Introduction
The safety and efficacy of medical devices hinge significantly on the integrity of their sterile packaging. As a critical barrier against contamination, sterile packaging plays an essential role throughout the medical device lifecycle, from manufacturing to patient use.
With various packaging solutions designed to withstand sterilization processes, advancements in technology have led to innovative materials and methods that enhance performance and compliance. However, navigating the complexities of regulatory standards and best practices remains a challenge for manufacturers.
This article delves into the fundamentals of sterile packaging, exploring the latest innovations, common pitfalls, and strategies for ensuring adherence to industry standards, all aimed at safeguarding patient health in an increasingly demanding healthcare environment.
Understanding Sterile Packaging: The Foundation of Medical Device Safety
Sterile packaging medical devices is a fundamental aspect of the medical equipment lifecycle, serving as a vital barrier that shields against environmental factors that could jeopardize sterility. The primary purpose of sterile packaging medical devices is to maintain the device in a contamination-free condition until it is ready for use. Different kinds of clean barriers, including pouches, trays, and blisters, serve the purpose of sterile packaging medical devices, as they are intended to withstand sterilization processes while providing sufficient protection during storage and transport.
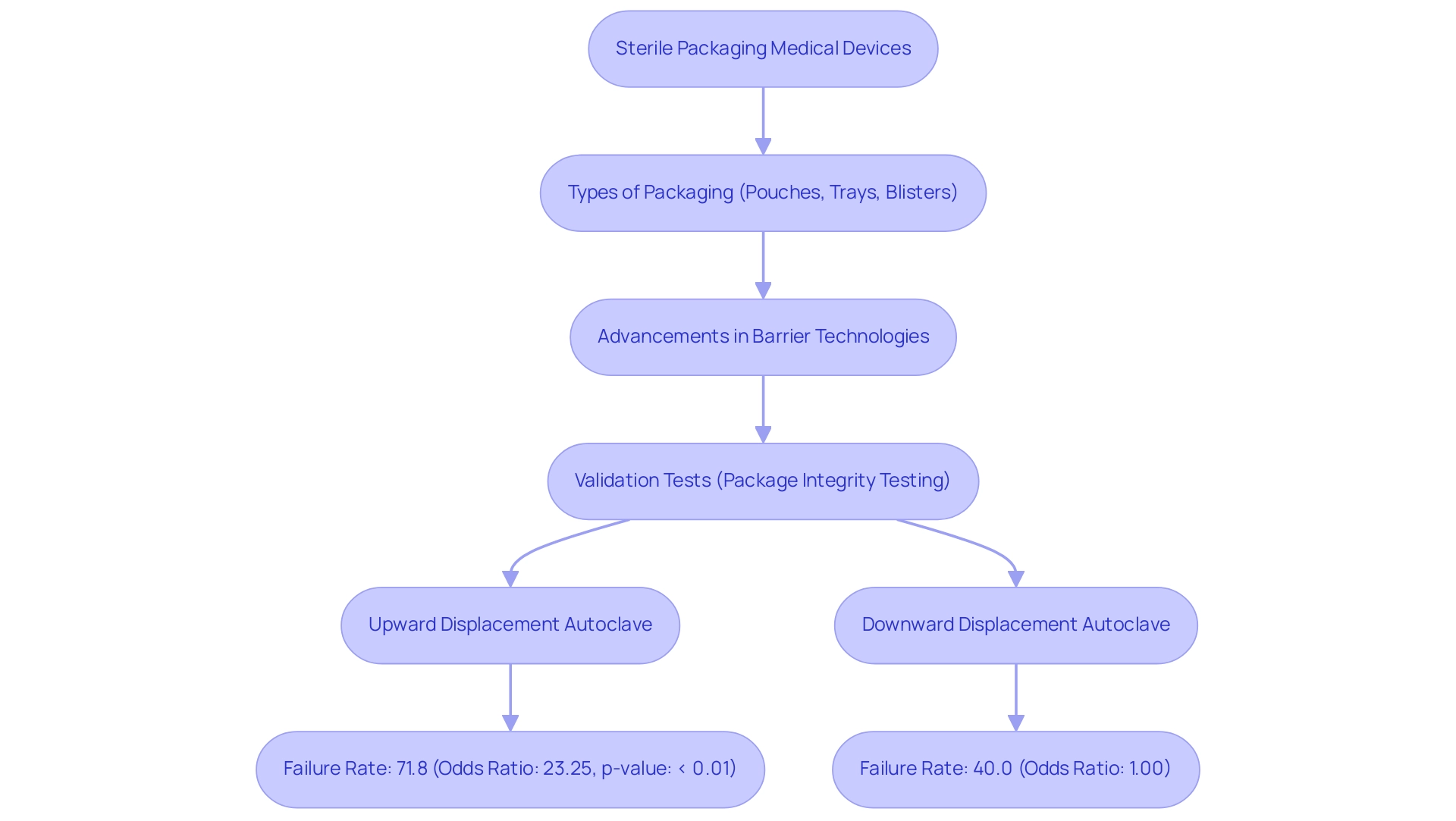
