Overview
The article underscores best practices for utilizing study design optimization tools in clinical research, highlighting their critical role in enhancing research efficacy and reliability. It asserts that these tools, encompassing statistical software and AI-driven platforms, effectively tackle prevalent challenges such as participant recruitment and regulatory compliance. Consequently, this leads to improved trial outcomes and heightened patient safety.
Introduction
In the realm of clinical research, the design of a study can indeed determine its success. As the landscape grows increasingly complex, researchers are increasingly turning to innovative study design optimization tools to enhance their methodologies and outcomes. By leveraging artificial intelligence for efficient patient recruitment and employing advanced analytics for real-time data monitoring, these tools are fundamentally transforming the conduct of clinical trials.
Understanding and implementing these resources enables researchers to navigate common challenges, adhere to regulatory standards, and ultimately improve the reliability and integrity of their findings. This article explores the significance of effective study design, the pivotal role of technology, and best practices that can propel clinical research forward, ensuring that new medical devices and therapies reach the market swiftly and safely.
Understanding Study Design Optimization Tools in Clinical Research
Study design optimization tools encompass a diverse array of methodologies and technologies that empower researchers to plan and execute clinical studies with greater efficacy. These tools include statistical software for sample size calculations, advanced platforms for real-time data monitoring, and analytics solutions that optimize logistics. By utilizing study design optimization tools, researchers can enhance their research designs, ensuring they are robust, compliant, and capable of delivering reliable results.
For instance, platforms like StudyOptimizer not only forecast patient enrollment with precision but also enhance the management of logistics, which are crucial for adhering to timelines and budgets. Recent advancements in study design optimization tools have introduced features that leverage artificial intelligence to predict recruitment challenges and enhance site selection, significantly improving enrollment efficiency. As AI plays an increasing role in healthcare, it is essential to ensure that its implementation upholds the fundamental principles of medicine, including beneficence, non-maleficence, autonomy, and justice.
A relevant case analysis titled 'Recruitment and Enrollment Optimization' highlights how data can be leveraged to optimize recruitment strategies and overcome challenges. Sophisticated analytics allow project managers to pinpoint optimal locations and tackle possible obstacles, ultimately enhancing enrollment effectiveness.
Comprehending the particular features of study design optimization tools allows researchers to select the most appropriate choices for their distinct research needs. This tailored approach not only enhances trial outcomes but also prioritizes patient safety, ultimately contributing to the successful advancement of medical devices in the clinical landscape. As noted by Maureen Josephson, 'Bias has been defined as any systematic error in the design, conduct or analysis of an investigation that results in a mistaken estimate of an exposure's effect on the risk of disease.'
This highlights the significance of thorough research design and the possible pitfalls that study design optimization tools can help alleviate. By aligning these tools with bioaccess®'s mission to advance medical devices sooner through expertise and a tailored approach, including thorough research management services such as feasibility assessments, site selection, compliance reviews, and project oversight, researchers can significantly enhance the effectiveness of their evaluations.
The Importance of Effective Study Design in Clinical Research
Effective study design optimization tools are essential in medical investigations, serving as the foundation for acquiring valid and dependable results. A meticulously crafted design that utilizes these tools encompasses critical elements such as participant selection, randomization, and control measures, all vital for minimizing bias and safeguarding the integrity of collected data. Significantly, study design optimization tools, such as adaptive experimental designs, exemplify this principle by permitting modifications based on interim results, which can improve resource efficiency and accelerate decision-making processes.
In 2025, the importance of effective research design is underscored by the need for robust statistical methodologies. For instance, an examination of British orthopedic journals revealed that only 3 out of 49 papers (6.1%) reported conducting a statistical power analysis, highlighting a gap that can compromise the validity of clinical trials. Adequately powered research is essential for producing generalizable results, necessitating careful consideration of effect sizes and variability.
Moreover, integrating patient-centric approaches into research design can significantly enhance recruitment and retention rates, ultimately leading to richer and more comprehensive data. Researchers must prioritize the development of studies that not only fulfill regulatory requirements but also resonate with the needs and preferences of participants. This alignment improves participant involvement and raises the overall standard of research, ensuring that findings are both relevant and influential.
The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for medical studies in Latin America, supported by Colombia's Minister of Health, exemplifies the significance of strategic partnerships in enhancing research capabilities. Furthermore, GlobalCare Clinical Studies' collaboration with bioaccess™ has accomplished over a 50% decrease in recruitment duration and 95% retention rates, demonstrating the efficiency of thorough study management services.
As Lindus Health mentions, 'Ready to enhance your research process with extensive statistical analysis and expert guidance?' This emphasizes the significance of comprehensive statistical examination in medical studies. The importance of effective research design is further illustrated through case studies that utilize study design optimization tools, such as the 'Conclusion on Clinical Trial Statistics,' which demonstrate how thoughtful planning can lead to successful outcomes.
