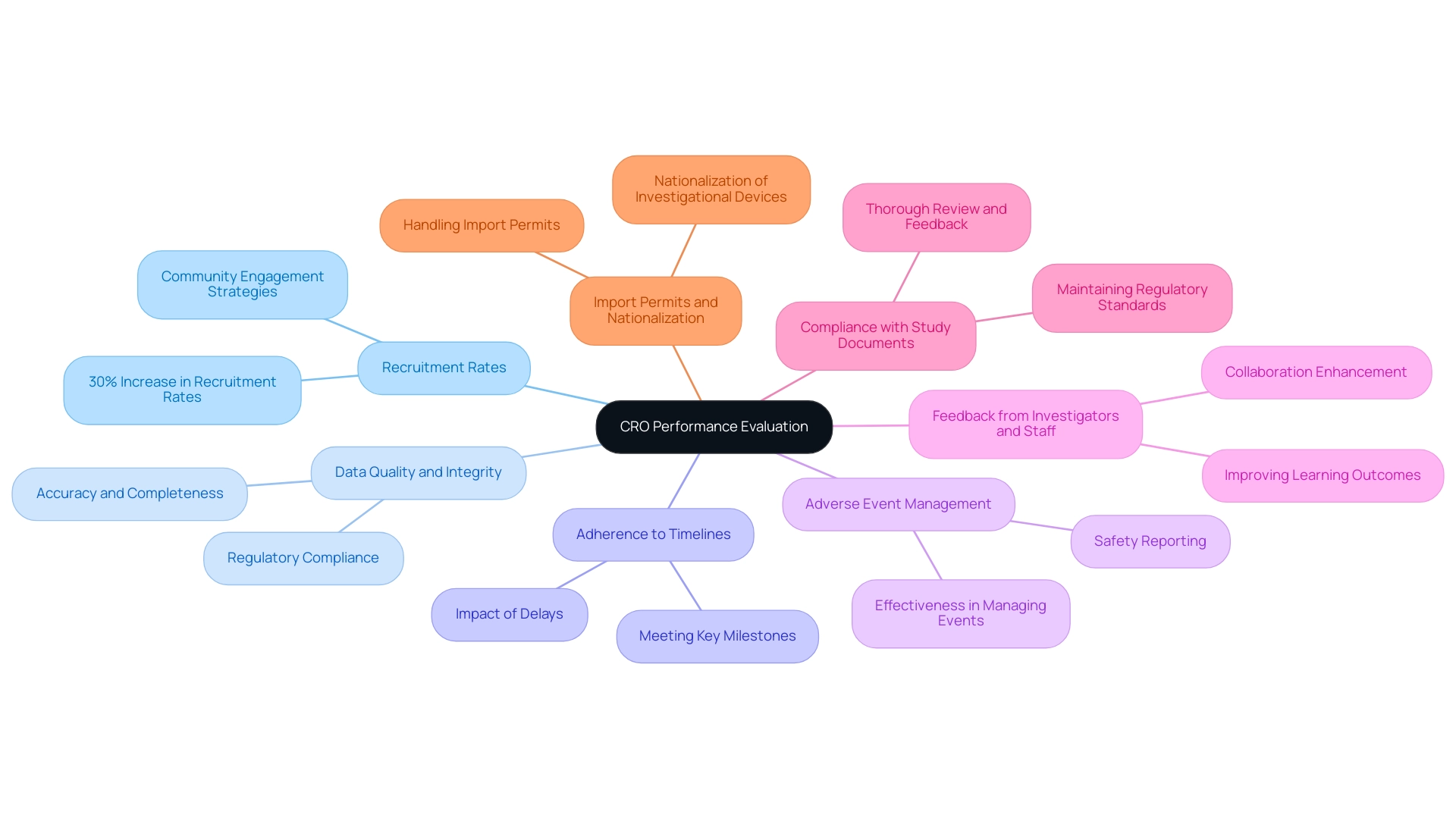
To maximize the effectiveness of a Contract Research Organization (CRO) in Panama, sponsors must establish clear performance metrics to assess success throughout the clinical trial. Key indicators include:
- Recruitment Rates: Track the speed and efficiency of patient recruitment relative to initial projections. Recent research has demonstrated a 30% increase in patient recruitment rates in Panama due to improved community engagement strategies, reflecting the CRO's capacity to generate a positive local impact.
- Data Quality and Integrity: Evaluate the accuracy and completeness of the data collected during the study—consistent data quality is vital for regulatory compliance and ensuring the validity of research outcomes.
