Overview
Standardized medtech trial designs play a pivotal role in ensuring reliable and consistent outcomes in clinical research. By reducing biases and streamlining data aggregation, these designs enhance the credibility of studies through clearly defined protocols. Furthermore, the adoption of standardized designs optimizes resource allocation, which ultimately leads to improved patient safety and the development of more effective medical technologies. As the medtech landscape evolves, it is essential to recognize the significant impact these trial designs have on addressing key challenges, reinforcing the necessity for collaboration and strategic action in the field.
Introduction
The landscape of medical technology is rapidly evolving, capturing the attention of stakeholders with the emergence of standardized trial designs as a cornerstone for reliable clinical research. These frameworks not only enhance the reproducibility of results but also streamline the path to regulatory approval by minimizing biases and variability, generating interest in their implementation.
As the industry embraces advancements in methodologies, the desire for structured protocols becomes increasingly clear, particularly in multicenter studies where consistency is paramount. With the integration of digital tools and innovative approaches, the future of clinical trials promises improved patient engagement and data integrity, reinforcing the conviction that these developments will lead to groundbreaking advancements in Medtech.
This article delves into the key components that drive successful trial designs, from optimizing protocols to ensuring data security, ultimately guiding researchers toward a more efficient and effective clinical research process.
Understanding Standardized Trial Designs in Medtech
Standardized medtech trial designs are essential in the Medtech sector, ensuring that medical evaluations produce reliable and consistent outcomes. These designs typically include predefined protocols that clearly delineate the study's objectives, methodologies, and statistical analysis plans. By adhering to standardized medtech trial designs, researchers can significantly reduce biases and variability, which are crucial for obtaining regulatory approvals.
The randomized controlled trial (RCT) design, often considered the gold standard in clinical research, exemplifies this approach by facilitating robust comparisons between treatment groups. Furthermore, the implementation of standardized medtech trial designs streamlines data aggregation and meta-analysis, thereby enhancing the understanding of a medical device's efficacy and safety across diverse populations. Recent advancements in standardized medtech trial designs in 2025 underscore their importance, with research indicating that such designs yield more consistent outcomes and improved patient safety. For instance, the case study titled "Emerging Technologies in Digital Therapeutics" explored the integration of Extended Reality (XR) and Human-computer Interaction (HCI) methods in medical research, highlighting the necessity for interdisciplinary collaboration to foster the commercialization of digital therapeutics products.
Incorporating standardized medtech trial designs also optimizes resource allocation, enabling research teams to leverage existing frameworks and templates to expedite the planning and execution phases of clinical studies. This strategic approach not only conserves time but also significantly reduces costs associated with design and implementation. As the Medtech landscape evolves, the focus on standardized medtech trial designs will continue to intensify, underscoring their vital role in advancing medical technologies and improving patient outcomes.
As emphasized by the International Digital Therapeutics Alliance (DTA), digital therapeutics is "health software that treats or alleviates diseases, disorders, conditions, or injuries by generating and providing medical interventions that have a significant positive therapeutic impact on patient health." However, it is crucial to recognize the limitations in current research, including reliance on ClinicalTrials.gov information, which may have led to omitted records and a lack of analysis on real-world research. With over 20 years of experience in the Medtech sector, bioaccess® is committed to delivering tailored strategies that facilitate the acceleration of medical devices for companies in the industry, offering comprehensive clinical trial management services that encompass feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and reporting.
Key Components of Successful Multicenter Imaging Studies
Successful multicenter imaging investigations hinge on several crucial elements, including standardized imaging protocols, effective communication among sites, and robust information management systems. Standardized imaging protocols are indispensable, as they ensure that all participating centers utilize the same techniques and equipment, which is vital for maintaining consistency in quality. For example, discrepancies in MRI machines or settings across different centers can lead to non-comparable images, ultimately resulting in unreliable conclusions.
Statistics indicate that the median sample sizes of experimental fMRI research have increased by +0.74 participants annually, underscoring the growing importance of standardized protocols and information management in accommodating larger participant groups.
Effective communication is pivotal in coordinating efforts across multiple sites. Regular meetings and updates are instrumental in addressing any issues that may arise during the research, ensuring that all sites remain aligned with the research's objectives. Furthermore, implementing a centralized information management system can significantly streamline data collection and analysis. This system enables real-time observation of research progress and data integrity, enhancing the overall quality of the information gathered and promoting prompt decision-making throughout the process.