Significantly, progress in barrier technologies has resulted in the creation of innovative solutions such as Amcor's ACT2100, introduced in June 2021, which improves performance in healthcare applications by effectively addressing containment needs. Validation tests, such as package integrity testing, are essential in ensuring that the sterile packaging medical devices retain their sterility throughout their shelf life. This thorough testing is essential, particularly given that in Model 2, the upward displacement autoclave demonstrated a troubling failure rate of 71.8% with an odds ratio of 23.25 and a p-value of < 0.01, emphasizing the need for dependable contamination-free solutions.
In contrast, the downward displacement autoclave showed a 40.0% failure proportion with an odds ratio of 1.00, providing a balanced view of the effectiveness of different sterilization methods. Moreover, Riverside's recent separation from Shawpack illustrates strategic shifts in the healthcare product wrapping sector, highlighting the significance of innovation in clean wrapping. With North America expected to possess the largest market share in the sterile packaging medical devices market by 2025, the influence of clean wrapping on microbial contamination rates cannot be overstated, as it is essential to ensuring healthcare instrument safety and effectiveness.
Regulatory Compliance in Sterile Packaging: Meeting Industry Standards
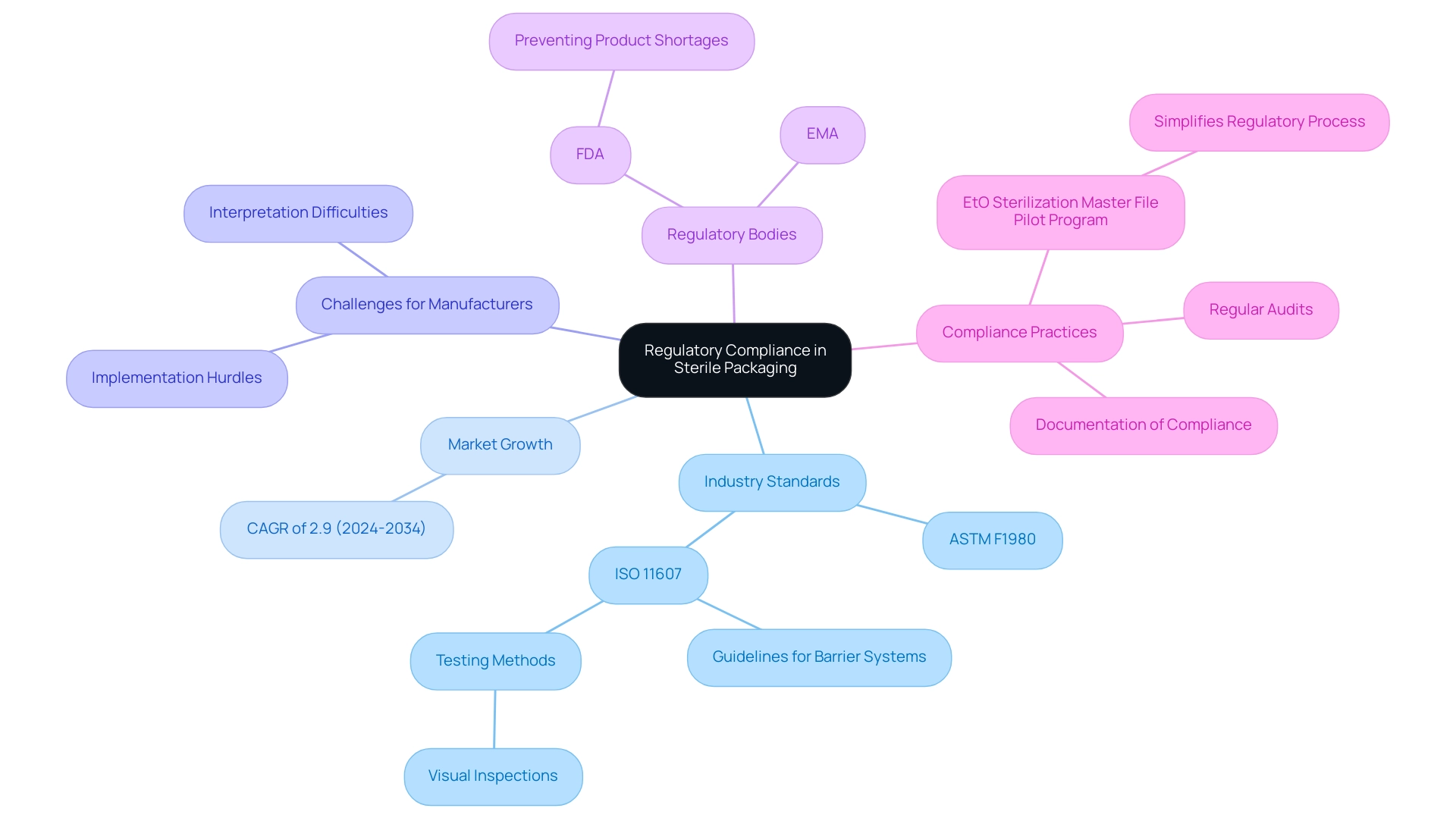
Following industry standards like ASTM F1980, which regulates the containment of terminally sterilized healthcare products, and ISO 11607, which specifies criteria for containment materials, is crucial for guaranteeing the safety and effectiveness of sterile packaging medical devices. The sterile container market in Spain is anticipated to expand at a compound annual growth rate (CAGR) of 2.9% from 2024 to 2034, reflecting an increasing demand for compliance in the industry. However, ISO 11607 presents challenges for manufacturers, including difficulties in interpretation and practical implementation hurdles that can complicate adherence to its guidelines.
Regulatory bodies like the FDA and EMA frequently update guidelines based on emerging research and technological advancements, making it imperative for organizations to stay informed about these changes. As FDA Commissioner Scott Gottlieb, M.D., mentioned in a recent statement, the agency is actively taking measures to prevent potential shortages of healthcare products while ensuring safe and effective sterilization practices, particularly considering shutdowns impacting major sterilization facilities. Regular audits and evaluations of container processes for sterile packaging medical devices are vital to maintaining compliance, thereby safeguarding patient safety and enhancing the credibility of medical device manufacturers.
Notably, ISO 11607 also provides guidelines for maintaining the integrity of barrier systems in sterile packaging medical devices through specific testing methods, such as visual inspections for defects, which are crucial for compliance practices. Moreover, thorough documentation of compliance efforts is essential for regulatory submissions and inspections, acting as a record of adherence to the latest FDA and EMA guidelines concerning clean product compliance. A practical example of regulatory simplification is illustrated by the EtO Sterilization Master File Pilot Program for PMA holders, which allows Class III medical device manufacturers to reference a Master File submitted by their sterilization provider in post-approval reports, thereby streamlining the regulatory process.
