Introduction
In the complex landscape of clinical research, biostatistics emerges as an indispensable discipline that underpins the validity and reliability of study outcomes. As researchers strive to design robust trials and analyze intricate datasets, the application of biostatistical principles becomes crucial in ensuring that findings are not only scientifically sound but also relevant to a diverse population.
This article delves into the multifaceted role of biostatistics in clinical research, exploring:
- Best practices for implementation
- The significance of sample size determination
- The importance of data integrity
Furthermore, it highlights the ethical considerations that must guide statistical practices, ensuring that the rights and welfare of participants are upheld throughout the research process. By understanding and applying biostatistical methods effectively, researchers can enhance the credibility of their studies, ultimately leading to improved healthcare outcomes and advancements in medical knowledge.
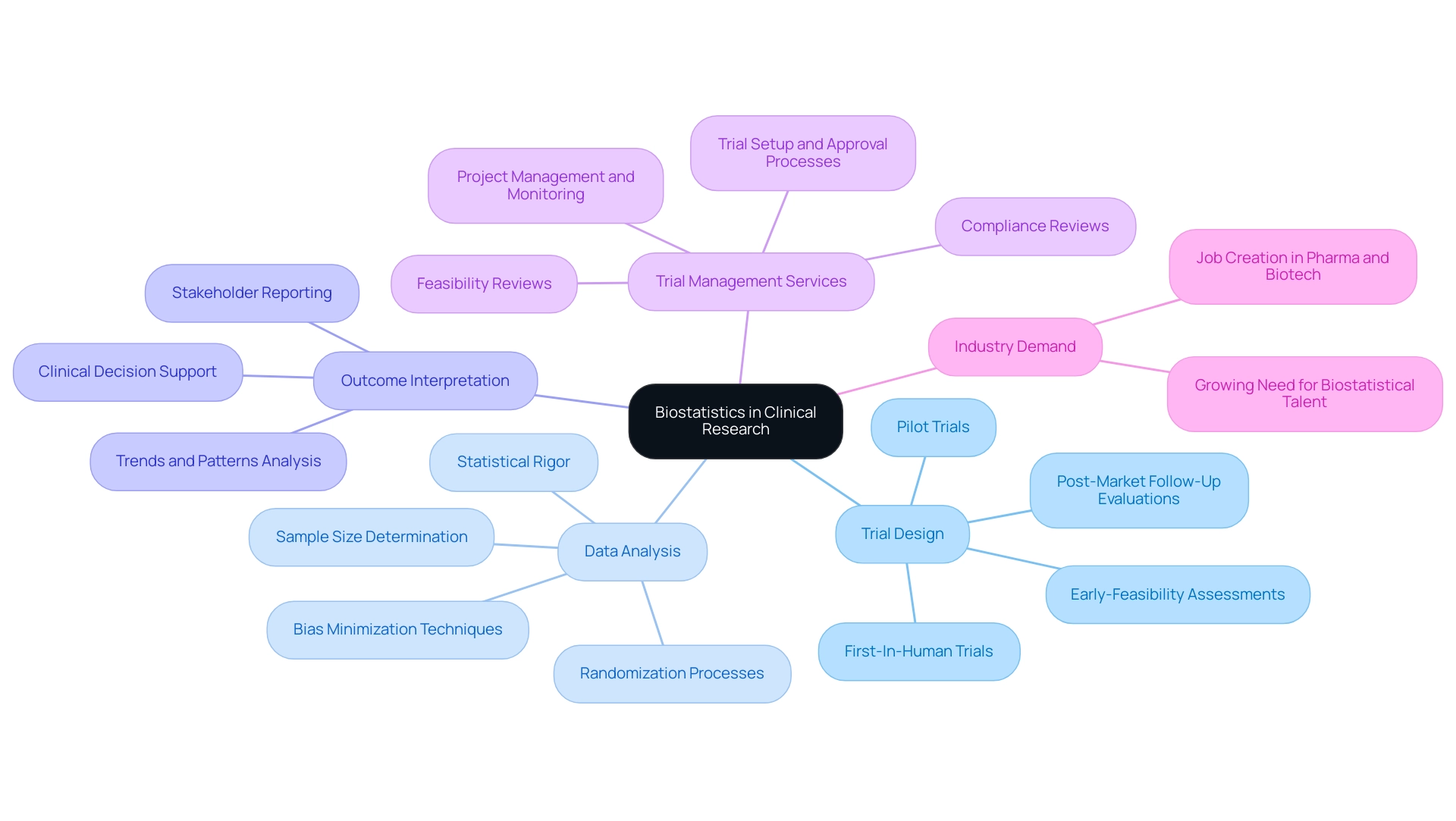
The Essential Role of Biostatistics in Clinical Research
Clinical biostatistics services serve as the foundation of medical investigations, playing a crucial role in design, data analysis, and the interpretation of outcomes. Its methodologies ensure that trials are not only scientifically valid but also yield findings that are reliable and applicable to diverse populations. In the realm of expedited medical device trials, especially in Latin America, the proficiency of companies like bioaccess® in performing:
- Early-Feasibility Assessments
- First-In-Human Trials
- Pilot Trials
- Post-Market Follow-Up Evaluations
illustrates the significance of thorough trial management services.