In addition to these practices, bioaccess offers extensive management services for research activities, encompassing:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup processes
- Import permits
- Project oversight
- Reporting on significant and minor adverse events
These services are designed to assist researchers in navigating the complexities of clinical trials, ensuring adherence to regulatory standards and enhancing overall research efficiency, including compliance with country requirements.
An evaluation of recent research revealed that consistent demographic reporting—factoring in sex, gender, race, ethnicity, and age—can significantly enhance the applicability and impact of biomedical research findings. The case analysis titled "Recommendations for Enhanced Demographic Reporting" advocates for open access to demographic information and a shift in research practices to emphasize diversity. As Hannah Pearl noted, "Demographic reporting varies as a function of disease studied, participant age range, funding, and publisher," highlighting the critical importance of diversity in participant selection and reporting.
Moreover, there is a positive trend in research focusing on AI implementation in medical imaging, which highlights the relevance of current advancements in the field and the necessity for comparable evaluations to monitor efficiency effects.
In summary, the integration of standardized imaging protocols, effective communication strategies, and robust information management systems, along with standardized medtech trial designs, are essential components that contribute to the success of multicenter imaging studies. By adhering to these best practices, researchers can ensure high-quality information collection and analysis, ultimately advancing the field of medical technology.
Optimizing Protocol Design for Multicenter Trials
Enhancing protocol design for multicenter studies requires a multifaceted approach that emphasizes clarity and adaptability, particularly with standardized medtech trial designs. Defining clear and measurable endpoints is paramount within these designs, as these benchmarks directly align with the study's objectives and facilitate consistent data collection across all participating sites. Research indicates that standardized medtech trial designs with well-defined endpoints significantly increase the likelihood of success, underscoring the necessity for precision in this area.
With over 20 years of experience in the Medtech sector, bioaccess® underscores the importance of these practices in achieving favorable outcomes through its comprehensive clinical study management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF) in Latin America.
In addition to endpoint clarity, standardized medtech trial designs must incorporate flexibility within the protocol to accommodate the diverse capabilities of various sites and the unique characteristics of patient populations. This adaptability is especially vital in studies utilizing standardized medtech trial designs, where variations can influence integrity and overall trial outcomes.
Equipping site staff with comprehensive training materials and resources is another critical strategy. Thorough training on the protocol, information collection methods, and compliance requirements can substantially minimize variability and errors in reporting. This proactive approach not only enhances the quality of data but also fosters a culture of compliance and accountability among site personnel.
Moreover, the use of standardized medtech trial designs allows for real-time modifications based on interim results, thereby improving the study's efficiency and effectiveness. As noted by Obioha C. Ukoumunne, the expert working group emphasizes the need for collaboration and clarity in protocol development.
Additionally, referencing the case study titled "The Importance of Internal Review in Protocol Development" illustrates the significance of internal reviews in ensuring protocol clarity and compliance, aligning with the section's focus on optimizing protocol design. By integrating these optimization strategies, researchers can significantly enhance the probability of achieving successful outcomes in multicenter studies, supported by standardized medtech trial designs, ultimately advancing the development of innovative medical technologies. Furthermore, recommendations for the design of multi-arm multi-stage studies, including a minimum set of items for Statistical Analysis Plans, should be considered to provide a more comprehensive view of best practices in protocol design.
This article aims to serve as a comprehensive guide for designing a research protocol, particularly relevant for new researchers in the field.
Ensuring Data Management and Security in Clinical Trials
Ensuring effective information management and security in clinical trials is essential for safeguarding sensitive patient details and upholding the integrity of the study. A robust information management plan is crucial, detailing procedures for collection, storage, and analysis. This plan should encompass protocols for information entry, validation, and quality control, which are vital for minimizing errors and ensuring accuracy.
In the face of escalating cyber threats, prioritizing information security measures is imperative. Utilizing encryption for both information storage and transmission, implementing stringent access controls, and conducting regular security audits are effective strategies to protect sensitive information. Furthermore, it is essential to train staff on protection protocols and ensure compliance with regulations such as HIPAA, fostering a culture of security within the research team.
Statistics suggest that the Medical Study Information Management Services sector in the US is expected to attain a market size of $8.9 billion by 2025, with a growth rate of 7.2% CAGR from 2019 to 2024, emphasizing the rising need for specialized information management services in medical studies. Data breaches in medical research are a growing concern, with a significant percentage of organizations reporting incidents that compromise patient confidentiality. As Lauren Houston states, "It is essential to ensure the scientific rigor of medical studies to assess information quality management processes, guarantee the correctness of results, and minimize mistakes."
By prioritizing data management and security, researchers not only enhance the credibility of their findings but also protect the rights of participants, ultimately contributing to the advancement of medical technology and patient care.