By utilizing study design optimization tools, investigators have reported enhanced findings in medical investigations, highlighting the potential for innovation in methodologies. As the landscape of clinical studies evolves, embracing these best practices will be essential for advancing medical devices and ensuring that clinical trials yield meaningful insights. bioaccess®'s expertise and customized approach aim to help advance medical devices sooner, providing valuable solutions for companies in the medtech industry.
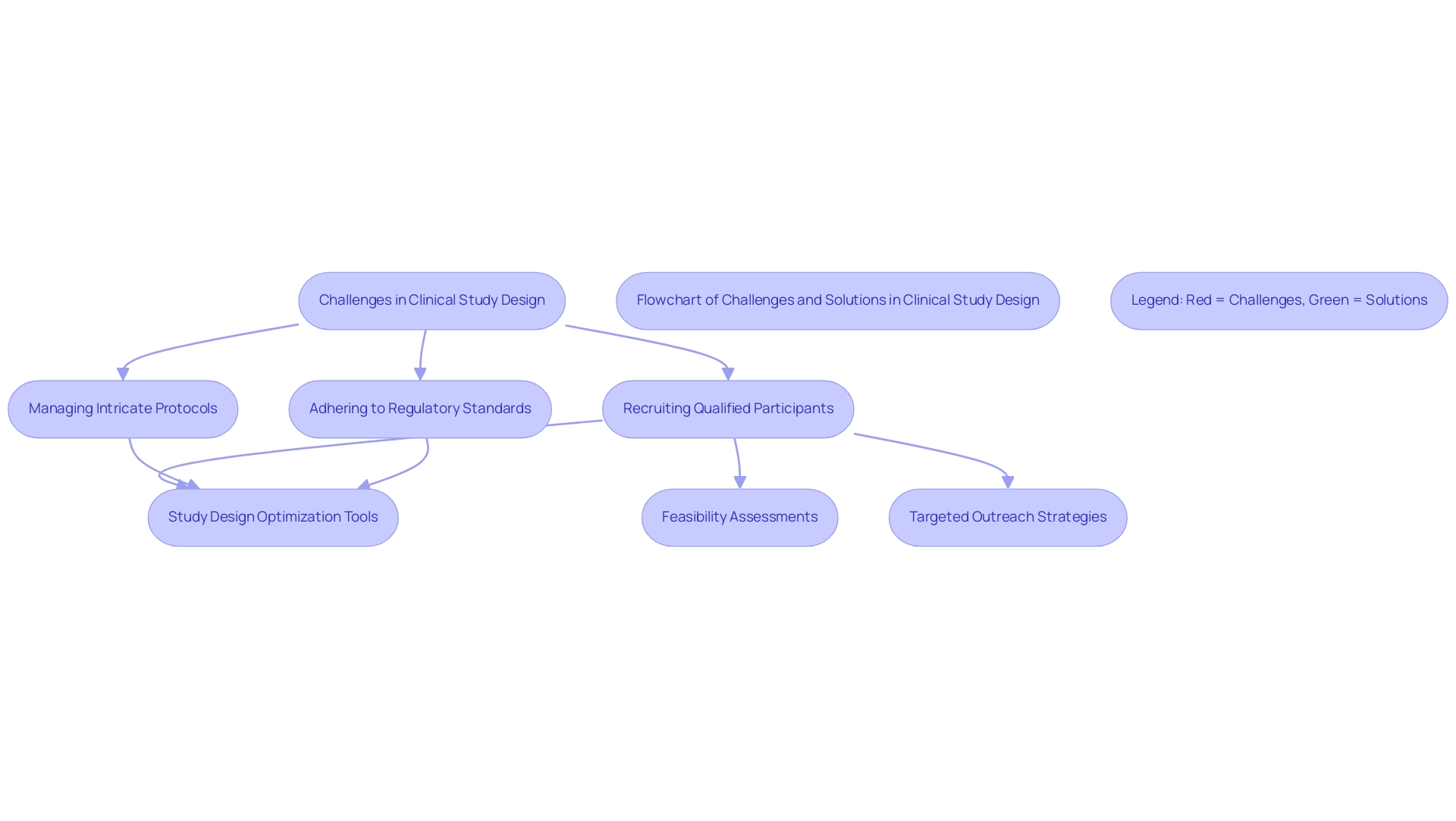
Common Challenges in Clinical Research Study Design
Clinical study design frequently encounters a myriad of challenges that can impede progress and compromise outcomes, emphasizing the necessity for study design optimization tools. Among the most pressing issues are:
- Difficulties in recruiting qualified participants
- Managing intricate protocols
- Adhering to regulatory standards
Notably, less than 10% of health funding is allocated to address issues impacting 90% of the global population, underscoring the urgent need for focused efforts in participant recruitment. This statistic highlights the critical gap in funding that can hinder progress in medical research, particularly for underrepresented populations.
The growing intricacy of medical studies, driven by technological advancements and regulatory modifications, often results in prolonged schedules and increased expenses. This situation necessitates the adoption of study design optimization tools as part of efficient project management strategies. Bioaccess provides extensive management services for research projects, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting
These services are designed to simplify the research process and enhance the likelihood of favorable results.