Our service capabilities encompass:
- Feasibility and selection of research locations and principal investigators
- Compliance reviews of trial documents
- Trial setup and approval processes involving ethics committees and health ministries
- Import permits
- Thorough project management and monitoring
As Dr. Arzu Onar-Thomas from the St. Jude Department of Biostatistics notes, 'Sometimes, a trial design is primarily guided by the limitations in sample size and may be less optimal statistically. In those cases, we prioritize the chance of appropriately answering the key trial questions with the understanding that we may be less confident in the rest of the results.'
This highlights the critical balance between statistical rigor and practical feasibility in the clinical biostatistics services provided for clinical trial design. Moreover, determining the optimal sample size is crucial, as it balances cost with statistical power, significantly impacting the integrity of the research. Biostatistics assists in randomization processes and the selection of suitable collection techniques, which are crucial for minimizing biases.
The role of biostatisticians in providing clinical biostatistics services is vital for interpreting complex data sets, as they uncover trends and patterns that inform medical decisions and treatment protocols, enhancing the reliability and impact of medical studies. Additionally, the demand for biostatistical talent continues to grow in the industry, prompting companies to place qualified candidates in pharma, biotech, and CROs. As the field evolves, the integration of advanced biostatistical methods further enhances the robustness and reliability of trials, contributing to job creation, economic growth, and healthcare improvement within local economies.
Best Practices for Implementing Biostatistical Methods in Research
Implementing biostatistical methods effectively in clinical research requires adherence to several best practices, particularly within the framework of clinical biostatistics services and comprehensive clinical trial management. Here are essential considerations:
- Engage a Biostatistician Early: Involving a biostatistician from the inception of the research design is essential. Their expertise assists in formulating hypotheses, selecting appropriate research designs, and accurately determining sample sizes necessary for achieving statistical power. As a biostatistician once remarked, "Involving biostatisticians early in research design is essential to the success of clinical trials."
- Ensure Quality: Robust information collection methods are imperative to minimize errors. This encompasses comprehensive training for information gatherers, using validated tools, and applying ongoing monitoring procedures to ensure accuracy throughout the research. Our clinical biostatistics services offer comprehensive oversight in these areas, ensuring compliance with country requirements.
- Choose Appropriate Statistical Methods: The statistical analyses should be specifically tailored to align with the study objectives and types of information. Researchers should leverage advanced software tools capable of managing complex analyses, ensuring the correct application of pertinent statistical tests.
- Maintain Regulatory Compliance: Adherence to regulatory guidelines set forth by authorities such as INVIMA, Colombia's National Food and Drug Surveillance Institute, is non-negotiable. This includes thorough documentation and validation of all statistical methods used in the study. INVIMA's role as a Level 4 health authority by PAHO/WHO further emphasizes the importance of rigorous compliance.
- Conduct Sensitivity Analyses: Performing sensitivity analyses is crucial to assess the robustness of findings against potential biases or variations in data handling. This practice significantly enhances the credibility of findings, providing a deeper understanding of their implications.