Furthermore, bioaccess provides extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. This highlights the significance of reducing risks and preventing expensive setbacks in research studies. The trend of research sponsors delegating to trusted firms with established histories further underscores the necessity for efficient information management processes.
Addressing the standardization of data management processes as a regulatory challenge is crucial for understanding the broader context of standardized medtech trial designs in research studies.
Addressing Recruitment Challenges in Clinical Research
Recruitment challenges in clinical research can profoundly impact the success of clinical studies. To effectively tackle these challenges, it is crucial to develop a targeted recruitment strategy that not only identifies but also engages potential participants. Utilizing digital platforms, social media, and community outreach initiatives can significantly raise awareness about the study and its benefits, making it easier for potential participants to connect with the research opportunity.
As bioaccess® aims to advance medical devices sooner through its expertise and customized approach, simplifying the enrollment process becomes a vital strategy to enhance participation rates. This can be accomplished by:
- Reducing the number of necessary visits
- Supplying clear and concise details about the study's purpose and procedures
- Providing incentives for participation
Moreover, working with healthcare providers and patient advocacy organizations can enhance outreach to underrepresented communities, thus increasing diversity within research studies.
The partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for research studies in Latin America, backed by Colombia's Minister of Health, illustrates the significance of strategic alliances in addressing recruitment challenges. This collaboration focuses on various types of studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
These studies are essential for reliable medical research. The requirement of well-resourced and well-organized research studies is crucial for ensuring the integrity of the scientific process and prioritizing safety.
Implementing these strategies not only enhances recruitment efforts but also ensures that studies are adequately staffed to meet their objectives. As Elisa Cascade, Chief Product Officer at Advarra, observed, "One of the greatest motivators of research interest was a patient’s lack of satisfaction with their medication, including bothersome side effects." This highlights the importance of addressing participant concerns to improve engagement.
Additionally, the recent case study on the CatBoost regressor demonstrated its effectiveness in predicting recruitment success, showcasing how data-driven approaches can enhance recruitment strategies. As the landscape of clinical research evolves, concentrating on effective participant engagement and targeted recruitment strategies will be essential for the success of clinical studies in 2025 and beyond.
Navigating Regulatory Compliance in Medtech Trials
Navigating regulatory compliance in Medtech studies demands a thorough understanding of relevant guidelines and standards. Researchers must possess expertise in the regulations set forth by authorities such as the FDA and EMA, along with Good Clinical Practice (GCP) guidelines. This foundational knowledge is vital for designing research that not only meets regulatory expectations but also facilitates the preparation of essential documentation for submissions.
Establishing a robust compliance monitoring system is crucial to ensure adherence to regulatory requirements throughout the process. Such a system should include:
- Regular audits
- Comprehensive training for staff on compliance issues
- Established communication channels for reporting any deviations from the protocol
A recent review revealed that only 9% of research, specifically 14 analyses, included compliance as a factor in their evaluation, highlighting the pressing need for increased awareness and rigorous statistical methods to adjust for compliance in device trials.
By prioritizing regulatory compliance, researchers can significantly enhance the credibility of their work and foster smoother interactions with regulatory bodies.
Moreover, case analyses, such as 'Statistical Methods for Analyzing Compliance,' have illustrated that many investigations fail to justify their choice of analysis methods concerning compliance, with only a small percentage indicating changes in significance when compliance is considered. This underscores the urgent need for more stringent statistical practices in compliance analysis. As emphasized by Professor A Davies, with support from Professors T A Prevost and A Davies, the importance of robust statistical techniques cannot be overstated in maintaining the integrity of medical studies.
In this context, bioaccess offers extensive clinical trial management services that include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
With experts like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, bioaccess is exceptionally equipped to navigate the complexities of regulatory compliance effectively in 2025 and beyond. The methodologies employed by bioaccess, such as tailored training programs and systematic audits, ensure that clients receive not only compliance but also enhanced research integrity.
Furthermore, thorough data extraction and checks for anomalies in the data are critical components in ensuring the reliability of compliance analysis. Testimonials from satisfied clients and successful case studies further illustrate bioaccess's effectiveness in managing research studies, providing compelling evidence of their capabilities.