For instance, a case study titled "Impact of Knowledge on Clinical Study Participation" revealed that both patients and researchers frequently lack adequate understanding of clinical study processes. This knowledge gap significantly affects enrollment rates and the integrity of trial results, underscoring the importance of enhancing awareness among all stakeholders. Effective communication among stakeholders—including sponsors, regulatory bodies, and study teams—is crucial for navigating these challenges.
Implementing robust project management tools and fostering a collaborative environment can greatly improve the capacity to address recruitment and protocol management issues by utilizing study design optimization tools.
As highlighted by Sara Fernandes-Taylor, "Statisticians and methodological experts should be consulted during the study design, analysis, and manuscript writing phases to enhance the quality of the study and to ensure the clear and appropriate application of quantitative methods." This statement emphasizes the significance of specialist participation in overcoming the challenges of medical studies.
Moreover, non-adherence to best practices in trials can lead to biased outcomes, compromising the validity and integrity of the study. To address recruitment challenges, strategies such as targeted outreach, educational initiatives, and leveraging technology for participant engagement can be instrumental. Furthermore, bioaccess underscores the importance of comprehensive reporting, which includes research status, inventory management, and monitoring serious and non-serious adverse events.
By tackling these typical challenges in trial study design with study design optimization tools, organizations can enhance their study planning processes, ultimately resulting in more successful outcomes. Additionally, understanding the role of INVIMA in regulatory adherence is crucial for conducting research studies in Colombia, ensuring that all investigational devices meet the required standards.
Navigating Regulatory Requirements in Clinical Research
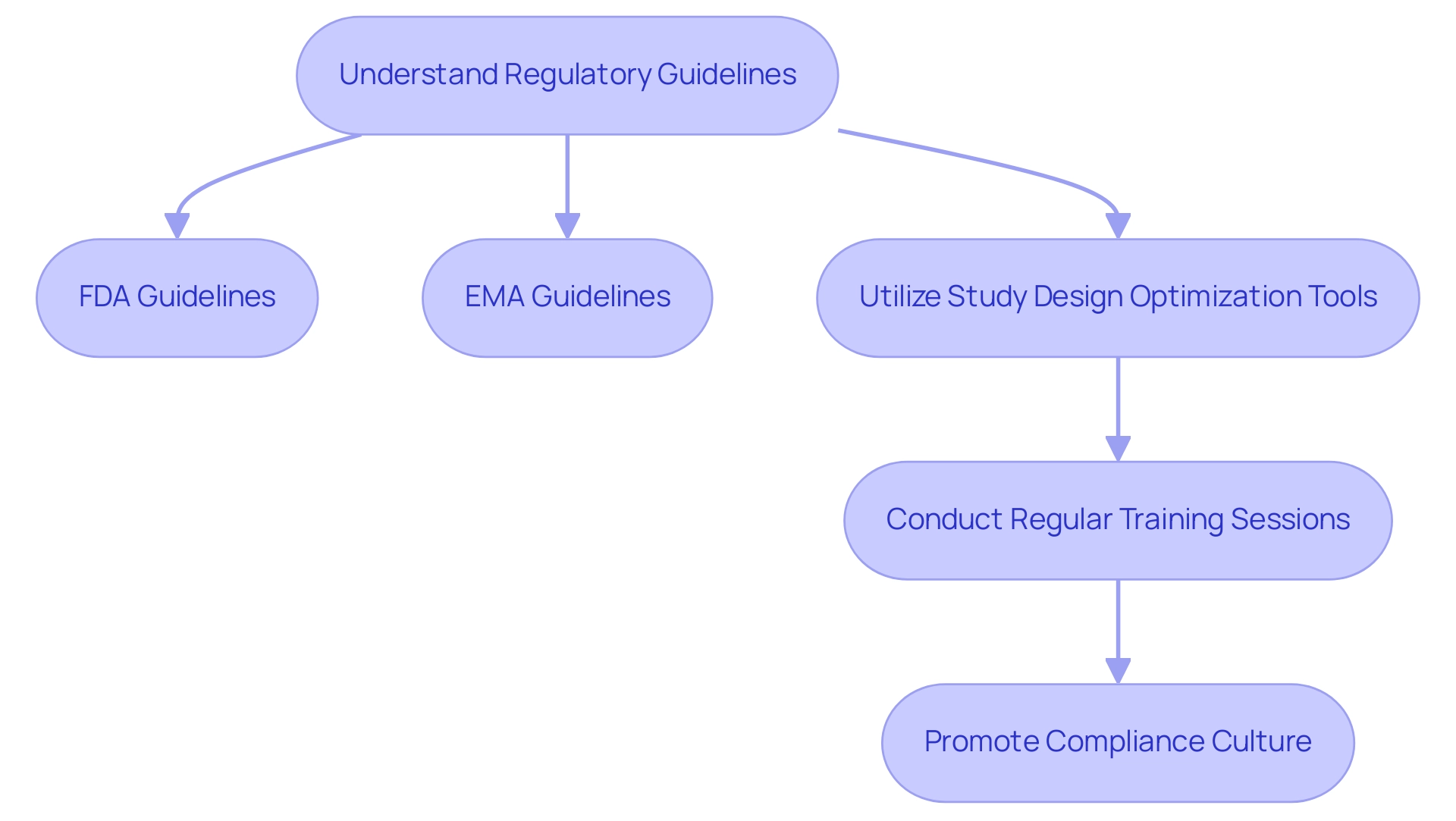
Navigating the regulatory landscape is a crucial element of medical investigation, demanding meticulous attention to detail and a thorough understanding of the guidelines established by regulatory bodies such as the FDA and EMA. These guidelines dictate essential aspects of trials, including protocols, informed consent processes, and data management practices.