- Report Findings Transparently: When sharing study findings, it is vital to clearly articulate the statistical methods utilized and the rationale behind their application. Transparency in reporting fosters trust within the scientific community and enables others to replicate the research, thus contributing to the body of knowledge.
- Feasibility and Site Selection: A key component of trial management is the feasibility and selection of research locations and principal investigators (PIs). Engaging with experienced PIs ensures that the research is conducted efficiently and effectively, adhering to both ethical standards and regulatory requirements.
- Import Permits and Nationalization: Understanding the import permit process and the nationalization of investigational devices is crucial for compliance. Our services help in navigating these regulatory pathways to ensure that all investigational products are appropriately authorized for use in trials.
The significance of biostatistics in health investigations is highlighted by educational programs, such as the 12-lecture series on biostatistics and bioinformatics for cancer analysis, which stresses the essential knowledge needed for effective design. Moreover, case examples such as the one on Artificial Intelligence in Health Informatics demonstrate real-world uses and factors of biostatistics in research, emphasizing how AI can improve data analysis and interpretation. By following these best practices, researchers can effectively utilize clinical biostatistics services to enhance the validity and reliability of their trials, ultimately leading to improved patient outcomes.
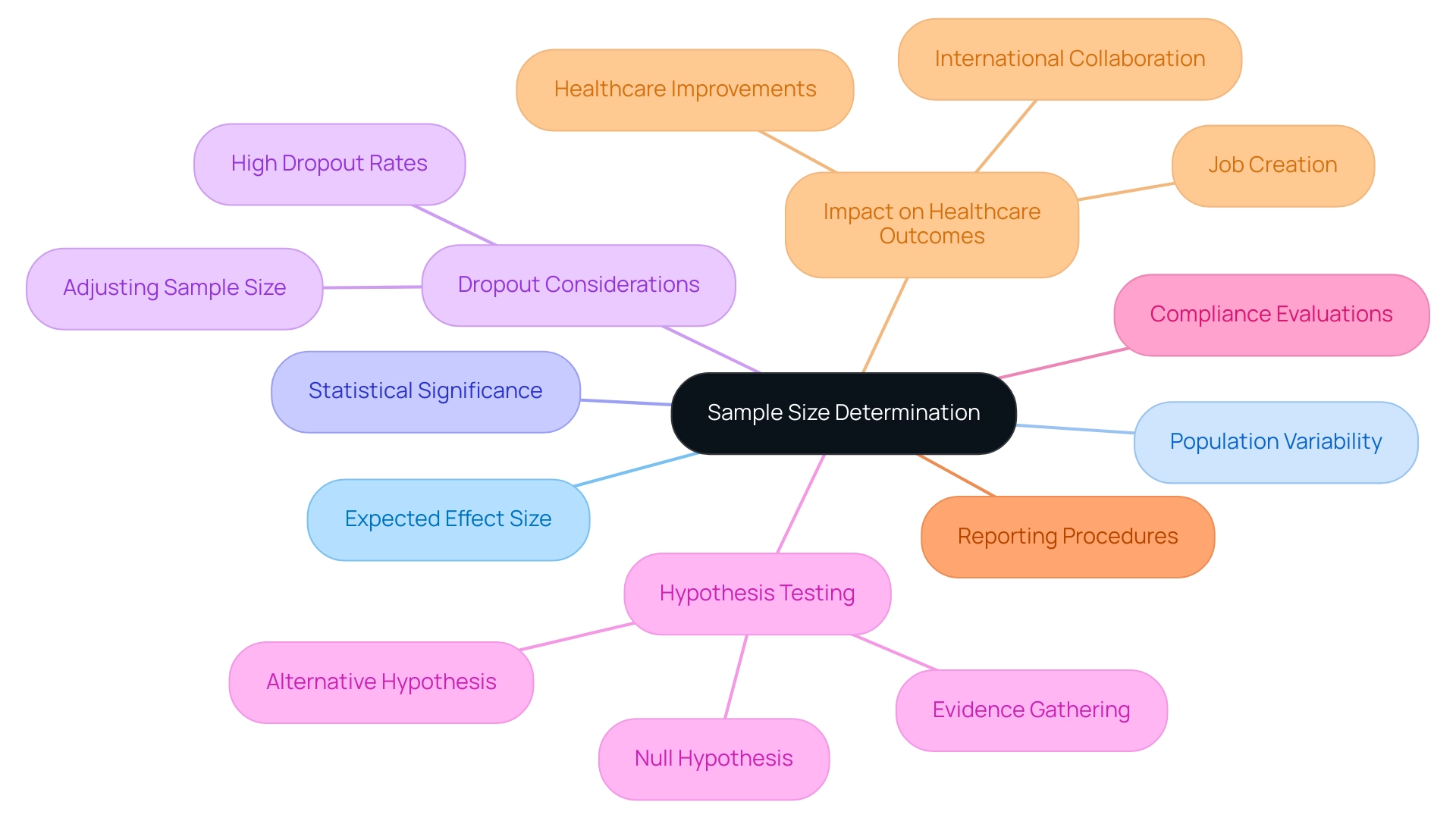
The Importance of Sample Size Determination
Precise sample size determination, a cornerstone of successful trials, is crucial for clinical biostatistics services to ensure the ability to detect a significant treatment effect when one exists. A common error in this process is underestimating the sample size needed to account for expected dropouts, particularly when dropout rates are high. An underpowered study risks failing to reveal significant results, while an excessively large sample can lead to unnecessary resource expenditure.
To calculate an appropriate sample size, researchers must consider several critical factors, which are often analyzed through clinical biostatistics services, including:
- The expected effect size
- Population variability
- The desired level of statistical significance
In the context of medical trials, the significance of these calculations cannot be overstated. As Dr. Prakash Patel, Executive Editor, emphasizes, 'A well-calibrated sample size is essential for drawing credible conclusions that can influence clinical practice.'