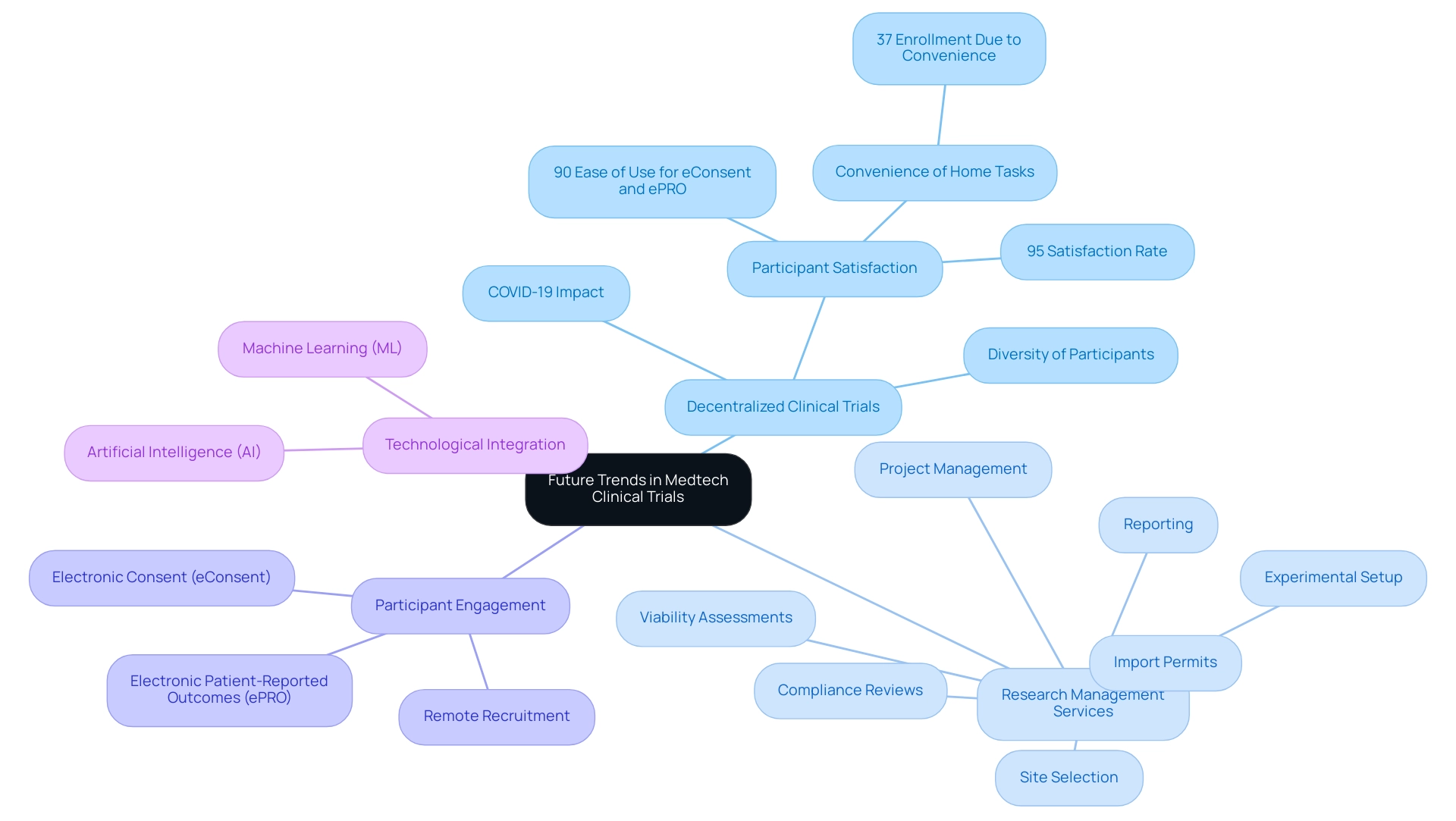
Future Trends in Medtech Clinical Trials
The Medtech research landscape is undergoing a significant transformation, propelled by technological advancements and evolving regulatory frameworks. A prominent trend is the growing acceptance of decentralized clinical trials (DCTs), which leverage digital tools to enhance remote patient observation and streamline data collection. This innovative approach not only boosts patient engagement but also broadens access to studies, particularly for individuals in underserved regions.
The surge in popularity of DCTs is closely associated with the COVID-19 pandemic, which necessitated new methodologies for conducting research while prioritizing participant safety.
In this context, comprehensive research management services, such as those offered by bioaccess, are vital. These services encompass:
- Viability assessments
- Site selection
- Compliance reviews
- Experimental setup
- Import permits
- Project management
- Reporting
Such capabilities ensure that trials are executed efficiently and in accordance with local regulations, ultimately contributing to the success of clinical research initiatives.