- Adherence to Timelines: Monitor whether the CRO meets key milestones and deadlines outlined in the project plan. Timely execution is crucial, as delays can adversely affect academic success and costs.
- Adverse Event Management: Examine the CRO's effectiveness in managing adverse events and safety reporting. A proactive approach is essential for participant safety and regulatory compliance.
- Feedback from Investigators and Staff: Solicit feedback from local investigators and research personnel on their collaboration with the CRO. Positive relationships can significantly enhance collaboration and improve overall learning outcomes.
- Compliance with Study Documents: Ensure that the CRO provides thorough review and feedback on study documents to comply with country requirements. This is critical for maintaining regulatory standards and facilitating smooth trial operations.
- Import Permits and Nationalization: Assess the CRO's capability in handling import permits and the nationalization of investigational devices, which are essential for the smooth execution of clinical trials.
As Albert Einstein once said, 'Try not to become a person of success, but rather try to become a person of value.' This principle underscores the importance of focusing on meaningful metrics rather than just outcomes. Additionally, a case study on Curo's engagement with residents through Insight Hub illustrates how effective communication and feedback can enhance satisfaction and foster community, principles that apply equally to evaluating CRO performance.
By consistently evaluating these performance metrics, sponsors can ensure their CRO aligns with research objectives. This comprehensive approach not only enhances trial success but also contributes to local economic growth, healthcare improvements, and international collaboration.
Conclusion
Panama's emergence as a leading destination for first-in-human studies is underscored by its strategic advantages, including a supportive regulatory environment and a rich clinical research infrastructure. As highlighted, the country boasts over 50 active clinical trial sites, with expectations for further growth in 2024. The successful execution of these trials is contingent upon overcoming challenges such as regulatory hurdles and communication barriers.
By fostering partnerships with experienced Contract Research Organizations (CROs) that understand the local landscape, sponsors can navigate these complexities more effectively.
Selecting the right CRO is crucial; factors such as regulatory knowledge, patient recruitment capabilities, and quality assurance practices significantly impact the success of clinical trials. The commitment to ethical standards and effective communication with local stakeholders enhances the potential for successful outcomes. Understanding the regulatory framework is equally important, as it ensures compliance and builds public trust in the research process.
In conclusion, the strategic collaboration between Medtech companies, local investigators, and regulatory bodies in Panama can yield significant advancements in healthcare. By leveraging the strengths of this dynamic ecosystem, sponsors can not only enhance the efficiency of their clinical trials but also contribute to the broader goals of improving healthcare access and innovation in the region. As Panama continues to evolve as a clinical research hub, the emphasis on collaboration and adherence to best practices will be vital in unlocking the full potential of first-in-human studies.
Frequently Asked Questions
Why is Panama considered a leading destination for first-in-human studies?
Panama is recognized for its strategic geographic location, strong regulatory framework, and expanding research infrastructure, along with over 50 active research sites and a diverse patient population, particularly in urban areas.
What challenges do Medtech companies face in Panama?
Medtech companies encounter challenges such as regulatory hurdles, language barriers, and fragmentation of resources that can impede progress.
How has the Panamanian Ministry of Health improved the research trial approval process?
The Ministry has implemented guidelines to streamline the approval process for research trials, enhancing efficiency for sponsors.
What collaborations are helping to expedite Medtech innovations in Panama?
Collaborations, such as that between Greenlight Guru and bioaccess™, aim to expedite the entry of Medtech innovations into the region.
What are the benefits of the regulatory environment in Panama for clinical research?
The regulatory environment offers clarity, tax incentives, and partnerships with local institutions, enhancing Panama's appeal for first-in-human trials.
What factors should be considered when selecting a Contract Research Organization (CRO) in Panama?
Key factors include regulatory knowledge, experience with first-in-human trials, local network and resources, quality assurance practices, patient recruitment capabilities, service offerings, and cost-effectiveness.
What is the success rate of first-in-human trials in Panama?
The success rate for first-in-human trials in Panama exceeds 75%.
What are the key regulations for research studies in Panama?
Key regulations include obtaining ethical approval, clinical study registration, acquiring informed consent, timely adverse event reporting, and regular monitoring and auditing of trials.
How can local stakeholder collaborations improve first-in-human trials in Colombia?
Collaborations with local investigators, research institutions, regulatory bodies, community leaders, and leveraging local knowledge can enhance recruitment, streamline processes, and improve participant engagement.
What performance metrics should sponsors establish for evaluating a CRO in Panama?
Sponsors should track recruitment rates, data quality and integrity, adherence to timelines, adverse event management, feedback from investigators, compliance with study documents, and handling of import permits and nationalization.

