Introduction
As the landscape of clinical research evolves, Argentina emerges as a compelling destination for first-in-human studies, offering a unique blend of regulatory expertise, diverse patient populations, and cost-effective solutions. Stakeholders in the medical and research fields must navigate a complex array of ethical and logistical considerations to ensure successful trial outcomes. Understanding the intricacies of local regulations, such as those set forth by the ANMAT, and partnering with experienced Contract Research Organizations (CROs) like bioaccess® can significantly enhance the efficiency and credibility of clinical trials.
This article delves into the critical requirements for conducting first-in-human studies in Argentina, highlighting the importance of selecting the right CRO, adhering to regulatory compliance, and fostering effective partnerships to maximize research potential and drive innovation in healthcare.
Understanding the Unique Requirements for First-in-Human Studies in Argentina
Navigating the complexities of first-in-human studies necessitates careful selection of a Contract Research Organization (CRO) that is well-versed in the ethical, regulatory, and logistical nuances specific to Argentina. Key considerations include:
- Regulatory Framework: A thorough understanding of the ANMAT (National Administration of Drugs, Foods and Medical Technology) regulations is essential, particularly with updates for 2024 that emphasize stricter compliance and transparency in clinical trial processes. Familiarity with these regulations ensures compliance and facilitates smoother trial processes.
- Comprehensive Clinical Trial Management Services: With over 20 years of experience in Medtech, bioaccess® offers a robust suite of services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). These capabilities are essential in ensuring that research is conducted efficiently and adheres to all regulatory requirements.
- Cultural Sensitivity: Recognizing the diverse local patient populations is crucial, as this understanding significantly influences recruitment and retention strategies. Engaging with community norms and values can enhance participant trust and cooperation.
- Ethical Considerations: It is imperative that the CRO adheres to stringent ethical guidelines, particularly concerning informed consent processes and patient safety protocols. Upholding these ethical standards not only safeguards participants but also enhances the credibility of the research.
Recent news emphasizes the growing attention on first-in-human research in Argentina, with a significant increase in clinical experiments being registered. According to Luis Gabriel Cuervo, "Findings from this research suggest that knowledge rather than attitude is the foremost factor impeding registration." This underscores the importance of educational initiatives to promote compliance with registration standards.