To ensure compliance, researchers should adopt a systematic approach to regulatory submissions. This includes utilizing study design optimization tools, such as checklists and templates, specifically designed to align with regulatory expectations. Regular training sessions and updates on evolving regulatory requirements are vital for study teams, enabling them to remain informed and agile in response to changes.
By promoting a culture of adherence, organizations can significantly reduce risks related to regulatory non-compliance, thus improving the credibility and integrity of their investigative efforts.
Statistics reveal that as of recent reports, the number of registered trials in non-U.S. regions has reached 172,209, accounting for 50% of all projects globally. This underscores the growing importance of understanding and adhering to diverse regulatory frameworks across different jurisdictions. Furthermore, maintaining electronic documents and data archives for a minimum of 25 years is essential as global trials continue to evolve, ensuring that all data remains accessible and verifiable.
Incorporating blockchain technology can further enhance data integrity and traceability in trials by creating tamper-proof records of data. This technological progress aids in adherence and enhances the overall dependability of health-related outcomes.
Case examples, such as the partnership between bioaccess® and Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia, emphasize the importance of openness and ethical standards in preserving scientific integrity. Effective management of regulatory compliance strategies is vital for ensuring credible results, reinforcing the need for study design optimization tools in navigating the complexities of the Latin American Medtech landscape.
bioaccess® plays a crucial role in bridging the gap between innovative Medtech companies and the potential for conducting studies in Latin America. By providing customized solutions and knowledge, including viability and choice of study location and lead investigator, bioaccess® assists Medtech startups in navigating the regulatory environment efficiently with study design optimization tools, ensuring that their assessments are not only compliant but also set for success. This encompasses thorough procedures for enhancing medical device evaluations, such as regulatory adherence, project oversight, and reporting, which are vital for utilizing study design optimization tools to achieve successful results.
Leveraging Technology for Study Design Optimization
Technology is revolutionizing the enhancement of educational frameworks in healthcare investigations, with artificial intelligence (AI) and machine learning (ML) leading the charge. These advanced study design optimization tools have the capacity to process extensive datasets, uncovering patterns and predicting outcomes, which significantly enhances design decisions. For instance, AI-driven platforms have proven their efficacy in patient recruitment by efficiently analyzing electronic health records to identify eligible participants, thereby streamlining the recruitment process.
A recent case analysis titled 'Virtual Messaging for Recruitment in Research' demonstrated that optimized email campaigns substantially increased enrollment rates compared to traditional methods, underscoring the potential of technology in enhancing recruitment efforts.
In collaboration with GlobalCare Clinical Research, bioaccess™ has expanded its ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and a retention rate exceeding 95%. This partnership illustrates how leveraging local knowledge can enhance patient involvement and simplify trial procedures.
However, despite these advancements, challenges remain in the study environment. Ensuring data quality, maintaining participant engagement, and addressing the digital divide are critical issues that researchers must navigate. As Bloss emphasized, there is an urgent need to revamp the existing human protection system to better accommodate these advancing technologies.