Furthermore, understanding that the treatment effect is assessed by comparing mean responses in treatment groups is crucial, as evidence must be gathered to either reject the null hypothesis or fail to reject it, without concluding that the null is true. Utilizing advanced software tools for power analysis can significantly streamline this process, allowing for more efficient and accurate calculations. Furthermore, it is essential to consider potential dropouts or cases of non-compliance, adjusting the sample size accordingly to maintain the integrity of the research.
For example, the case study titled 'Statistical Hypothesis Testing in Clinical Trials' illustrates the process of hypothesis testing, where a new treatment is compared to the current standard of care. By prioritizing sample size determination, researchers enhance the robustness of their findings and contribute to more dependable medical evidence, ultimately supporting better healthcare outcomes with clinical biostatistics services. Moreover, effective medical trials play a vital role in impacting local economies through job creation and healthcare improvements, fostering international collaboration within the medtech sector.
It is also vital to perform compliance evaluations, guarantee appropriate trial arrangements, and establish rigorous reporting procedures throughout the study to uphold high standards of medical information management.
Data Management and Integrity in Clinical Trials
Upholding information integrity is essential in clinical trials, as it fundamentally influences the validity of research findings. A thorough information management plan should include protocols for collection, storage, and analysis. Key practices include:
-
Establishing Standard Operating Procedures (SOPs): Clear SOPs are essential; they outline information collection methods, entry processes, and validation checks, which serve to minimize errors.
-
Utilizing Electronic Information Capture (EDC) Systems: EDC systems enhance the efficiency of information collection and enable real-time monitoring, facilitating the swift identification of discrepancies. This is crucial, given that collaborative research efforts can accelerate the development of new treatments by 15%.
-
Conducting Regular Reviews: Implementing routine audits is necessary to swiftly identify and rectify errors, ensuring that the information remains accurate and reliable throughout the study lifecycle.
-
Implementing Information Security Measures: Protecting sensitive information through encryption, access controls, and regular backups is imperative to prevent unauthorized access or loss of information. As highlighted by Datavant,
Medical research requires end-to-end visibility to generate insights from comprehensive patient journeys gathered before, during, and after the trial.