By carefully addressing these requirements, stakeholders can ensure that their selected CRO is sufficiently prepared to handle the challenges linked to first-in-human research in Argentina, ultimately promoting successful results.
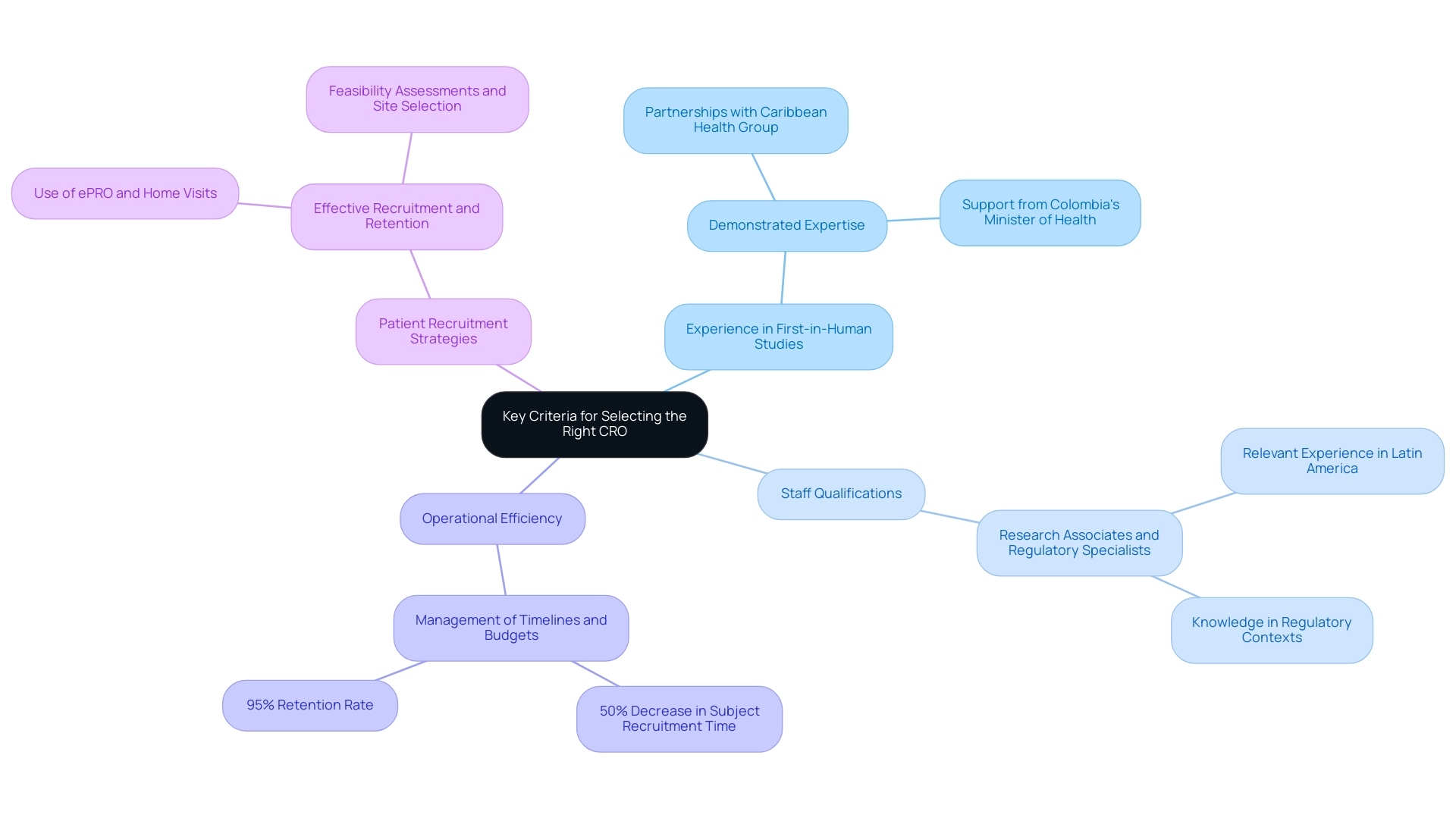
Key Criteria for Selecting the Right CRO for Clinical Trials
Choosing the appropriate Contract Research Organization (CRO) for medical experiments, particularly first-in-human initiatives, is essential for securing favorable results. Here are the key criteria to consider:
- Experience in First-in-Human Studies: It is essential to partner with a CRO that has demonstrated expertise in conducting first-in-human trials. For instance, bioaccess™ has formed partnerships with Caribbean Health Group and GlobalCare Clinical Trials to improve the implementation of studies in Colombia, demonstrating its ability to address the unique challenges linked with these studies effectively. This initiative is further supported by Colombia's Minister of Health, who promotes enhancing medical research in the region.
- Staff Qualifications: Assess the qualifications of the CRO’s personnel, including research associates and regulatory specialists. Their pertinent experience is crucial in managing the intricacies of research in Latin America, as demonstrated by bioaccess™'s extensive knowledge in the region, especially in regulatory contexts essential for medical device evaluations.
- Operational Efficiency: An assessment of the CRO's processes for managing timelines and budgets is vital. For example, GlobalCare Clinical Studies reported over a 50% decrease in subject recruitment time and a 95% retention rate through its partnership with bioaccess™, emphasizing the significance of high operational efficiency and strong data management practices.
- Patient Recruitment Strategies: The ability to effectively recruit and retain participants is critical. As highlighted by Antidote, "Utilizing tools and technology that reduce the number of site visits — such as electronic patient-reported outcomes (ePRO) and home visits — may enhance enrollment for the patients that research teams have the most difficulty contacting." Creative approaches such as these, backed by bioaccess™'s extensive management services for research, including feasibility assessments, site selection, and regulatory adherence, can significantly improve enrollment, especially for difficult-to-access patient groups.
By thoughtfully utilizing these standards and evaluating partners such as bioaccess™, stakeholders can make educated choices when choosing a CRO, thus improving the chances of successful research results.