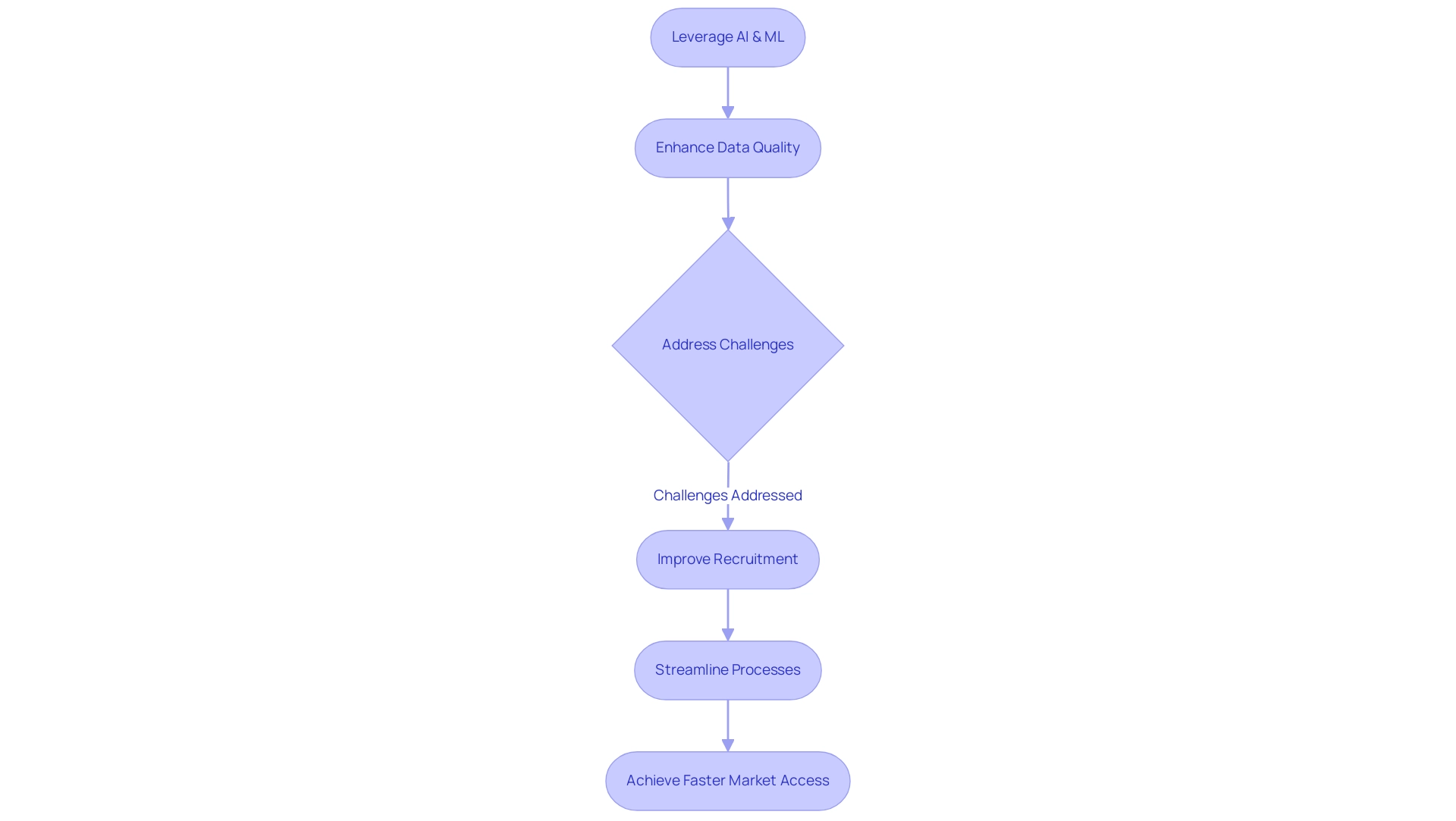
The incorporation of study design optimization tools further enables investigators to dynamically manage project progress, allowing for prompt modifications that improve efficiency. This capability not only enhances the quality of research designs but also aids in cost reduction and shortened timelines associated with medical investigations. Recent research indicates that the use of AI and ML in medical evaluations can lead to a 30% increase in enrollment rates compared to conventional methods, highlighting the transformative potential of these technologies in medical inquiry.
Moreover, the application of AI in research experiments can leverage study design optimization tools to enhance patient recruitment, improve research design, boost data quality, and enable continuous monitoring, facilitating faster market access for new therapies.
As we approach 2025, the role of AI and ML in research design is expected to expand, with ongoing advancements promising even greater efficiencies. By utilizing study design optimization tools, researchers can refine their research designs, ensuring that innovative medical devices and therapies reach the market more swiftly and effectively. Additionally, bioaccess®'s expertise in managing various types of research, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Trials, along with their capabilities in trial setup, compliance reviews, and reporting, positions them as a key player in driving global health improvement through international collaboration and innovation in Medtech.
Furthermore, the collaboration with IDx Technologies strengthens bioaccess's role in AI-driven disease detection, particularly in ophthalmology, further reinforcing their commitment to advancing clinical studies.