By prioritizing strong information management practices, researchers can uphold the integrity of trials, which is essential for effective clinical biostatistics services, thereby ensuring that their findings significantly contribute to medical knowledge. The preparation of a Statistical Analysis Plan (SAP) exemplifies this; a well-structured SAP delineates statistical methods, endpoints, handling of information, and reporting, ultimately leading to standardized and reliable clinical biostatistics services that are crucial for the success of a trial. This is where Quanticate’s Clinical Data Management Team plays a crucial role, providing tailored solutions that guarantee high-quality information across various unified platforms, including EDCs.
Furthermore, the function of social media in spreading best practices and innovations in data management cannot be ignored, as platforms like LinkedIn and Twitter promote knowledge sharing and community involvement among healthcare professionals.
Statistical Reporting Standards in Clinical Research
In the realm of clinical research, strict adherence to statistical reporting standards, notably the Consolidated Standards of Reporting Trials (CONSORT), is imperative for fostering transparency and reproducibility. Key elements that researchers must report include:
-
Research Design: It is crucial to provide a detailed account of the research design, encompassing randomization methods, blinding procedures, and control measures.
This allows readers to critically assess the validity of the findings.
-
Statistical Methods: Researchers should meticulously outline the statistical techniques employed for data analysis, including any adjustments made for confounding variables.
This detailed reporting is essential for understanding the basis upon which conclusions are drawn.
-
Results Presentation: Results must be presented clearly and concisely, utilizing tables and figures to enhance understanding.
Incorporating confidence intervals and p-values offers additional context for interpreting the findings.
-
Discussion of Limitations: Recognizing the constraints of the research is vital.
Researchers should openly discuss potential biases and the implications for the generalizability of the results.
By rigorously adhering to these statistical reporting standards, researchers not only enhance the credibility of their studies but also contribute to the wider distribution of valuable knowledge in healthcare through the use of clinical biostatistics services. This commitment to transparency is reflected in ongoing initiatives, such as the creation of guidelines for reporting trials that integrate Western and Chinese medicine, which highlights the significance of culturally relevant variables, as noted by Peter Feldman from National Ageing Research Australia, who stated,
This project to develop clinical biostatistics services as part of an extension of CONSORT and SPIRIT reporting guidelines will focus on the reporting of variables relevant to ethnically, culturally, and linguistically diverse participants in randomized controlled trials (RCTs).
Additionally, the TIDieR-Children and Adolescents for pediatric trial interventions, registered in 2025, highlights the growing emphasis on tailored reporting standards for specific populations. Furthermore, a fillable checklist is available online to assist authors and reviewers, and a draft checklist for the guidelines on Integrated Chinese and Western Medicine is currently under review. Such efforts are essential in guaranteeing that medical studies are both thorough and reachable to varied communities.
Ethical Considerations in Biostatistical Practices
Ethical considerations are paramount in the application of clinical biostatistics services within clinical research. Researchers bear the responsibility of ensuring that their statistical methods uphold the rights and welfare of participants. Key ethical principles include:
- Informed Consent: It is essential that participants receive comprehensive information regarding the study’s objectives, procedures, potential risks, and benefits prior to consenting to participate. Biostatistical practices must respect the autonomy of participants, ensuring they fully comprehend the implications of the study. As Jennifer Van Mullekom, a professor of practice in the Department of Statistics at Virginia Tech, articulates, "In addition, they will report key elements of their small group discussion to the larger group," which underscores the importance of transparent communication and feedback mechanisms in research contexts.
- Information Privacy: Safeguarding the confidentiality of participant information is crucial. This involves anonymization and secure storage practices to safeguard sensitive information. Researchers must be clear about how information will be utilized and shared, reinforcing trust with study participants.
- Integrity of Reporting: Researchers must maintain honesty and transparency when reporting statistical results derived from clinical biostatistics services. This includes avoiding selective reporting or data manipulation aimed at achieving preferred outcomes. Ethical vigilance is necessary to prevent misleading interpretations, such as p-hacking and overfitting. It is crucial that researchers do not overstate the significance of their results and are transparent about uncertainties, which is vital for maintaining ethical integrity.
- Oversight by Ethics Committees: Engaging with ethics review boards is vital to ensure that clinical biostatistics services adhere to ethical standards throughout the research process. These committees play a crucial role in upholding the integrity of clinical trials and supporting clinical biostatistics services.