Navigating Regulatory Compliance and Local Expertise in Argentina
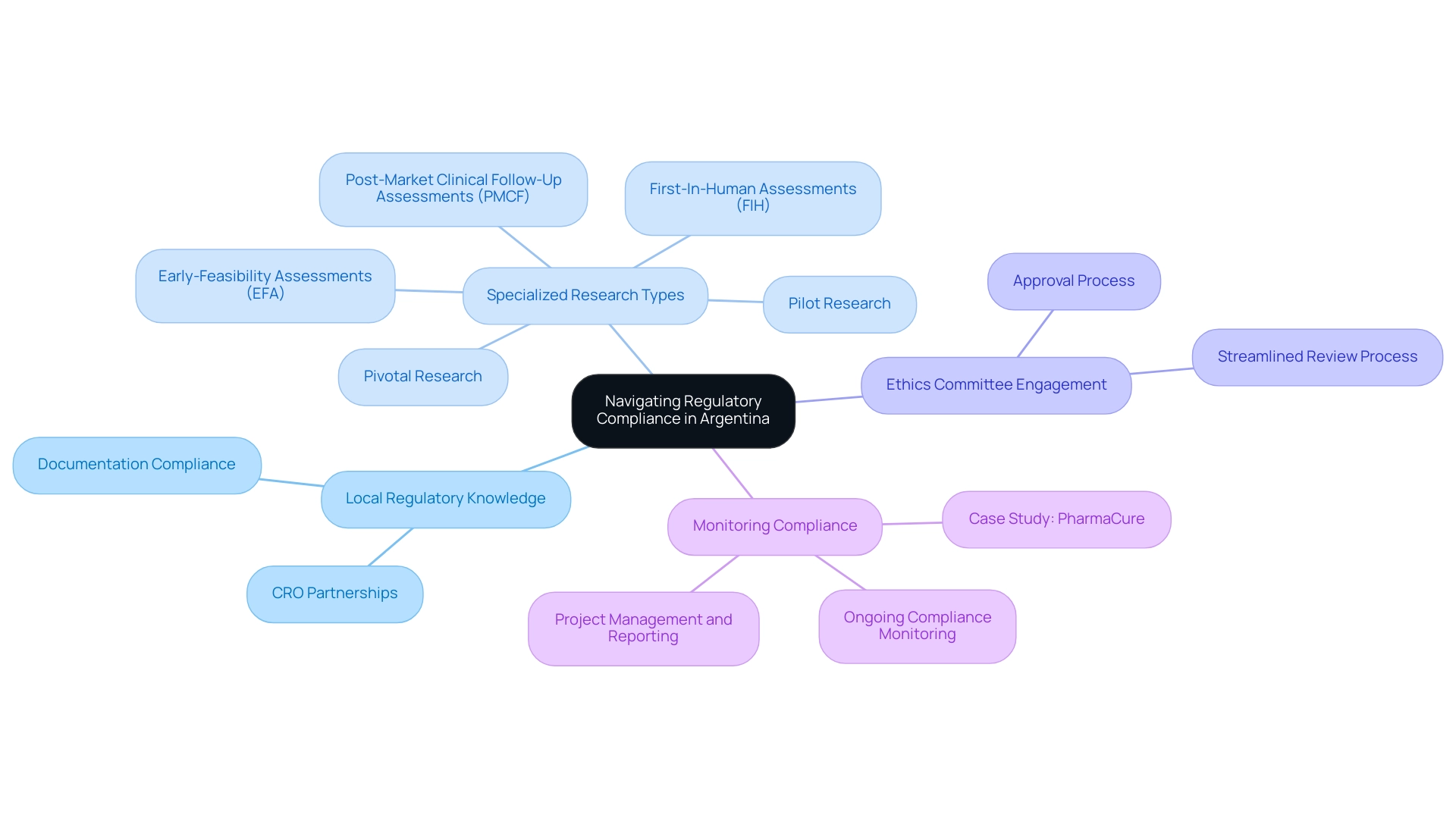
In Argentina, the regulatory environment for medical research is mainly overseen by ANMAT, which establishes thorough guidelines for carrying out investigations. To effectively navigate this framework, stakeholders should consider the following key elements:
- Local Regulatory Knowledge: Partnering with a Contract Research Organization (CRO) like bioaccess®, which has extensive expertise in ANMAT regulations, is essential for successful study management. With a proven track record of over 20 years in Medtech, bioaccess® ensures that all necessary documentation is submitted in compliance with local requirements.
- Specialized Research Types: Bioaccess® specializes in managing various types of research, including Early-Feasibility Assessments (EFA), First-In-Human Assessments (FIH), Pilot Research, Pivotal Research, and Post-Market Clinical Follow-Up Assessments (PMCF), providing a comprehensive approach to clinical research.