Best Practices for Implementing Study Design Optimization Tools
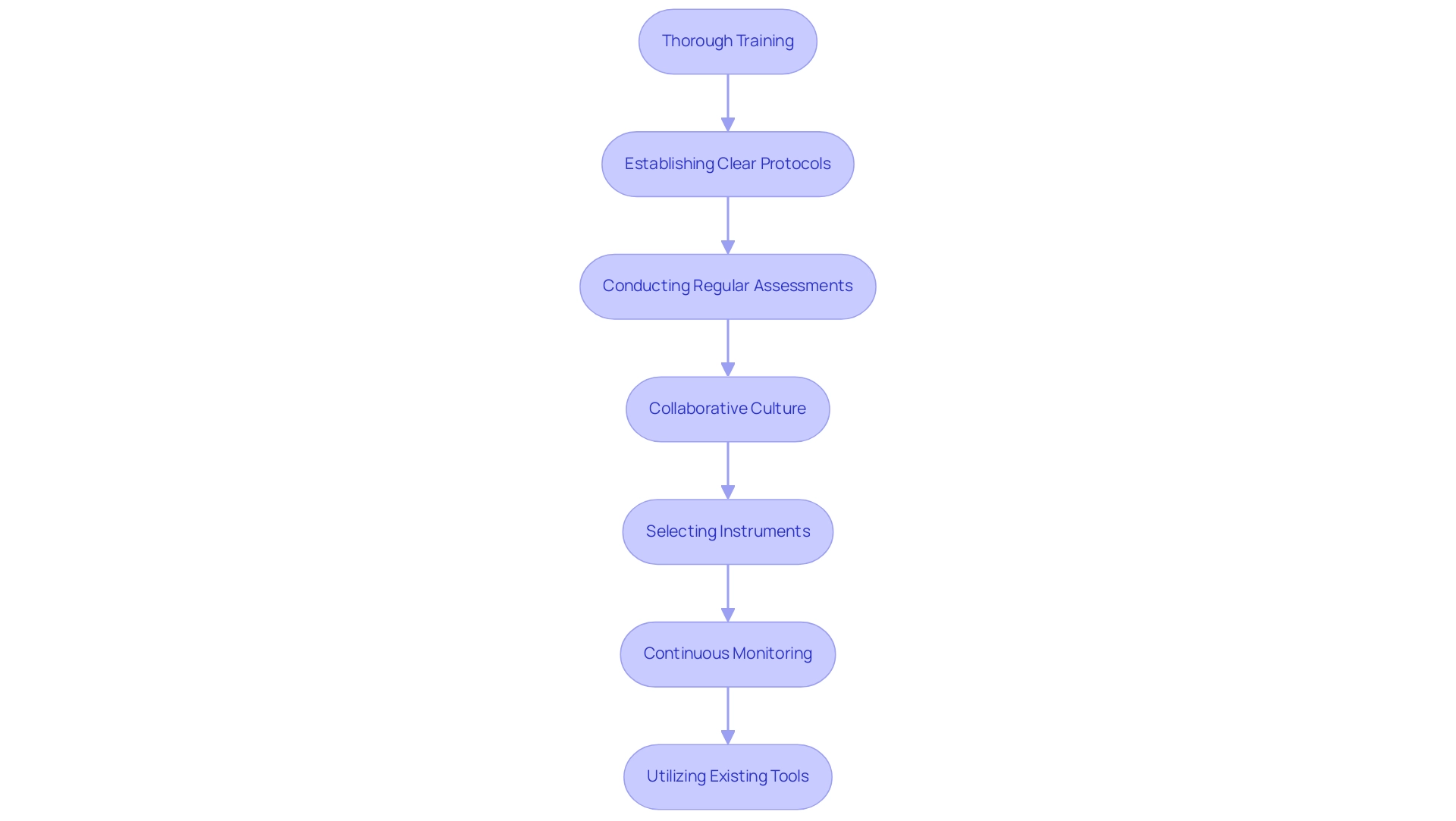
Implementing study design optimization tools requires a strategic framework to ensure their effectiveness. Essential best practices include:
- Thorough training for teams on the functionalities of these tools
- Establishing clear protocols for their application
- Conducting regular assessments of their impact on results
A collaborative culture among team members is crucial, as it encourages the sharing of insights and experiences related to tool usage, ultimately enhancing the overall process.
This collaborative approach is further bolstered by the benefits of cross-fertilizing expertise and shared infrastructures, significantly advancing healthcare. Selecting instruments tailored to the specific requirements of the project and the team's capabilities is vital. For instance, hybrid designs in execution analysis demonstrate how combining experimental and observational elements can simultaneously evaluate both effectiveness in practice and implementation strategies. This method not only facilitates the rapid translation of evidence into practice but also addresses effectiveness and implementation within a single investigation.
As bioaccess®, a leading contract service organization with over 20 years of experience in the Medtech field, points out, their insights into these strategies are invaluable for optimizing study designs, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
Moreover, continuous monitoring of the performance of study design optimization tools, along with necessary adjustments, enables researchers to maximize their benefits. As Sid Kumar noted, "Building an in-house experimentation platform is costly and time-consuming. Learn why leading companies now choose to buy instead—saving years and scaling faster."
This perspective highlights the advantages of leveraging existing tools rather than developing new ones from scratch. Additionally, utilizing study design optimization tools, such as freely available software, for designing and implementing dose-optimization trials can significantly enhance trial design. By incorporating these strategies, researchers can improve the design and execution of trials, ultimately advancing the healthcare domain and contributing to global health enhancement through international collaboration and innovation in Medtech.
For those interested in managing cookie preferences, you can change your cookie settings at any time by clicking the cookie consent banner. This allows you to revisit and modify your preferences or withdraw consent immediately. Different browsers have various methods for blocking and deleting cookies.