Best Practices for Designing Effective Sterile Packaging
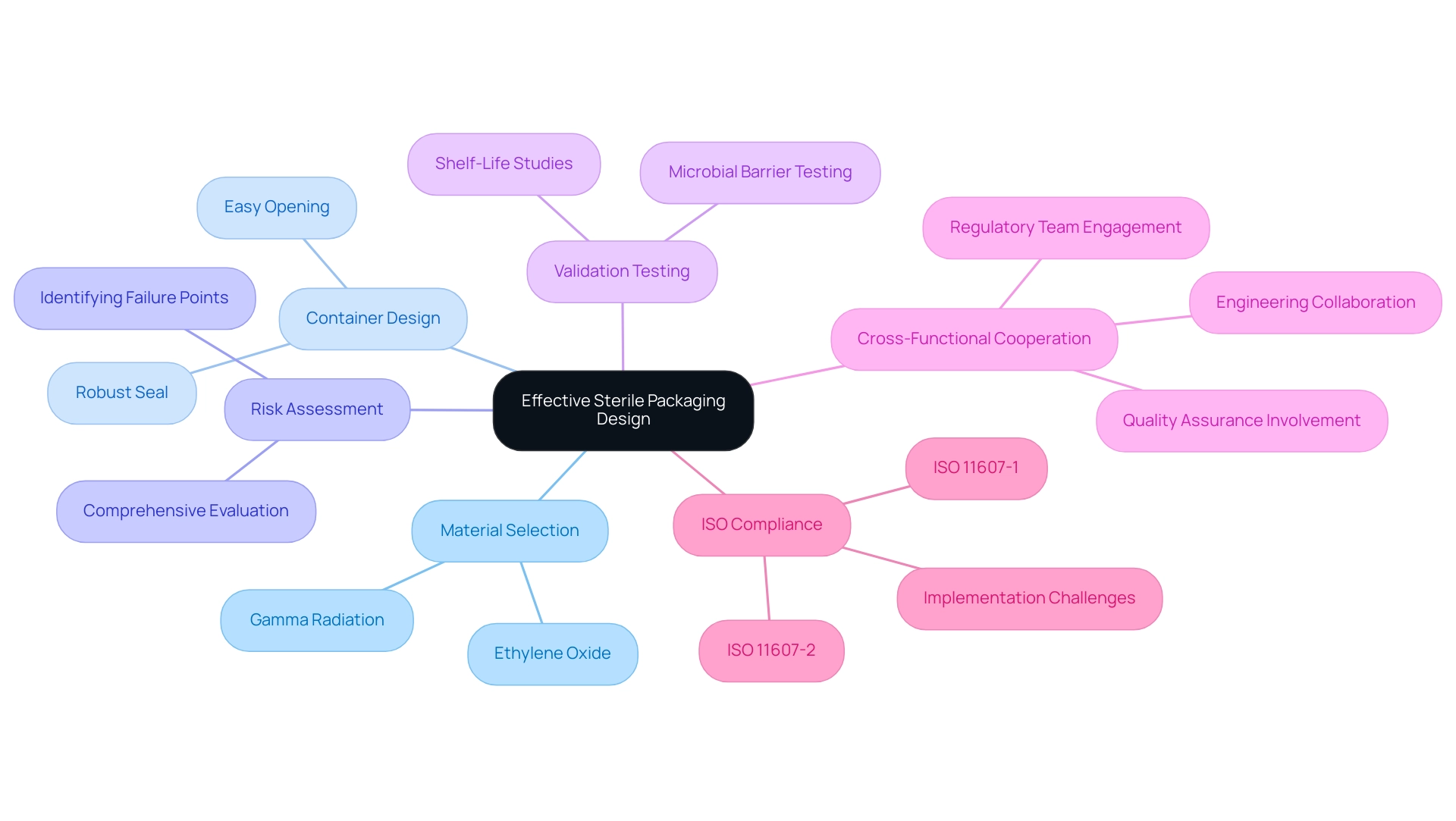
In the design of sterile packaging medical devices, it is crucial to select materials that align with the sterilization processes—such as ethylene oxide or gamma radiation—to maintain product integrity. The container design must not only facilitate easy opening but also secure a robust seal to prevent contamination. A comprehensive risk assessment during the design phase is vital as it identifies potential failure points that could compromise sterility.