- Ethics Committee Engagement: Gaining approval from local ethics committees is a prerequisite before initiating any clinical investigation. Recent updates indicate that the review process has become more streamlined, making it beneficial to select a CRO with established relationships with these committees. This can accelerate the approval process and enhance the ethical standards of the study.
- Monitoring Compliance: Ongoing compliance is critical throughout the duration of the research. Choose a CRO that implements robust monitoring processes to ensure that all regulatory requirements are continuously met. Bioaccess® is well-equipped to handle these needs, providing comprehensive project management and reporting services that maintain the integrity of the research data. A relevant case analysis is the recent examination carried out by PharmaCure, which successfully navigated ANMAT's compliance requirements, resulting in timely approvals and adherence to ethical standards.
Focusing on these elements not only mitigates the risks associated with regulatory non-compliance but also enhances the overall credibility and success of the research. With recent updates indicating a reduction of the inspection period for orphan drugs from 90 days to 70 days, timely and effective navigation of these regulatory pathways is more crucial than ever. Utilizing the knowledge of specialists such as Katherine Ruiz, a noted authority in regulatory affairs for medical devices, can greatly improve the chances of studies in this changing environment.
Benefits of Conducting Clinical Trials in Argentina
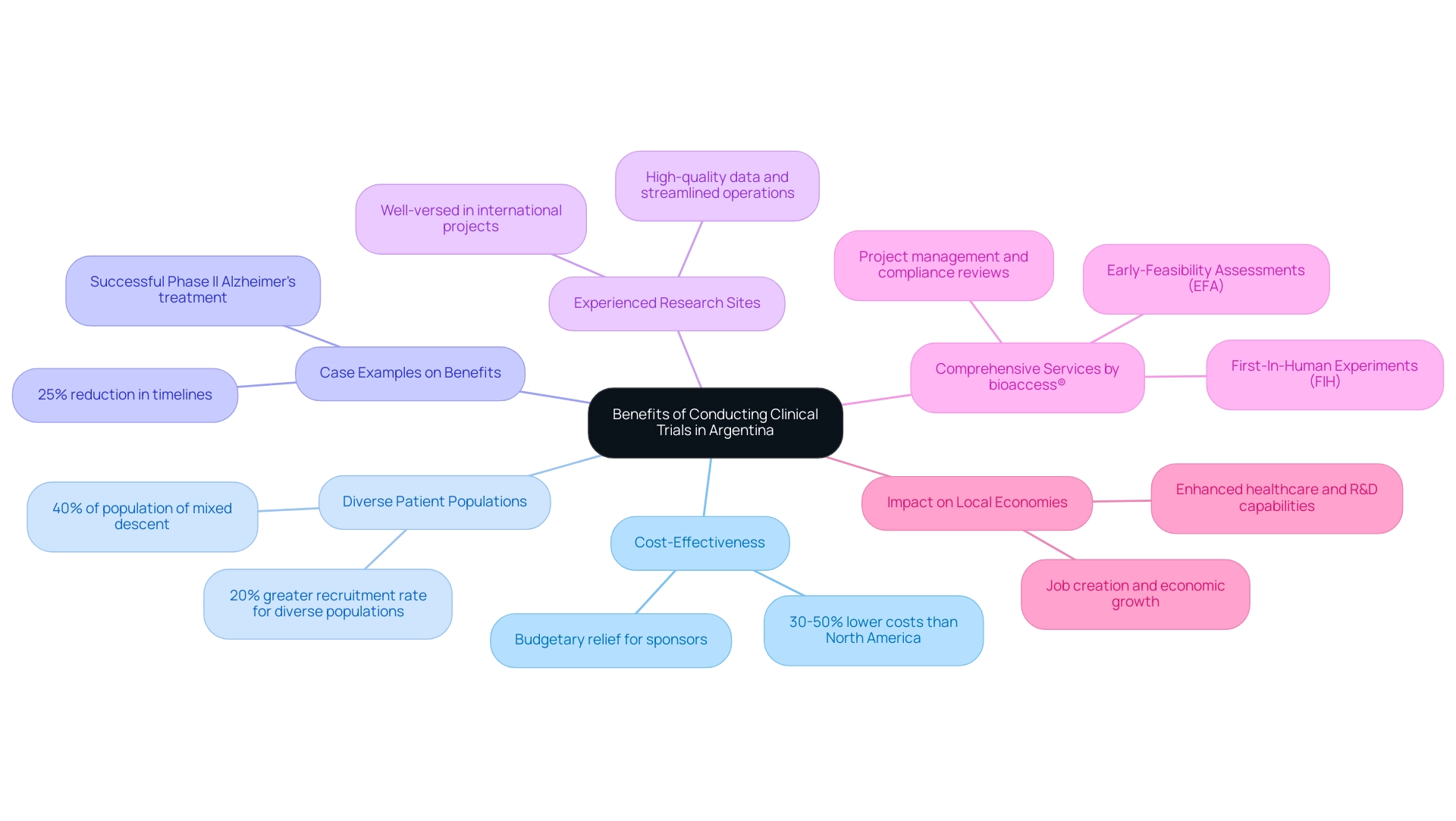
Conducting research in Argentina offers numerous benefits that render it an attractive choice for sponsors, especially for first-in-human and early feasibility projects:
- Cost-Effectiveness: The financial environment for research in Argentina is significantly advantageous, with expenses typically projected to be 30-50% less than those encountered in North America. This cost disparity can provide significant budgetary relief for sponsors, allowing for more extensive research endeavors or the allocation of resources to other essential areas of development.
- Diverse Patient Populations: Argentina's rich demographic diversity, with over 40% of its population being of mixed European and Indigenous descent, enables researchers to engage with a wide range of patient populations. This variety is crucial for studying different health conditions and understanding how various demographic factors can influence treatment outcomes, ultimately leading to more robust and generalizable results. For example, a recent research indicated that medical experiments carried out in Argentina achieved a 20% greater recruitment rate for diverse populations compared to experiments in North America.