For detailed instructions, please refer to the following links:
- Chrome: https://support.google.com/accounts/answer/32050
- Safari: https://support.apple.com/en-in/guide/safari/sfri11471/mac
- Firefox: https://support.mozilla.org/en-US/kb/clear-cookies-and-site-data-firefox?redirectslug=delete-cookies-remove-info-websites-stored&redirectlocale=en-US
- Internet Explorer: https://support.microsoft.com/en-us/topic/how-to-delete-cookie-files-in-internet-explorer-bca9446f-d873-78de-77ba-d42645fa52fc
If you are using any other web browser, please visit your browser’s official support documents.
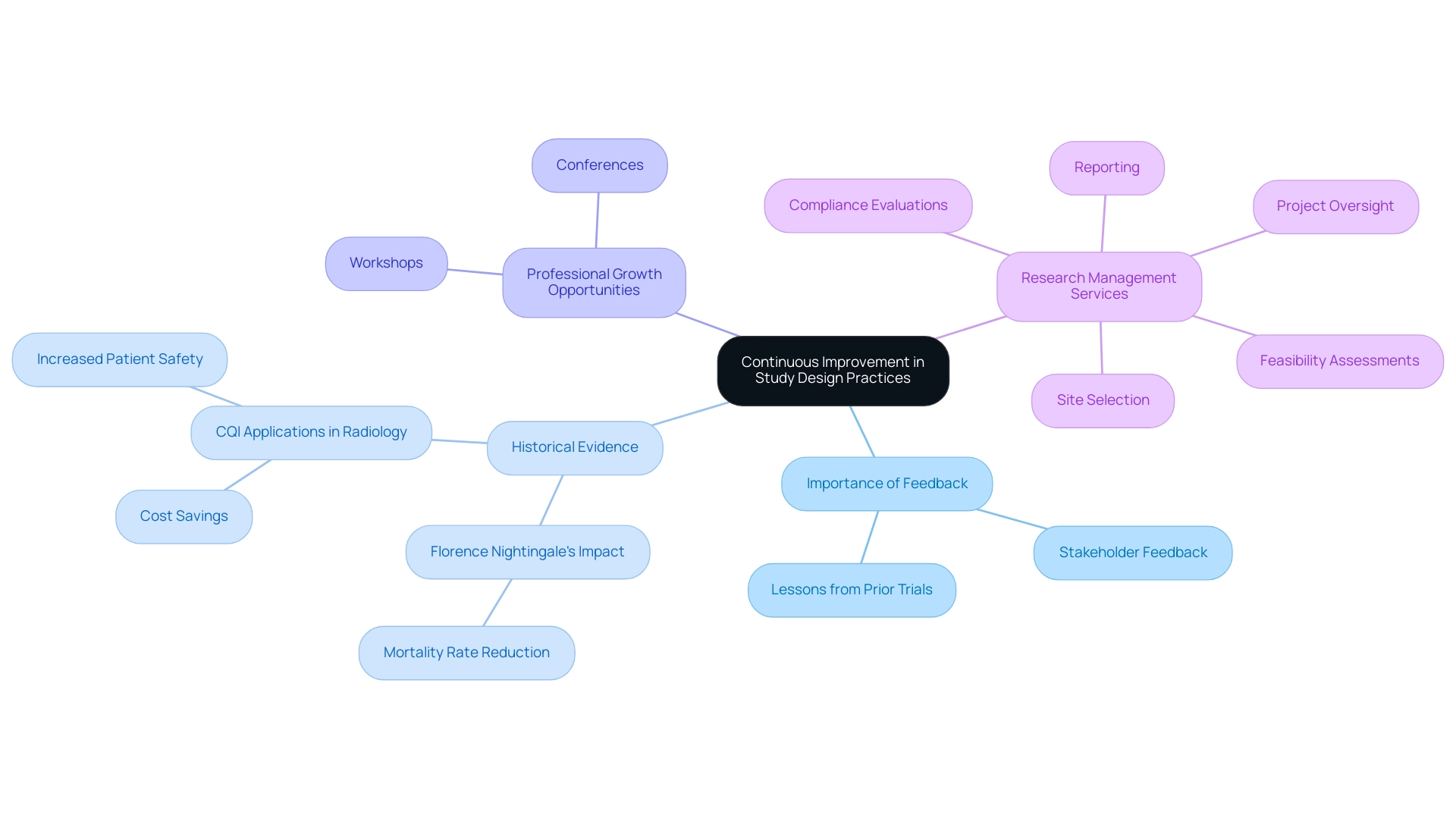
Continuous Improvement in Study Design Practices
Continuous enhancement in research design practices is essential for navigating the dynamic landscape of clinical investigation, particularly through the use of study design optimization tools. Researchers must routinely evaluate and refine their research designs by actively incorporating feedback from stakeholders and lessons learned from prior trials, employing these optimization tools. This iterative approach not only identifies areas for enhancement but also facilitates the adoption of tools that can significantly improve outcomes.