Additionally, employing validation tests, including microbial barrier testing and shelf-life studies, confirms that the sterile packaging medical devices effectively protect the medical device throughout its intended lifespan. Highlighting cross-functional cooperation among engineering, quality assurance, and regulatory teams can result in innovative solutions that tackle a variety of challenges. As ISO 11607 describes, especially in its two sections (ISO 11607-1 and ISO 11607-2), keeping updated on recent advancements in materials and optimal practices for hygienic design will be crucial for improving safety and adherence.
Manufacturers must also navigate the challenges of interpreting and practically implementing these standards. For instance, the case study titled 'Design and Development Considerations in ISO 11607' illustrates how adherence to design principles and material selection criteria can optimize container configurations for sterile packaging medical devices and ensure compatibility with sterilization methods, thereby enhancing overall product safety.
Common Pitfalls in Sterile Packaging and How to Avoid Them
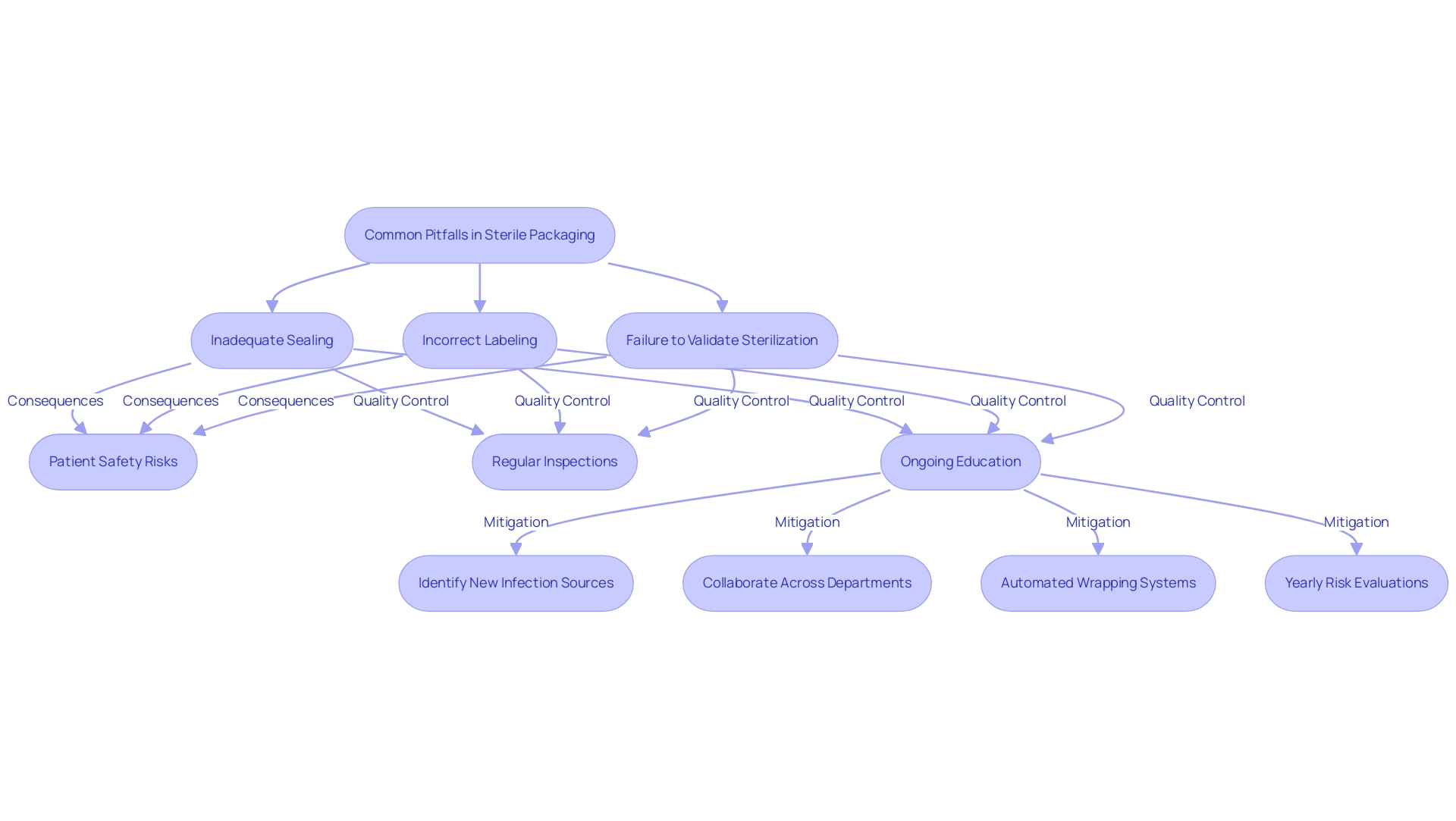
Common pitfalls in the sterile packaging of medical devices can significantly impact patient safety, with inadequate sealing, incorrect labeling, and the failure to validate sterilization processes being among the most critical issues. For instance, research indicates that instruments that are not satisfactorily cleaned pose a substantial risk, illustrated by a Waldχ value of 2.482, an odds ratio of 0.967, and a p-value of 0.010. Such findings emphasize the necessity for meticulous attention to container integrity.
As highlighted by industry expert YC,
We would like to thank the CSSD staff members who handled surgical instrument preparation during June-December 2018 in our hospital,
the dedication of staff is essential in maintaining high standards. To mitigate these risks, organizations must implement stringent quality control measures, including:
- Regular inspections and audits of the processes involved in sterile packaging medical devices.
- Ongoing education and training for all personnel involved, which are crucial in fostering an understanding of the importance of maintaining sterility.
This includes identifying new potential sources of infection and collaborating across departments to address them effectively. Embracing automated wrapping systems can further reduce human error and enhance consistency, ultimately contributing to the safety of sterile packaging medical devices and improving patient outcomes. Furthermore, as suggested in the case study 'Reducing Risks in Sterile Processing,' performing yearly risk evaluations of clean supply systems can offer valuable insights into possible weaknesses.
By addressing these common issues through a comprehensive approach, healthcare organizations can significantly enhance their clean processing environments.