The STOPCoV research serves as a prime example of DCT effectiveness, designed to evaluate the long-term antibody response of preventative COVID-19 vaccines and to gather data for booster decision analysis. By incorporating remote recruitment, electronic consent (eConsent), and at-home sample collection devices, the study achieved an impressive 95% participant satisfaction rate. Furthermore, 90% of participants found eConsent and electronic patient-reported outcomes (ePROs) user-friendly, while 37% highlighted the convenience of completing tasks from home as a crucial factor in their enrollment.
This underscores the potential of DCTs to attract diverse participant groups and enhance overall efficiency.
Moreover, the integration of artificial intelligence (AI) and machine learning (ML) is revolutionizing study design and execution. These technologies refine patient recruitment strategies, simplify analysis, and facilitate more informed decision-making processes. As regulatory bodies adapt to these innovations, it is imperative for researchers to remain flexible and proactive in incorporating these methodologies into their study designs.
Leonard Sacks, Associate Director for Clinical Methodology at the FDA, emphasizes that "existing regulations need to be applied to the data that remote technology produces in the interest of patient safety, privacy, and data integrity in technology-enabled decentralized studies." By staying informed about these trends, including the recent launch of a decentralized clinical trial site network by Syneos Health® to support high-quality DCTs, researchers can adeptly navigate the evolving Medtech landscape and position themselves for success in future clinical trials.
Conclusion
The evolution of standardized trial designs is reshaping the Medtech landscape, providing a robust framework that enhances the reliability and reproducibility of clinical studies. By minimizing biases and variability, these designs streamline the path to regulatory approval while improving patient safety and data integrity across multicenter studies. The integration of digital tools and innovative methodologies paves the way for more efficient trial processes, ensuring that researchers can effectively engage patients and collect high-quality data.
Key components such as standardized protocols, effective communication, and comprehensive data management systems are crucial for the success of multicenter trials. By prioritizing clarity and adaptability in protocol design, researchers can adeptly navigate the complexities of clinical trials, ultimately leading to better outcomes and advancements in medical technology. Furthermore, addressing recruitment challenges through targeted strategies and leveraging digital platforms significantly enhances participant engagement and diversity, both of which are essential for the integrity of clinical research.
Looking ahead, the adoption of decentralized clinical trials and the integration of artificial intelligence will continue to transform the Medtech clinical trial landscape. As researchers embrace these trends and prioritize regulatory compliance, they will enhance the credibility of their studies and contribute to the advancement of innovative medical technologies. By focusing on these key areas, stakeholders can ensure that clinical trials remain effective, efficient, and aligned with the evolving needs of patients and the healthcare industry.
Frequently Asked Questions
Why are standardized medtech trial designs important in the Medtech sector?
Standardized medtech trial designs are essential because they ensure that medical evaluations produce reliable and consistent outcomes, reduce biases and variability, and are crucial for obtaining regulatory approvals.
What elements are typically included in standardized medtech trial designs?
Standardized medtech trial designs typically include predefined protocols that outline the study's objectives, methodologies, and statistical analysis plans.
What is the randomized controlled trial (RCT), and why is it significant?
The randomized controlled trial (RCT) is considered the gold standard in clinical research because it facilitates robust comparisons between treatment groups, enhancing the reliability of the study outcomes.
How do standardized medtech trial designs benefit data analysis?
They streamline data aggregation and meta-analysis, which enhances the understanding of a medical device's efficacy and safety across diverse populations.
What advancements in standardized medtech trial designs were noted in 2025?
Recent advancements have shown that standardized medtech trial designs yield more consistent outcomes and improved patient safety.
Can you provide an example of a case study related to standardized medtech trial designs?
The case study titled 'Emerging Technologies in Digital Therapeutics' explored the integration of Extended Reality (XR) and Human-computer Interaction (HCI) methods in medical research, emphasizing the need for interdisciplinary collaboration.
How do standardized medtech trial designs optimize resource allocation?
They allow research teams to leverage existing frameworks and templates, which expedites the planning and execution phases of clinical studies, conserving time and reducing costs.
What role does the International Digital Therapeutics Alliance (DTA) play in digital therapeutics?
The DTA emphasizes that digital therapeutics is health software that generates medical interventions with a significant positive therapeutic impact on patient health.
What limitations exist in current research on standardized medtech trial designs?
Limitations include reliance on ClinicalTrials.gov information, which may lead to omitted records and a lack of analysis on real-world research.
What services does bioaccess® offer in relation to clinical trials?
Bioaccess® provides tailored strategies for accelerating medical devices, including feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and reporting.