For instance, a systematic review methodology that involved searches of 11 electronic databases examined 23 pieces of research on Continuous Quality Improvement (CQI) applications in radiology. All studies reported improvements in various operational metrics, including cost savings and increased patient safety. Such evidence highlights the positive impact of continuous improvement initiatives in medical research.
As Florence Nightingale famously stated, "Within six months of Nightingale’s team of nurses implementing and documenting various quality improvements, the mortality rate dropped from 42.7% to 2.2%." This historical example demonstrates the profound effects that quality enhancements can have on outcomes in medical environments.
Furthermore, participating in professional growth opportunities—such as workshops and conferences—provides researchers with insights into the latest trends and best practices regarding study design optimization tools. At bioaccess, we ensure that medical studies are conducted with high safety and efficiency standards while maintaining data integrity. Our extensive research management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
All customized to address the specific requirements of each project.
We utilize methodologies such as agile project management and risk-based monitoring to enhance our service delivery.
As the field evolves, fostering a mindset of continuous improvement enables researchers to leverage study design optimization tools to ensure that their study designs remain effective, efficient, and responsive to the ever-changing needs of the healthcare landscape. By prioritizing iterative processes and ongoing education, researchers can enhance the quality and impact of their clinical trials, leveraging study design optimization tools to position bioaccess as a leader in Medtech clinical research in Latin America, with a focus on innovation and regulatory excellence.
Conclusion
In the rapidly evolving landscape of clinical research, the significance of effective study design is paramount. This article has elucidated how study design optimization tools—such as advanced analytics and artificial intelligence—can markedly enhance the planning and execution of clinical trials. By leveraging these innovative resources, researchers can significantly improve patient recruitment, streamline trial logistics, and ensure adherence to regulatory standards, ultimately yielding more reliable and impactful findings.
Furthermore, the discussion has highlighted the critical necessity for adaptive trial designs and robust statistical methodologies to minimize bias and protect the integrity of data. By integrating patient-centric approaches and fostering strategic partnerships, researchers can bolster participant engagement and retention, which are essential for achieving successful outcomes. Moreover, addressing prevalent challenges such as recruitment difficulties and regulatory complexities through comprehensive project management and effective communication can further optimize study designs.
As technology continues to advance, the integration of real-time data monitoring and AI-driven solutions will assume an increasingly pivotal role in clinical research. By embracing these best practices and persistently refining study designs, researchers can ensure that innovative medical devices and therapies reach the market more swiftly and safely. The commitment to continuous improvement and adaptation in study design practices will ultimately propel the future of clinical research, enhancing the quality of insights gained and advancing global health initiatives.
Frequently Asked Questions
What are study design optimization tools?
Study design optimization tools are methodologies and technologies that help researchers plan and execute clinical studies more effectively. They include statistical software for sample size calculations, real-time data monitoring platforms, and analytics solutions for optimizing logistics.
How do study design optimization tools enhance research designs?
These tools enhance research designs by ensuring they are robust, compliant, and capable of delivering reliable results. They help in forecasting patient enrollment, managing logistics, and addressing recruitment challenges.
What role does artificial intelligence play in study design optimization?
Recent advancements have introduced AI features that predict recruitment challenges and improve site selection, significantly enhancing enrollment efficiency in clinical studies.
Can you provide an example of how data can optimize recruitment strategies?
The case analysis titled 'Recruitment and Enrollment Optimization' demonstrates how data analytics can be used to identify optimal locations and tackle obstacles, ultimately enhancing enrollment effectiveness.
Why is understanding the features of study design optimization tools important for researchers?
Comprehending the specific features of these tools allows researchers to select the most suitable options for their unique research needs, leading to improved trial outcomes and prioritizing patient safety.
What critical elements are included in a well-crafted study design?
A well-crafted study design includes participant selection, randomization, and control measures, all of which are vital for minimizing bias and maintaining the integrity of collected data.
What is the significance of statistical power analysis in research design?
Statistical power analysis is essential for producing generalizable results. A lack of power analysis can compromise the validity of clinical trials, as evidenced by a study showing only 6.1% of orthopedic papers reported conducting one.
How can patient-centric approaches affect research design?
Integrating patient-centric approaches can significantly enhance recruitment and retention rates, leading to richer and more comprehensive data, while ensuring studies resonate with participants' needs and preferences.
What are some successful collaborations in enhancing research capabilities?
Collaborations like that between bioaccess™ and Caribbean Health Group, and GlobalCare Clinical Studies with bioaccess™, have demonstrated significant improvements in recruitment duration and retention rates through effective study management services.
What is the overall impact of utilizing study design optimization tools in clinical studies?
Utilizing these tools can enhance findings in medical investigations, promote innovation in methodologies, and ensure that clinical trials yield meaningful insights, ultimately advancing medical devices in the clinical landscape.