Innovations in Sterile Packaging: Shaping the Future of Medical Device Safety
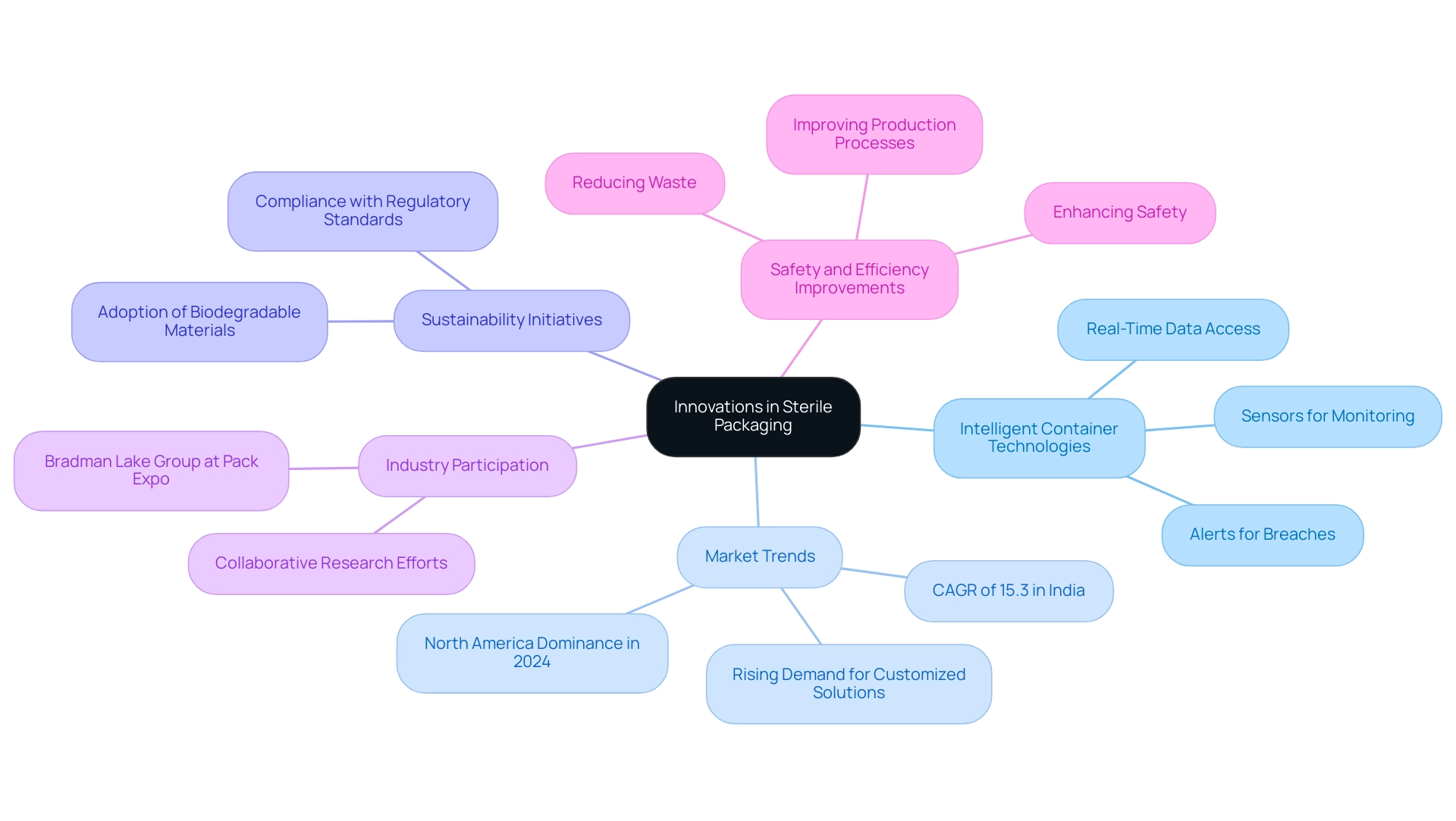
Recent advancements in hygienic wrapping have ushered in the era of intelligent container technologies, which utilize sensors to continuously monitor critical environmental conditions such as temperature and humidity. These innovations enable manufacturers to access real-time data regarding container integrity, allowing for immediate alerts to potential breaches in sterility. As the worldwide pharmaceutical sector grows, especially due to the increasing occurrence of infectious illnesses and elderly demographics, the demand for sterile packaging medical devices is rising.
Significantly, the hygienic container market in North America is anticipated to dominate in 2024, highlighting the importance of these advancements. Additionally, the sterile container market in India is expected to grow at a CAGR of 15.3%, illustrating the dynamic nature of the global market. Furthermore, the demand for customized medical containers is on the rise, with manufacturers tailoring designs to meet specific customer requirements and the unique characteristics of medical devices and pharmaceuticals.