- Case Analysis Insights: The case analysis titled "Ethical Considerations in the Use of Biostatistical Techniques" highlights the necessity for ethical vigilance in biostatistics. It emphasizes that researchers must be transparent about the limitations of their techniques and avoid overstating the significance of their results, ensuring that interpretations are appropriate and ethical.
By prioritizing these ethical considerations, researchers not only preserve the integrity of their studies but also contribute to the responsible advancement of medical knowledge. Understanding the ethical landscape in biostatistics is essential in fostering trust and credibility in clinical research.
Conclusion
Biostatistics plays a pivotal role in clinical research, serving as the backbone that supports the integrity of study designs, data analyses, and the interpretation of results. By implementing best practices such as early engagement of biostatisticians, ensuring data quality, and adhering to regulatory compliance, researchers can enhance the reliability of their findings. Moreover, accurate sample size determination and robust data management practices further solidify the foundation of clinical trials, ensuring that studies are not only scientifically valid but also relevant to diverse populations.
The importance of ethical considerations in biostatistics cannot be overstated. Upholding the rights and welfare of participants through informed consent, data privacy, and integrity in reporting is essential for maintaining trust in the research process. By prioritizing these ethical principles, researchers contribute to the responsible advancement of medical knowledge, fostering an environment of transparency and accountability.
Ultimately, the effective application of biostatistical methods is crucial for improving healthcare outcomes and advancing medical knowledge. By understanding and applying these principles, researchers can significantly enhance the credibility of their studies, leading to better-informed clinical decisions and ultimately benefiting patients and communities alike. The integration of advanced biostatistical techniques will continue to shape the future of clinical research, driving innovation and ensuring that findings translate into meaningful healthcare improvements.
Frequently Asked Questions
What are clinical biostatistics services and why are they important?
Clinical biostatistics services are essential for medical investigations, as they provide a foundation for the design, data analysis, and interpretation of outcomes. These methodologies ensure that trials are scientifically valid and yield reliable findings applicable to diverse populations.
What specific services does bioaccess® provide in expedited medical device trials?
Bioaccess® specializes in various trial management services including Early-Feasibility Assessments, First-In-Human Trials, Pilot Trials, and Post-Market Follow-Up Evaluations.
What capabilities are included in clinical biostatistics services?
The capabilities include feasibility and selection of research locations and principal investigators, compliance reviews of trial documents, trial setup and approval processes involving ethics committees and health ministries, import permits, and thorough project management and monitoring.
How does sample size affect trial design according to Dr. Arzu Onar-Thomas?
Dr. Arzu Onar-Thomas notes that trial design may be influenced by sample size limitations, which can lead to less optimal statistical outcomes. The focus should be on appropriately answering key trial questions, even if confidence in other results is lower.
Why is determining the optimal sample size crucial in clinical research?
Determining the optimal sample size is vital as it balances cost with statistical power, significantly impacting the integrity of the research.
What role do biostatisticians play in clinical trials?
Biostatisticians are crucial for interpreting complex data sets, uncovering trends and patterns that inform medical decisions and treatment protocols, which enhances the reliability and impact of medical studies.
What best practices should be followed when implementing biostatistical methods in clinical research?
Key best practices include engaging a biostatistician early, ensuring quality data collection, choosing appropriate statistical methods, maintaining regulatory compliance, conducting sensitivity analyses, transparently reporting findings, selecting feasible research sites, and understanding import permits.
How does regulatory compliance impact clinical biostatistics services?
Adherence to regulatory guidelines, such as those from INVIMA in Colombia, is critical. It ensures thorough documentation and validation of statistical methods, which is essential for the credibility of the study.
What is the significance of conducting sensitivity analyses in clinical trials?
Sensitivity analyses assess the robustness of findings against potential biases or variations in data handling, enhancing the credibility of results and providing a deeper understanding of their implications.
How can researchers ensure transparency in reporting study findings?
Researchers should clearly articulate the statistical methods utilized and the rationale behind their application when sharing study findings, fostering trust within the scientific community and enabling replication of research.