- Case Examples on Benefits: A significant case example from 2023 highlighted a successful Phase II medical evaluation for a new Alzheimer's treatment conducted in Buenos Aires. This examination not only highlighted the effectiveness of the treatment but also illustrated the streamlined processes and local expertise available in Argentina, leading to a 25% reduction in timelines compared to similar research in the U.S.
- Experienced Research Sites: Many research organizations (CROs) in Argentina are well-versed in conducting international projects, possessing the necessary expertise to deliver high-quality data and streamline operations. This experience not only guarantees adherence to regulatory requirements but also boosts the efficiency of testing processes, thereby enhancing timelines and results.
- Comprehensive Services by bioaccess®: Through bioaccess®, sponsors can utilize extensive management services for their projects, including Early-Feasibility Assessments (EFA), First-In-Human Experiments (FIH), feasibility evaluations, site selection, compliance reviews, setup, import permits, project management, and reporting. With more than 20 years of experience in Medtech, bioaccess® offers a tailored approach that guarantees trials are carried out seamlessly, complying with local regulations while optimizing efficiency.
- Impact on Local Economies: Furthermore, the execution of medical research generates waves of economic advantages in local communities, encouraging job creation, stimulating economic growth, enhancing healthcare, and improving research and development capabilities. Such initiatives not only contribute to the local economy but also enhance global acknowledgment of the region's research capabilities.
These compelling benefits position Argentina as a strategic choice for sponsors in the research arena, significantly impacting CRO selection and overall project success.
Building Effective Partnerships with Your Chosen CRO
To cultivate successful partnerships with your chosen Contract Research Organization (CRO), particularly in the context of first-in-human and early-feasibility investigations in Latin America, consider implementing the following strategies:
- Open Communication: Initiate and maintain transparent communication channels from the beginning. This ensures that both parties are aligned on project objectives and expectations, which is crucial for effective collaboration. As Kris Kowdley, MD, AGAF, FAASLD, FACP, FACG, emphasizes, clear communication is critical for successful outcomes in research trials.
- Regular Updates: Establish a schedule for frequent meetings to review progress, address challenges, and make necessary adjustments to the study protocol. This proactive approach facilitates timely decision-making and enhances project agility, allowing teams to respond effectively to emerging needs.
- Collaborative Problem-Solving: Create a cooperative atmosphere where both teams can collectively tackle any issues that may arise during the experiment. This promotes innovative solutions and fortifies the partnership.