The adoption of biodegradable and eco-friendly materials is also gaining momentum, aligning with sustainability initiatives while ensuring compliance with regulatory standards. Organizations should actively explore these advancements, as they have the potential to enhance safety, reduce waste, and improve overall efficiency in production processes. Participation in industry conferences and collaborative research efforts, such as those showcased by the Bradman Lake Group at Pack Expo International 2024, can provide valuable insights into the latest developments in sterile packaging medical devices, ensuring organizations remain at the forefront of these critical trends.
Conclusion
The significance of sterile packaging in the medical device lifecycle cannot be overstated. It serves as a vital barrier that safeguards devices from contamination, ensuring their safety and efficacy from manufacturing to patient use. With advancements in technology leading to innovative materials and methods, manufacturers must remain vigilant in adhering to regulatory standards such as ASTM F1980 and ISO 11607. These standards are essential not only for compliance but for maintaining the integrity of sterile barrier systems, which is crucial for patient safety.
Navigating the complexities of sterile packaging design requires a proactive approach, including:
- Thorough risk assessments
- Validation testing
- Cross-functional collaboration
By understanding common pitfalls—such as inadequate sealing and failure to validate sterilization processes—organizations can implement stringent quality control measures to mitigate risks. Ongoing education and the integration of smart packaging technologies further enhance safety by providing real-time monitoring of packaging conditions.
As the healthcare landscape evolves, embracing innovations in sterile packaging is essential for meeting the increasing demand for safety and efficacy in medical devices. The shift towards customized and eco-friendly packaging solutions reflects a growing commitment to sustainability and compliance. By staying informed and adaptable, manufacturers can not only safeguard patient health but also contribute to the advancement of the medical device industry in a dynamic and challenging environment.
Frequently Asked Questions
What is the primary purpose of sterile packaging for medical devices?
The primary purpose of sterile packaging for medical devices is to maintain the device in a contamination-free condition until it is ready for use.
What types of clean barriers are used for sterile packaging of medical devices?
Different kinds of clean barriers used for sterile packaging include pouches, trays, and blisters, which are designed to withstand sterilization processes while providing protection during storage and transport.
What advancements have been made in sterile packaging technologies?
Advancements include the development of innovative solutions like Amcor's ACT2100, which enhances performance in healthcare applications by effectively addressing containment needs.
Why are validation tests important for sterile packaging?
Validation tests, such as package integrity testing, are essential to ensure that sterile packaging retains sterility throughout its shelf life, which is critical for patient safety.
What were the failure rates observed in different sterilization methods?
The upward displacement autoclave demonstrated a failure rate of 71.8%, while the downward displacement autoclave showed a 40.0% failure rate, highlighting the effectiveness of different sterilization methods.
What is the significance of compliance with industry standards like ASTM F1980 and ISO 11607?
Compliance with these standards is crucial for ensuring the safety and effectiveness of sterile packaging for medical devices, as they regulate the containment of terminally sterilized healthcare products and specify criteria for containment materials.
What challenges do manufacturers face regarding ISO 11607?
Manufacturers face challenges including difficulties in interpreting the standard and practical implementation hurdles that can complicate adherence to its guidelines.
How do regulatory bodies like the FDA and EMA influence sterile packaging practices?
Regulatory bodies frequently update guidelines based on emerging research and technological advancements, making it essential for organizations to stay informed about these changes to ensure safe and effective sterilization practices.
What role does documentation play in compliance with regulatory standards?
Thorough documentation of compliance efforts is essential for regulatory submissions and inspections, serving as a record of adherence to FDA and EMA guidelines regarding clean product compliance.
What is the EtO Sterilization Master File Pilot Program?
The EtO Sterilization Master File Pilot Program allows Class III medical device manufacturers to reference a Master File submitted by their sterilization provider in post-approval reports, streamlining the regulatory process.