Moreover, utilizing bioaccess®’s expertise in managing trials—including early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies—can significantly improve your collaboration with CROs. bioaccess® provides extensive services including feasibility and selection of research locations and primary investigators, compliance evaluations, setup, import permits, and project management. With a proven track record of over 20 years in Medtech, bioaccess® is equipped to navigate the complexities of regulatory environments, ensuring compliance and streamlined processes. By adopting these strategies, stakeholders can significantly enhance their partnerships, ultimately leading to better results in research. The success of initiatives, such as Kris Kowdley, MD’s presentation of late-breaking data for Iqirvo® at the American Association for the Study of Liver Diseases (AASLD), underscores the critical role of effective communication and collaboration in advancing research and development in clinical trials.
Conclusion
Navigating the complexities of first-in-human studies in Argentina requires a strategic approach that encompasses regulatory compliance, ethical considerations, and effective partnerships. The importance of selecting a knowledgeable and experienced Contract Research Organization (CRO) cannot be overstated, as it plays a pivotal role in ensuring trial success. With the evolving landscape shaped by ANMAT regulations, stakeholders must prioritize CROs like bioaccess®, which offer comprehensive clinical trial management services and possess a deep understanding of local practices.
The benefits of conducting clinical trials in Argentina further reinforce its appeal as a prime location for research. From cost advantages to the rich diversity of patient populations, Argentina provides a conducive environment for innovative studies. The ability to leverage local expertise and established relationships with ethics committees enhances the efficiency and credibility of the research process, ultimately driving better outcomes.
In conclusion, the successful execution of first-in-human studies in Argentina hinges on meticulous planning, adherence to regulatory frameworks, and the cultivation of strong partnerships with experienced CROs. By focusing on these critical elements, stakeholders can maximize research potential and contribute to the advancement of healthcare innovation, positioning Argentina as a key player in the global clinical research arena.
Frequently Asked Questions
What is the importance of selecting a Contract Research Organization (CRO) for first-in-human studies in Argentina?
Choosing the right CRO is essential for securing favorable results in first-in-human studies, as they must be knowledgeable about the ethical, regulatory, and logistical nuances specific to Argentina.
What regulatory framework must CROs understand in Argentina?
CROs must have a thorough understanding of ANMAT (National Administration of Drugs, Foods and Medical Technology) regulations, especially with updates for 2024 that emphasize stricter compliance and transparency in clinical trial processes.
What services does bioaccess® offer for clinical trials?
bioaccess® provides a robust suite of services including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring efficient research conduct and adherence to regulatory requirements.
Why is cultural sensitivity important in first-in-human studies?
Recognizing the diverse local patient populations influences recruitment and retention strategies, enhancing participant trust and cooperation in clinical trials.
What ethical considerations should CROs adhere to?
CROs must follow stringent ethical guidelines, especially regarding informed consent processes and patient safety protocols, to safeguard participants and enhance the credibility of the research.
What recent trends have been observed in first-in-human research in Argentina?
There has been a significant increase in registered clinical experiments, highlighting the need for educational initiatives to promote compliance with registration standards.
What experience is essential for a CRO conducting first-in-human studies?
A CRO must have demonstrated expertise in conducting first-in-human trials, as seen with bioaccess™'s partnerships that enhance study implementation in regions like Colombia.
How do staff qualifications impact CRO effectiveness?
The qualifications of research associates and regulatory specialists are crucial in managing research complexities, particularly in the regulatory contexts of Latin America.
What operational efficiencies should a CRO demonstrate?
A CRO should manage timelines and budgets effectively, as evidenced by GlobalCare Clinical Studies' significant improvements in subject recruitment and retention through collaboration with bioaccess™.
What strategies can enhance patient recruitment in clinical studies?
Utilizing tools and technology that minimize site visits, such as electronic patient-reported outcomes (ePRO) and home visits, can improve participant enrollment, especially for hard-to-reach patient groups.
What are the benefits of conducting research in Argentina?
Argentina offers cost-effectiveness, diverse patient populations, experienced research sites, and comprehensive services from CROs like bioaccess®, making it an attractive option for sponsors.
How can stakeholders cultivate successful partnerships with CROs?
Strategies include maintaining open communication, scheduling regular updates, and fostering collaborative problem-solving to enhance project outcomes and research results.




